Preliminary assessment of microcirculation in the ascending aorta in patients with normal and dilated ascending aorta using laser Doppler perfusion monitoring
Abstract
Background: Microcirculation within the ascending aortic wall, supplied by the vasa vasorum, is increasingly recognized as a potential factor in the pathogenesis of aortic aneurysms. However, in vivo assessment remains challenging. This pilot study aimed to evaluate ascending aortic microcirculation intraoperatively using Laser Doppler Perfusion Monitoring (LDPM).
Material and methods: Twenty-four patients (18 males, 6 females) undergoing elective cardiac surgery were enrolled. LDPM was performed on the exposed ascending aorta prior to surgical manipulation. A probe was sutured to the aortic wall and perfusion was recorded in Perfusion Units (PU). A brief probe compression test was conducted to evaluate perfusion response.
Results: The mean LDPM value was 281.58 PU (males: 339.94 PU; females: 139.86 PU). In males, LDPM negatively correlated with age (r = –0.593, p = 0.0121), whereas a moderate but non-significant correlation was noted with BMI (r = –0.303, p = 0.2366). No significant associations were observed with hypertension, diabetes, smoking, or aortic dilation. A paradoxical increase in PU during probe compression occurred in 54% of patients.
Conclusions: LDPM provides real-time intraoperative assessment of aortic microcirculation. Age-related decline in perfusion may contribute to aortic wall vulnerability. Further studies with larger cohorts are needed to validate these findings.
Citation
Krysiak M, Łoś A, Pawlaczyk R, Janeczek M, Hellmann M. Preliminary assessment of microcirculation in the ascending aorta in patients with normal and dilated ascending aorta using laser Doppler perfusion monitoring. Eur J Transl Clin Med. 2025;8(1):5-9Introduction
Microcirculation and its impairment as elements of the pathophysiology of cardiovascular diseases is recently gaining attention in recent research. In the aortic wall the microcirculation is a network of small vessels (vasa vasorum) that supply its tissue with blood flow and nutrients. Impairments in this network are considered an early pathogenic factor in cardiovascular diseases, and may often precede structural changes, e.g. formation of an aneurysm. The microscopic scale of these vessels poses a significant technical challenge in the evaluation of microcirculation. Laser Doppler Perfusion Monitoring (LDPM) provides a non-invasive method to measure tissue perfusion and its dynamic response to stimuli. Recent studies have explored the potential involvement of microcirculatory dysfunction in conditions such as aortic aneurysm, ischemic heart disease and hypertrophic cardiomyopathy.
Prior applications of LDPM in cardiac surgery have primarily focused on myocardial perfusion during off-pump coronary artery bypass grafting (OPCABG) [1-2]. LDPM has been utilized more widely in neurology [3], hypertension [4], nephrology [5], and plastic surgery [6]. However, studies involving LDPM in the assessment of aortic microcirculation remain scarce. Herein, we present preliminary LDPM data obtained intraoperatively from cardiac surgery patients at a single institution. In this pilot study, we aimed to establish a method of direct measurement microcirculation within the wall of the ascending aorta intraoperatively using LDPM. To the best of our knowledge, no reports have been published on the use of LDPM measurements in the ascending aorta, apart from a single press release authored by our team [7].
Materials and methods
We enrolled patients undergoing cardiac surgery in accordance with the European Society of Cardiology (ESC) guidelines, provided that replacement of the ascending aorta with a vascular prosthesis was not planned. The study population consisted of 24 patients (18 males, 6 females). The mean age for males was 63.88 years (median 65.0), and for females 71.14 years (median 70.0) (Figure 1). Six patients had ascending aortic dilation (> 35 mm), 6 had diabetes mellitus (25%), 18 had hypertension (66.6%), 2 had chronic kidney disease (8.3%), 14 were current or former smokers (58.3%) and 10 had a body mass index (BMI) of ≥ 30 (41.67%). All participants provided informed and voluntary consent to participate in the study. The research protocol was approved by an independent bioethics committee for scientific research.
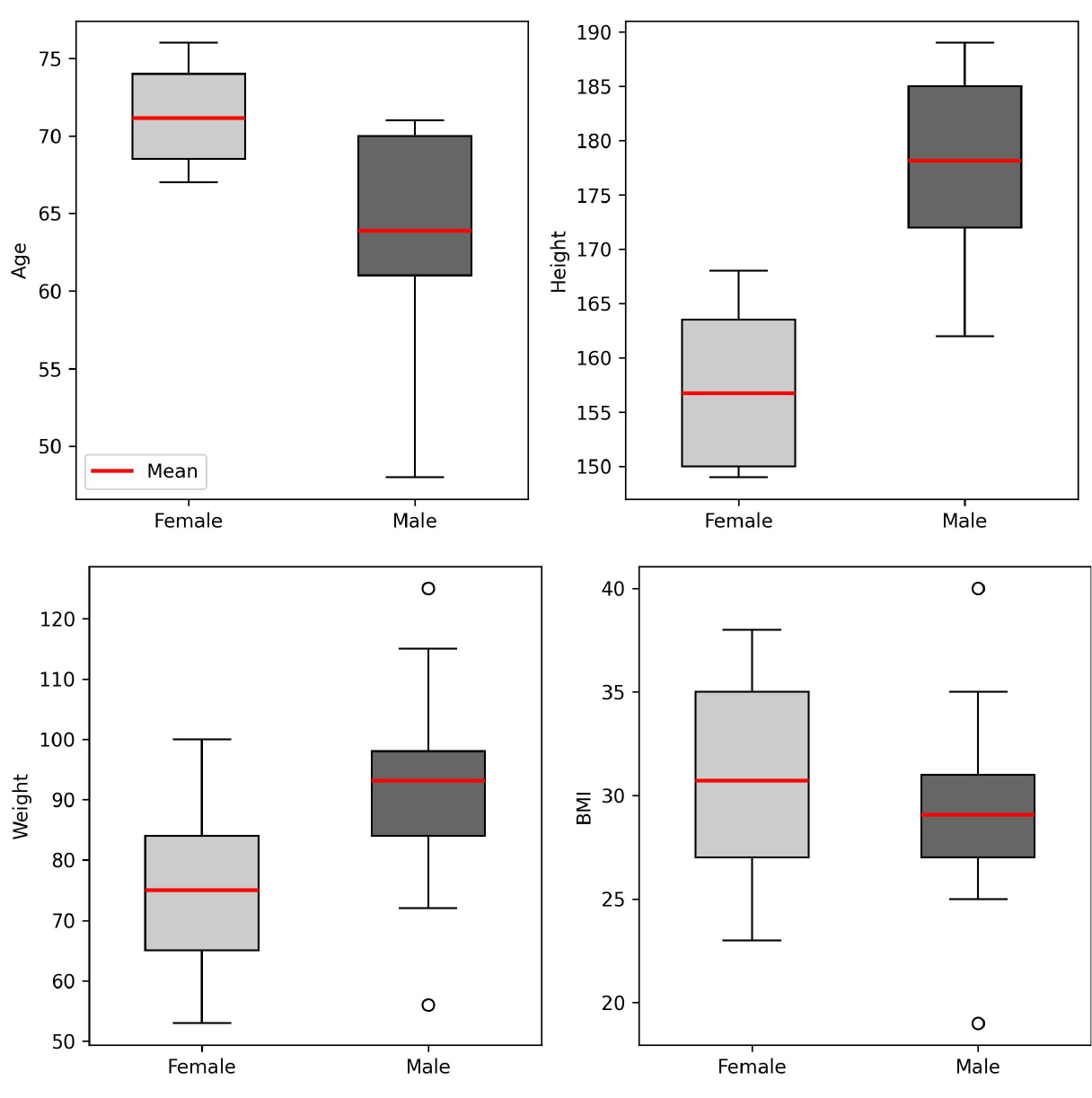
Figure 1. Population parameters: age (years), height (cm), weight (kg), BMI (kg/m²)
Surgical procedures were conducted by 1 of the 2 surgeons trained in the use of LDPM. Using laser Doppler technology, LDPM detects perfusion within within a tissue volume of approximately 1 mm³, penetrating up to 1 mm in depth. Perfusion values are reported in perfusion units (PU), which are not standardized and therefore allow for a relative comparison of measurements obtained with the same manufacturer’s equipment only.
All measurements were taken using the Probe 404-1 and Periflux 5000 base unit (PeriMed AB, Järfälla, Sweden). After exposure of the mediastinum and ascending aorta, the probe was sutured to the aortic wall using three single 5-0 stitches (Figure 2). Baseline perfusion was recorded for 1 minute. A compression test was then performed by applying pressure to the probe, and changes in perfusion were visualized graphically. Due to the need to maintain sterility in the surgical field, it was not possible to measure the amount of pressure applied during the probe compression test. However, the measurements were performed by only 2 cardiac surgeons, who aimed to apply a consistent amount of pressure in each test. While the compression test remains subjective, our goal was to minimize the variability resulting from differences between operators as much as possible. This test aimed to determine whether compression reduced the blood flow through the vasa vasorum, supporting the hypothesis that increased wall stress (e.g. in hypertension) may impair aortic wall perfusion, contributing to aneurysm formation [8].
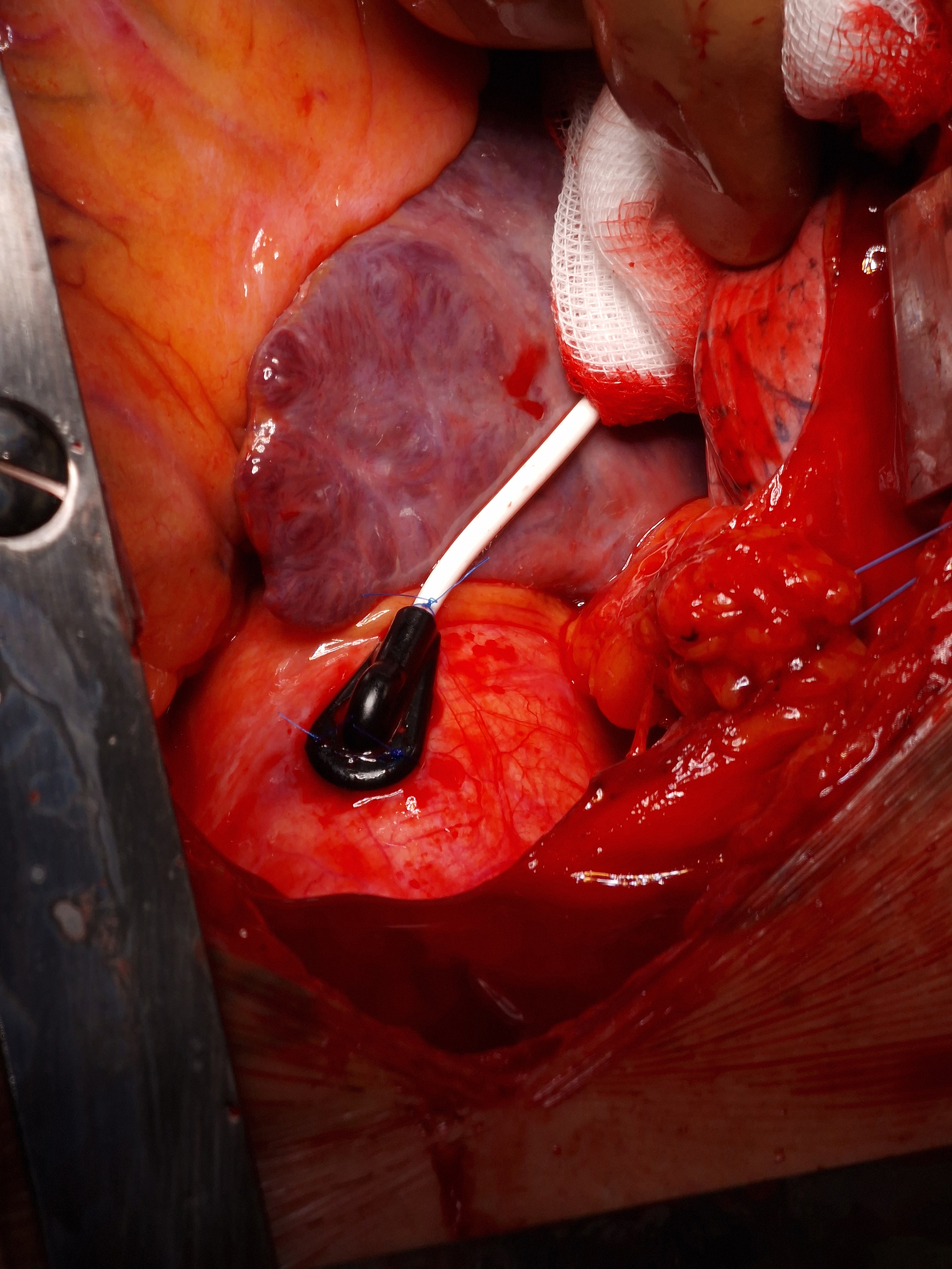
Figure 2. LDPM probe sutured to the ascending aorta
Results
The mean LDPM value in the study cohort was 281.58 PU, with a median of 215.5 PU. In male patients, the mean was 339.94 PU (standard deviation (SD) 241.2), and the median 252.0 PU, whereas in female patients the mean was 139.86 PU (SD 53.0), and median 149 PU (Figure 3). In males, LDPM negatively correlated with age (Spearman’s r = –0.593, p = 0.0121) (Figure 4); a moderate but non-significant correlation was noted in females (Spearman’s r = 0.429, p = 0.3374). A moderate but non-significant correlation between BMI and LDPM was found in males (Spearman’s r = –0.303, p = 0.2366), with no significant correlation in females.
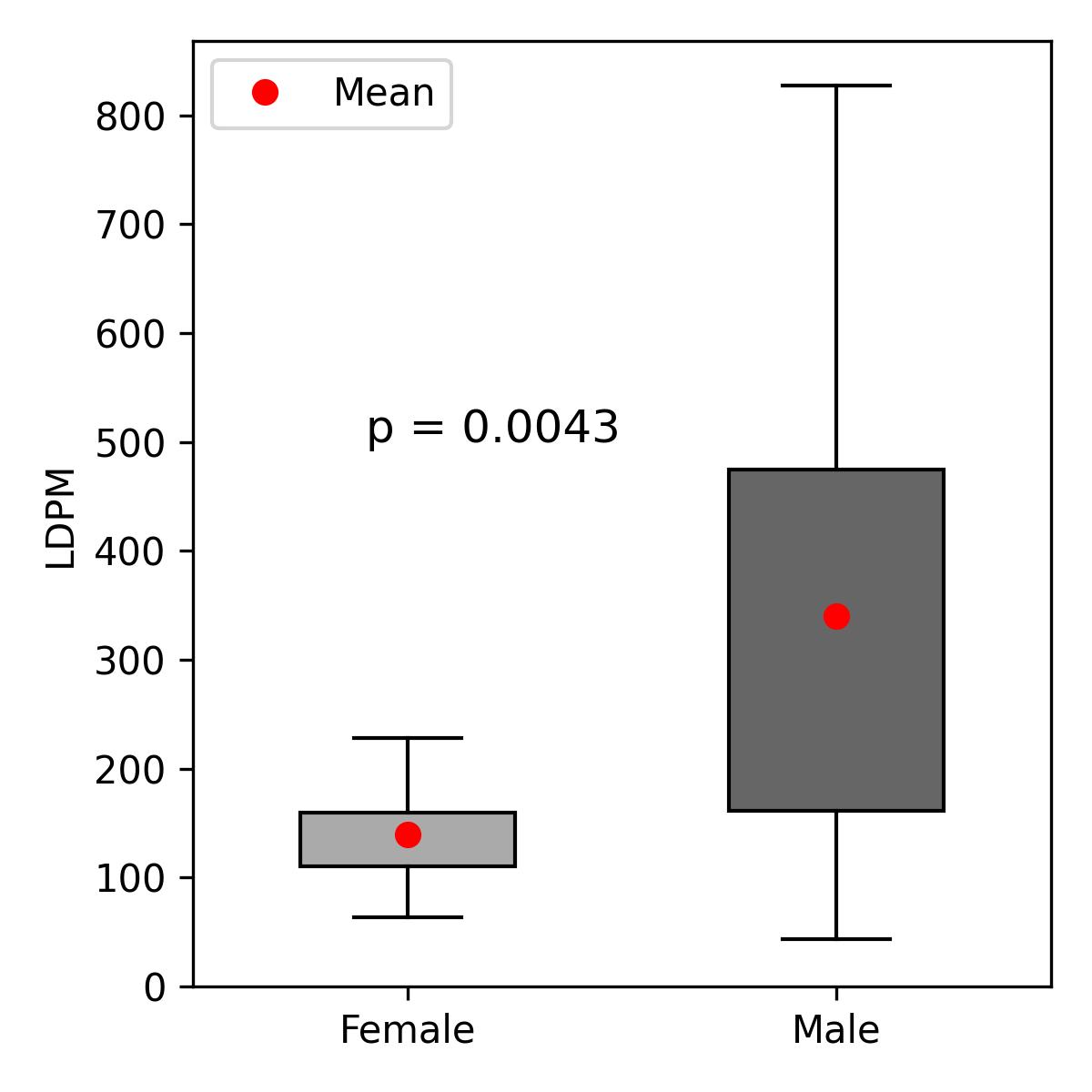
Figure 3. LDPM in the male and female populations
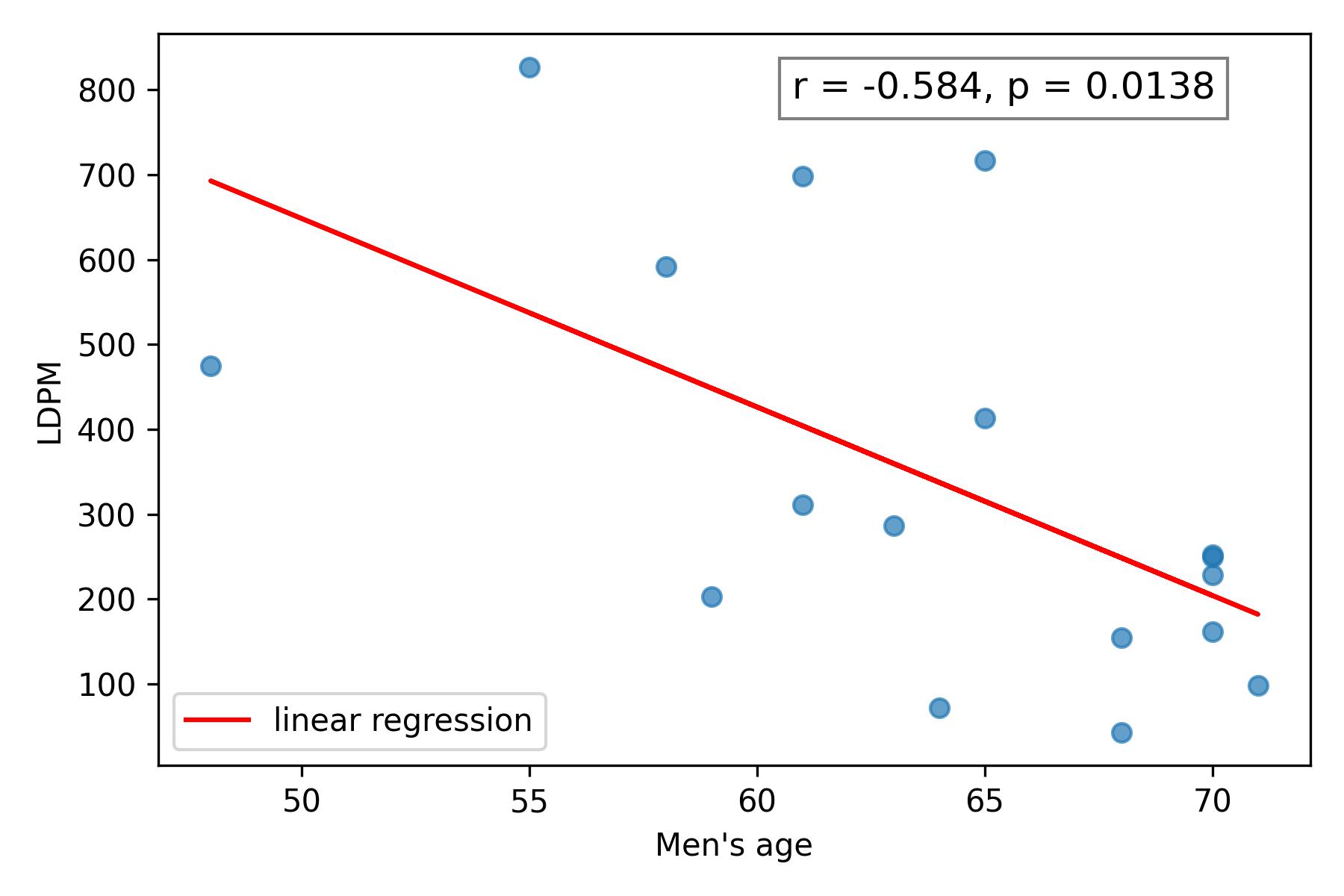
Figure 4. The relationship between LDMP and age in men
In the group with an enlarged ascending aorta, the mean LDPM value was 312.17 PU (SD 213.71 PU), with a median of 238.5 PU. In the group without aortic enlargement, the mean LDPM was 271.39 PU (SD 231.41 PU), and the median was 185.5 PU. No statistically significant difference was observed between the groups with and without ascending aortic enlargement (Mann-Whitney U test, p = 0.505). No significant correlations were observed between LDPM values and smoking (Pearson’s r = 0.199), diabetes mellitus (r = –0.09), or hypertension (r = –0.036). Interestingly, 54% of patients exhibited a paradoxical increase in PU during the compression test; the remaining patients showed the expected decrease in perfusion.
Discussion
Our microcirculatory assessment of the ascending aorta is an early but promising step toward understanding the pathophysiology of aneurysm formation. Cystic medial degeneration, a primary cause of ascending aortic aneurysms, is often associated with hypertension-induced vascular remodeling [9]. Hypertensive arteriopathy may extend to the aortic vasa vasorum, impairing their function. Similarly, poorly controlled diabetes is known to damage microvasculature in organs such as the retina and kidneys, raising the possibility that similar damage occurs in the aortic wall [10]. This pilot study suggests several promising directions for further exploration, including correlating intraoperative LDPM data with the histopathological analysis of aortic adventitia.
One of our aims was to evaluate the impact of hypertension and diabetes on ascending aortic microcirculation. However, our small sample size (n = 24) limited the statistical power and may explain the absence of statistically significant correlations. Depending on the assumed parameters, the sample size required to achieve adequate statistical power ranges from 75 to 128 participants. We anticipate that a study with 100 participants, combined with improved data collection, would enable the detection of statistically significant correlations and the drawing of meaningful conclusions.
Another limitation of LDPM is the laser’s penetration depth, which reaches a maximum of approximately 1 mm into the wall of the ascending aorta. As a result, individual differences in the thickness of the aortic wall layers, and the associated variability in tissue vascular density, may lead to discrepancies in measurements between patients. This represents an additional limitation of the LDPM, highlighting the need for further analysis and the development of standardized techniques for measurements. Another unresolved issue involves the heterogeneous response to the compression test, suggesting the need for further validation of this method.
Additionally, the use of a single perfusion probe significantly limited our measurement throughput, as each probe required prolonged sterilization between surgical procedures. In our study the aortic diameter was determined via echocardiography, a modality subject to operator variability [11]. Whereas computed tomography angiography would provide more precise measurements and may be advisable in future studies.
Conclusions
This pilot study demonstrates the feasibility of directly measuring microcirculation in the ascending aortic wall intraoperatively using LDPM. The observed negative correlation with age may reflect the age-related decline in aortic microvascular function. No statistically significant correlations were found between LDPM and aortic dilation, diabetes, hypertension, smoking, or BMI. Further investigations in larger cohorts with an extended parameter analysis are warranted to validate these preliminary findings.
Funding
The project was funded by the state budget, granted by the Ministry of Science and Higher Education as part of the program “Student Research Groups Create Innovations.”
Conflict of interest statement
The authors declare no financial relationships with any commercial entity that could have influenced the outcomes or interpretations presented in this manuscript, and report no conflicts of interest.

References
| 1. |
Piotrowski J, Anisimowicz L, Hellmann M. Laser Doppler flowmetry to assess myocardial microcirculation. Cardiol J [Internet]. 2020;27(2):197–9. Available from: https://journals.viamedica.pl/cardiology_journal/article/view/66850.
|
| 2. |
Hellmann M, Piotrowski J, Anisimowicz L, Cracowski J-L. A mystery of the myocardial microcirculation during coronary artery bypass grafting. Eur J Cardio-Thoracic Surg [Internet]. 2018;54(2):405–405. Available from: https://academic.oup.com/ejcts/article/54/2/405/4917834.
|
| 3. |
Wårdell K, Blomstedt P, Richter J, Antonsson J, Eriksson O, Zsigmond P, et al. Intracerebral Microvascular Measurements during Deep Brain Stimulation Implantation using Laser Doppler Perfusion Monitoring. Stereotact Funct Neurosurg [Internet]. 2007;85(6):279–86. Available from: https://karger.com/article/doi/10.1159/000107360.
|
| 4. |
Rizzoni D, Nardin M, Coschignano MA, Rossini C, De Ciuceis C, Caletti S, et al. Methods of evaluation of microvascular structure: state of the art. Eur J Transl Clin Med [Internet]. 2018;1(1):7–17. Available from: https://ejtcm.gumed.edu.pl/articles/10.
|
| 5. |
Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: Correlation with cardiovascular risk. Kidney Int [Internet]. 2006;70(1):157–64. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0085253815517523.
|
| 6. |
Łoś A, Walczak I, Bieńkowski M, Kutryb-Zając B, Hellmann M. New insight into the aortic microcirculation in coronary disease: Intraoperative laser Doppler flow measurement and vasa vasorum imaging. Polish Hear J [Internet]. 2024;82(10):1008–9. Available from: https://journals.viamedica.pl/polish_heart_journal/article/view/101739.
|
| 7. |
Sundheim LK, Sporastøyl AH, Wester T, Salerud G, Kvernebo K. Acute skin trauma induces hyperemia, but superficial papillary nutritive perfusion remains unchanged. Microcirculation [Internet]. 2017;24(7). Available from: https://onlinelibrary.wiley.com/doi/10.1111/micc.12389.
|
| 8. |
Chumachenko P V, Ivanova AG, Bagheri Ekta M, Omelchenko A V, Sukhorukov VN, Markin AM, et al. Condition ‘Vasa Vasorum’ in Patients with Thoracic Aortic Aneurysm. J Clin Med [Internet]. 2023;12(10). Available from: http://www.ncbi.nlm.nih.gov/pubmed/37240684.
|
| 9. |
Srivastava P, Gupta M, Mandal A. Cystic Medial Degeneration Leading to Aortic Aneurysm and Aortic Regurgitation. Hear India [Internet]. 2014;2(4):107. Available from: https://journals.lww.com/10.4103/2321-449X.146617.
|
| 10. |
Alnaser RI, Alassaf FA, Abed MN. Melatonin as a potential treatment option in diabetes complications. Eur J Transl Clin Med [Internet]. 2024;7(2):78–91. Available from: https://ejtcm.gumed.edu.pl/articles/192108.
|
| 11. |
Beetz NL, Trippel TD, Philipp K, Maier C, Walter-Rittel T, Shnayien S, et al. Discrepancy of echocardiography and computed tomography in initial assessment and 2-year follow-up for monitoring Marfan syndrome and related disorders. Sci Rep [Internet]. 2022;12(1):15333. Available from: https://www.nature.com/articles/s41598-022-19662-y.
|












