Abstract
Recent research indicates that disrupted sleeping and eating patterns along with ageing can contribute to the development of diabetes mellitus (DM) via effects on circadian rhythms and metabolic control. The use of anti- -DM drugs alone is insufficient to achieve control of the reduction in β-cell function, insulin resistance, inflammatory mediation, oxidative stress and the DM-related complications. To prevent micro- and macrovascular DM complications, researchers are exploring the therapeutic potential of melatonin, a hormone secreted from the pineal gland with peak at night-time. We searched the Cochrane Library, PubMed and Google Scholar databases for relevant articles. Review articles, clinical research and case studies about the impact of melatonin on DM complications, the anti-oxidant action of melatonin, anti-inflammatory action of melatonin and the combination of melatonin plus DM drugs were included in this review. One hundred three articles that met our selection criteria, published between November 2004 and January 2024 were analyzed. This review aims to summarize the literature regarding melatonin’s mechanisms of action and potential as a therapeutic option in the treatment of DM complications.
Citation
Alnaser R I, Alassaf F A, Abed M N. Melatonin as a potential treatment option in diabetes complications. Eur J Transl Clin Med. 2024;7(2):78-91Introduction
René Descartes described the pineal gland as “the seat of the soul.” Today we know that the pineal gland’s main role is to receive and convey information about the light-dark cycle through the secretion of melatonin, the so-called “darkness hormone” [1]. This endogenous hormone’s secretion is regulated by circadian rhythms [2]. In addition, it plays an important role in glucose metabolism, along with its anti-aging, anti-cancer, and anti-inflammatory properties [3-4]. When the circadian rhythm is disrupted, incorrect signals of the central circadian timing system are sent and thus disrupting the release of melatonin and glucose homeostasis, that can even lead to DM mellitus [5-6].
DM mellitus (DM) is a chronic disease characterized by elevation in blood sugar concentration (hyperglycemia). There are two main types of DM: type-1 DM mellitus (T1DM, triggered by an auto-immune disease) and type-2 DM mellitus (T2DM, due to insulin resistance) [7-10]. Interestingly, melatonin has been associated with both types of DM. In T1DM, elevated plasma levels of melatonin and reduced insulin levels are observed, whereas T2DM is characterized by lower melatonin levels. This is consistent with the finding that melatonin and insulin antagonize each other [3]. This is because in T1DM the pancreatic beta (β) cells are destroyed leading to decreased insulin production and hyperglycemia, while the enzyme activation cascade stimulates melatonin production. However, in T2DM low melatonin levels cause higher mRNA expression of the melatonin receptors and insulin resistance enhances the elevation of insulin, resulting in β-cell exhaustion and elevated glucagon leading to hyperglycemia [11]. Unhealthy diet and obesity are commonly known risk factors for T2DM, while recent studies suggest that shift work and sleep disorders can also raise the risk of T2DM by disrupting circadian rhythms [12]. Patients with T2DM are frequently shown to have micro- and macrovascular complications [13-14].
Chronic and uncontrolled hyperglycemia leads to the formation of advanced glycated end products (AGEs), leading to the generation of reactive oxygen species (ROS) and stimulation of inflammatory cascade and resulting in DM complications [15-16]. Melatonin has anti-oxidant properties and emerging evidence suggests it reduces DM complications via amelioration of oxidative damage [17]. In addition to its role as a ROS scavenger, melatonin can alleviate DM complications via several mechanisms described in this review.
Both direct and indirect anti-oxidant properties of melatonin can protect our bodies and maintain health. Additionally, melatonin can have a potential role in the protection of mitochondrial action, modulation of the immune system, improvement in circadian rhythm control, modulation of the immune system and neuroprotective actions [18]. Unfortunately, as we age the levels of melatonin in our bodies decrease and this decrease is even more pronounced in people with insulin resistance-related diseases e.g. DM [19].
In this review we aimed to summarize the literature regarding melatonin’s mechanisms of action and potential as a therapeutic option in the treatment of DM complications.
Material and methods
The literature search was performed, during the period between November 2023 and February 2024 on databases including the Cochrane Library, PubMed and Google Scholar. The keywords “melatonin”, “anti-oxidant”, “DM mellitus” and “complications” were used separately and in combination. Articles about the impact of melatonin on DM complications, the anti-oxidant action of melatonin, anti-inflammatory action of melatonin, and the combination of melatonin plus DM drugs were included in this review. We limited the search to articles published in English to ensure that the studies could be understood and assessed by the review team. Articles published in other languages and irrelevant to the research questions were excluded.
Results and discussion
The initial search returned 200 abstracts. After screening the abstracts, we conducted a thorough manual review of the 153 articles and found 103 that met our selection criteria, published between November 2004 and January 2024.
Mechanism of hyperglycemia toxicity
Chronic hyperglycemia can cause damage through several mechanisms e.g. increased glucose metabolism, decreased antioxidant enzyme capacity, protein kinase C (PKC) activation, irreversible protein glycation, AGE formation and an imbalance between ROS formation and elimination resulting in oxidative stress [20]. Glucose enters the cell and undergoes metabolism to generate electron donors used by mitochondria for energy production. However, when the glucose level exceeds the mitochondrial capacity, overload electron donors will form ROS e.g. superoxide [21].
Glutathione is a crucial antioxidant in cells but it needs to be reduced by glutathione reductase and this process requires a cofactor: nicotinamide adenine dinucleotide phosphate (NADPH). Hyperglycemia can lead to depletion of NADPH, thus limiting the generation of reduced glutathione and making cells susceptible to ROS activity. The increased hexosamine pathway flux is one of the associated mechanisms in DM, it contributes to complications and also generates ROS [22].
Hyperglycemia increases the formation of diacylglycerol (DAG), which activates the PCK pathway, promotes the expression of endothelial nitric oxide synthase and inhibits endothelin-1, thus supporting vasodilation. As tissues become insulin resistant, this pathway decreases the synthesis of nitric oxide and increases the synthesis of vasoconstrictor endothelin-1. Chronic hyperglycemia also directs the glycation of intracellular proteins, causing the formation of AGEs and affecting vascular function through increasing vascular wall stiffness [23].
Chronic hyperglycemia in the past can cause DM-related complications such as micro- and macrovascular diseases, even after normalizing blood glucose levels. This is due to the metabolic memory of tissues, including pancreatic β-cells. Thus, in order to control the progression of DM complications, it is important to control hyperglycemia [24].
Mechanism of anti-oxidative action of melatonin
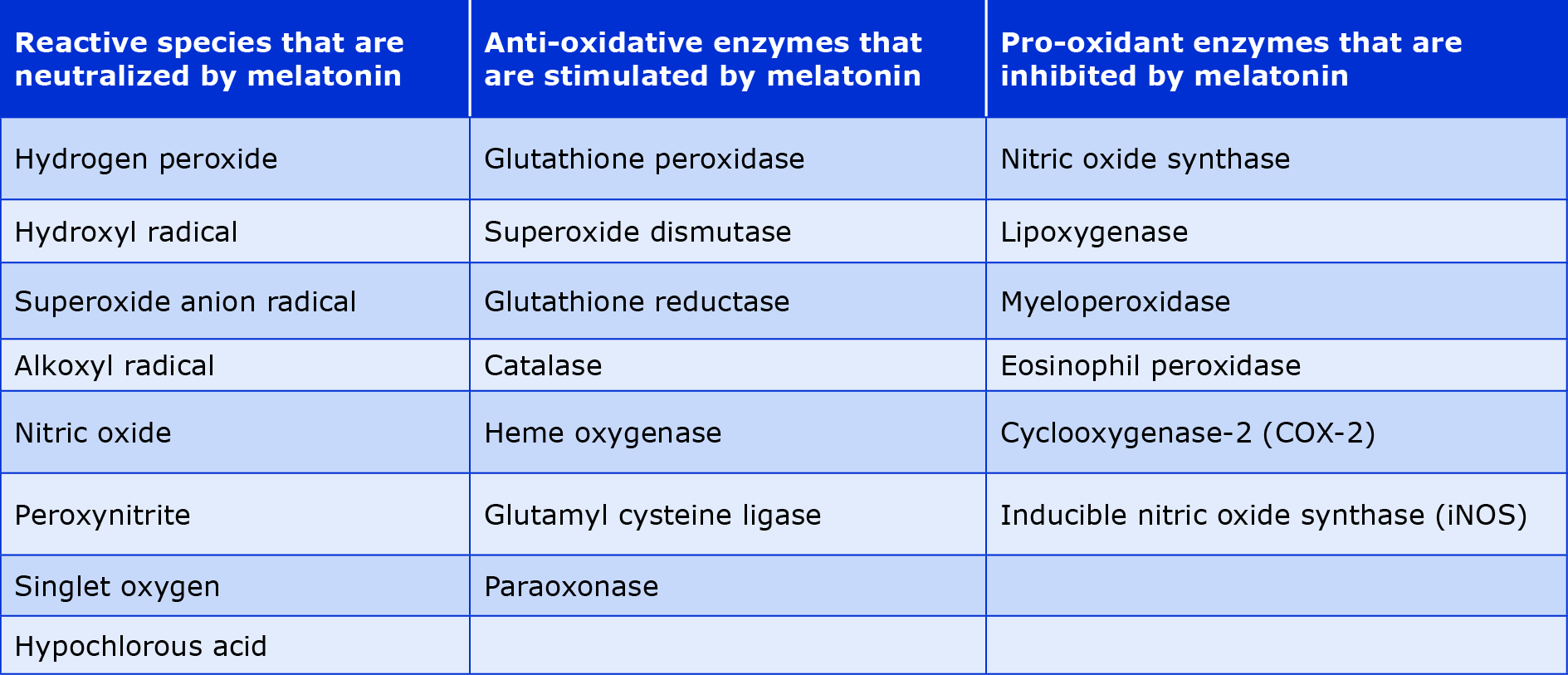
Beyond the role of biological clock synchronizer, melatonin has pleiotropic activity, e.g. as an antioxidant [25]. Because of its lipophilicity, melatonin can cross the blood-brain barrier as well as the cell membrane to enter several tissues. It acts as a direct scavenger of reactive oxygen and nitrogen species, thus protecting the cell from free radical damage [26]. Additionally, melatonin has indirect anti-oxidant activity by stimulating the expression of several antioxidant enzymes and glutathione (a crucial low molecular weight non-enzymatic antioxidant) that work cooperatively with other antioxidants to enhance the mitochondrial electron transport chain [27]. In summary, melatonin and its metabolites inhibit the activity or expression of genes involved in free radical generation and upregulates the genes involved in free radical detoxification, as shown in Table 1.
Melatonin scavenges free radicals by enhancing antioxidant enzyme activity, therefore it protects the mitochondrial cell membrane, improves the electron transport chain activity, and enhances adenosine triphosphate (ATP) production [28]. A consequence of reactive species activity is lipid peroxidation, a naturally occurring metabolic process of alteration in the structure and function of the cell membrane, leading to a change in membrane fluidity which is the pathomechanism of ageing and several diseases. Melatonin is a stabilizer and inhibitor of the lipid peroxidation process via its ROS scavenger activity [29]. Moreover, melatonin can chelate metals such as iron, copper, cadmium, aluminum, zinc and lead, thus preventing their oxidative action and regenerating the activity of biological proteins such as collagen and insulin [30].
In addition to the anti-oxidant action, melatonin can inhibit the activation of nuclear factor-kappa B (NF-kappaB) a transcriptional factor which decreases the expression of inflammatory mediators such as cyclooxygenase (COX-2), tumor necrosis factor 1 alpha (TNF-1α) and inducible nitric oxide synthase (iNOS) [27]. Thus, melatonin is potentially helpful for a range of inflammatory conditions. Melatonin exerts anti-inflammatory activity by binding to macrophages and lymphocytes, blocking the transcriptional factors that trigger proinflammatory cytokines including hypoxia-inducible factor (HIF), nuclear factor Erythroid2-related factor 2 (NRF2), cyclic adenosine monophosphate (cAMP), cAMP response element binding protein (CREB), signal transducer and activator of transcription (STAT), peroxisome proliferator-activated receptors (PPARs) and activator protein-1 (AP-1) [31].
Table 1. Antioxidant effects of melatonin [32]
Complications of diabetes
There are two major types of DM complications: microand macrovascular. The microvascular complications include retinopathy, nephropathy, and neuropathy. Whereas the macrovascular complications involve cerebrovascular disease, coronary artery diseases (CAD) and peripheral vascular disease [33-34] (Figure 1).

Figure 1. The potential roles of melatonin in DM complications
Chronic and poorly controlled hyperglycemia is the main factor that leads to DM complications which impair the quality of life and if not treated can even be life-threatening [35]. In addition to uncontrolled hyperglycemia, a sedentary lifestyle along with unhealthy diet, smoking, obesity, hypertension and hypercholesterolemia could increase the likelihood of developing micro- and macrovascular DM complications [36]. Inflammatory factors and external environmental factors that disrupt endocrine homeostasis may also play a major role in DM complications. As mentioned above, as individuals age, the secretion of the melatonin decreases, thus increasing the risk of DM complications [19].
Melatonin and microvascular complications
Diabetic retinopathy
Diabetic retinopathy (DR) is the major cause of blindness in patients with DM [37]. The pathophysiology of DR is linked to oxidative stress, inflammation, and autophagy [37]. The oxidative stress in the retina of a patient with DM is characterized by an elevation in ROS with a decrease in anti-oxidant enzymes e.g. superoxide dismutase. Additionally, hyperglycemia with retinal hypoxia resulting in overexpression of NADPH oxidase leads to overproduction of ROS. Moreover, the ROS activates the NF-kappaB transcriptional factor, which promotes the transcription of various genes, including those involved in the synthesis of nitric oxide and proinflammatory cytokines. Thus, using an anti-oxidant agent that increases the activity of superoxide dismutase can prevent the development of DR.
The other key factor of DR is chronic inflammation in which inflammatory cytokines including IL-1β, IL-8, IL-6, TNF1α, and monocytes chemoattractant protein-1 cause microvascular cell loss and damage of the blood-retina barrier [38]. Melatonin indirectly inhibits NF-kappaB and subsequent inflammatory mediators, thus reducing retinal lipid peroxidation and nitric oxide activity, inhibiting COX-2 activity and neutralizing hydroxyl radicals [39].
In DR, autophagy has dual roles: sustaining the function of the retina and also it has deteriorating effects in the progression of retinopathy. In the early stage of DR, autophagy protects neurons and the blood-retinal barrier from damage, whereas in advanced stage the overexpression of autophagy causes cell death and retinal damage. Hyperglycemia also induces autophagy inside the retinal gliocytes known as Müller cells and can also cause lysosomal dysfunction, which hinders the autophagy. This dysfunction can lead to the accumulation of cellular waste and stress within the Müller cells, which can result in the release of vascular endothelial growth factor (VEGF), a protein crucial for vascular function. Excess VEGF can cause abnormal blood vessel growth and leakage, leading to retinal damage and the development of DR [40].
In normal conditions, melatonin promotes autophagy for regulating cell survival, but during hyperglycemia and oxidative stress, melatonin indirectly inhibits autophagy through alleviating oxidative stress, inflammation, and endoplasmic reticulum stress and decreasing proinflammatory cytokines and VEGF [41-42].
Several clinical trials explored that the levels of melatonin secretion in proliferative DR patients are lower than in patients with non-proliferative DR and people without DM [43-45]. Wan et al. confirmed the lower level of plasma melatonin in DR and suggested that melatonin could be used as a biomarker for diagnosing this disease [46]. Moreover, administration of melatonin deactivates the microglia and prevents the death of epithelial cells in the retina via amelioration of oxidative stress, thus conserving the integrity of the inner blood-retinal barrier [47].
Diabetic nephropathy
Diabetic nephropathy (DN) involves kidney deterioration due to oxidative stress and is the major cause of end-stage renal disease in patients with DN worldwide [48]. It is characterized by glomerular hyperfiltration, nephron enlargement and mesangial cell hypertrophy, ultimately resulting in glomerulosclerosis [49]. Uncontrolled hyperglycemia with persistent ROS generation leads to apoptosis of several types of cells, including epithelial cells of the proximal tubule, damaging the nuclear DNA and mitochondria's genetic material that promotes loss of kidney function. Moreover, abnormal activation of the renin-angiotensin system (RAS) via various pathways such as PKC, adenosine monophosphate kinase (AMPK) and transforming growth factor-β (TGF-β) impact renal functions and lead to damage [50].
As an anti-oxidant, melatonin can reduce the oxidative stress markers of renal injury [51]. However, the nephroprotective activity of melatonin includes not only the anti-oxidant properties but is also demonstrated in reducing insulin resistance, decreasing blood glucose through improving insulin signaling, regulating glucose uptake by the renal tubules through its regulatory effect on glucose transporter (GLUT 1) and inhibiting the activation of RAS [52-54]. Therefore, by decreasing the loss of podocytes, reducing urinary microalbumin excretion, alleviating inflammation and fibrosis of the kidney, melatonin may increase renal recovery [42, 55]. Satari et al. investigated the positive effect of melatonin on improving metabolic parameters such as fasting blood glucose, total antioxidant capacity, glutathione levels, gene expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) and high-density lipoprotein (HDL) levels in patients with DN after 12 weeks of administration [56]. Additionally, melatonin can inhibit renal ischemia-reperfusion injury among renal transplant patients by improving kidney function, ameliorating oxidative stress and inflammation [57].
Diabetic neuropathy
Diabetic neuropathy (DNP) is a nerve damage disorder resulting from hyperglycemia that induces oxidative stress, it includes autonomic, proximal, focal and peripheral neuropathy. Peripheral neuropathy is the most common and represents the main cause of altered gait, neuropathic pain, foot ulceration, and amputation [58]. Hyperglycemia, dyslipidemia and insulin resistance can lead to DNP, which enhance the activation of several pathways e.g. PKC, polyol, AGE formation, poly adenosine diphosphate-ribose polymerase (PARP) and loss of insulin signaling [59]. These metabolic alterations lead to changes in gene expression, abnormal ion current (increased sodium channel activity and decreased potassium channel activity), mitochondrial dysfunction, together with inflammation and oxidative stress resulting in nerve damage and cell death [60]. The neuroprotective action of melatonin occurs via several mechanisms including oxidative scavenger, immune-modulator, and anti-nociceptive effects [61].
The anti-apoptotic effect of melatonin in DNP results from the stimulation of PKC/NF-kappaB pathways that leads to the expression of PTEN-induced putative kinase 1 (PINK1), a protein that maintains mitochondrial health [62]. In addition to the role of melatonin in upregulating antiapoptotic proteins such as B-cell CLL/lymphoma (BCL-2), mammalian target of rapamycin mTOR, NF-kappaB, and WNT signaling pathways in high glucose-treated Schwann cells [63]. Melatonin could inhibit the endoplasmic reticulum stress and insulin resistance in T2DM through inhibition of apoptosis signal-regulated kinase 1 (ASK1), a protein that mediates apoptosis and cell death [64]. Additionally, one of the clinical trials assessed the efficacy of melatonin as an adjunct to pregabalin (150 mg) once daily to relieve the pain of DNP. Participants received a daily dose of 3 mg of melatonin for 1 week, followed by a daily dose of 6 mg for 7 weeks. The melatonin-treated individuals showed a significant decrease in DNP pain compared to the control group [65].
Melatonin and macrovascular complications
Atherosclerosis is the key pathological mechanism of cerebrovascular, coronary and peripheral arterial diseases, affecting arteries all over the body. It is a condition of plaque buildup inside arterial walls leading to the narrowing of arterial lumen, decreasing blood flow, and potentially causing macrovascular complications. Hyperglycemia contributes to oxidative stress, inflammation, and endothelial dysfunction which collectively promote atherosclerosis in patients with DM [66].
Cerebrovascular disease
Cerebrovascular diseases including cognitive impairment, Alzheimer’s disease and dementia are all correlated with DM complications, resulting from elevated intracellular glucose levels that lead to auto-oxidation of glucose, excessive ROS formation, disruption of vascular function and reduction in the brain blood flow, leading to neuronal injury and dysfunction [67]. Additionally, melatonin can neutralize the ROS and reduce insulin resistance in the T2DM patient with Alzheimer’s disease [41, 68]. Furthermore, atherosclerosis in the cerebral arteries can obstruct and reduce blood flow to the brain causing stroke. A clinical study demonstrated the effectiveness of melatonin in improving functional and neurological recovery outcomes in patients with acute ischemic stroke when taken within 24 hours of the stroke symptom onset [69].
Coronary artery disease
Coronary artery disease (CAD) is considered the 3rd leading cause of mortality worldwide [70]. DM is a significant CAD risk factor in developed countries, with studies showing higher incidence of CAD among men and women with DM, than those without [71]. DM has a different impact on CAD mortality: women have a higher risk of mortality than men. Moderate hyperglycemia even without a DM diagnosis can increase the risk of CAD [72]. A meta-analysis by Berry et al. found significant differences in lifetime risks of cardiovascular disease among 257384 participants. ranked by, age, gender and race. They concluded that participants with high-risk factors (DM, hypertension, smoking, hypercholesterolemia) at any age were associated with substantially higher lifetime chances of CAD [73].
Atherosclerosis in the coronary arteries involves several pathological mechanisms, the major one is inflammation which triggers the formation of atherosclerotic plaque [74]. Deposition of the lipid, oxidative stress, endothelial dysfunction and differentiation of the vascular smooth muscles contribute to plaque formation [75]. The positive effects of melatonin on insulin resistance and lipid profile render it a good mediator for managing coronary artery disease in patients with DM [76]. Moreover, a link between melatonin secretion and reduction in systolic blood pressure has been shown. However no such association was found with diastolic blood pressure, indicating that the mechanisms that are not fully understood at this time [77].
Peripheral artery disease
Peripheral artery disease (PAD) is a complete or partial occlusion of the peripheral artery in the limb by an atherosclerosis plaque. PAD is the main cause of non-healing ulcers, limb amputation and mortality, particularly in patients with DM [78]. Animal studies have explored the effect of melatonin on heat-induced inflammatory and coagulation responses and they found that melatonin normalized platelet morphology and elevated fibrinogen levels following thermal injury [79-80]. In healthy young men, melatonin was associated with a significant reduction in fibrinogen levels, but no change in factor VII:C (a coagulation regulatory factor) and D-dimer levels [81]. Besides, melatonin could attenuate D-dimer elevations, suggesting limited thrombus formation and reducing atherothrombotic risk [82].
Platelet hyperactivity, increased prothrombotic coagulation factor activity, and impaired fibrinolysis are the main causes of an elevated thrombotic tendency in the context of low-grade chronic inflammation. The prothrombotic and inflammatory environment commonly observed in atherothrombotic disease may be favorably modulated by melatonin, thanks to its anti-inflammatory and antioxidative activity [83]. The anti-inflammatory, anti-hypertensive, anti-oxidative and anti-thrombotic properties of melatonin, make it a potential therapeutic option for reducing the risk of arterial occlusion in patients with DM. Otamas et al. showed the role of melatonin in preventing platelet aggregation and disruption of the clotting cascade by changing the structure of fibrin clots, in addition to promoting fibrinolysis [83].
Melatonin and the treatment of diabetes mellitus
The reduction of melatonin levels due to exposure to light at night and aging may lead to T2DM [84]. For that reason, the diurnal system could be regarded as a target for reducing the incidence of insulin resistance and DM [85]. The regulation of blood glucose by melatonin occurs through the direct binding to melatonin receptors (the receptors of melatonin are present in the pancreatic β-cells which modulates the secretion of insulin) [86]. Moreover, melatonin receptors are also present in other tissues, e.g. adipose, muscle and liver. Binding to these receptors causes different effects depending on the tissue type, e.g. in adipose tissue and muscle melatonin modulates the glucose transporters’ expression, thus regulating glucose absorption by these tissues, while in the liver such binding will decrease hepatic gluconeogenesis [87-88].
Several human studies have revealed the efficacy of melatonin supplements in controlling fasting blood glucose when administered in low doses and for a short period (about 3 months) [89-91]. According to the evidence, taking melatonin supplements can improve glycemic control, which can enhance the existing DM treatment [37]. A randomized clinical trial on patients with DM and CAD found that melatonin improved glycemic control, high-density lipoprotein-cholesterol, blood pressure, C-reactive protein, nitric oxide, malondialdehyde, glutathione and even mental health parameters (measured via Beck Depression Inventory) [90]. However, additional studies are needed to evaluate the effects of long-term administration of high dose melatonin on DM.
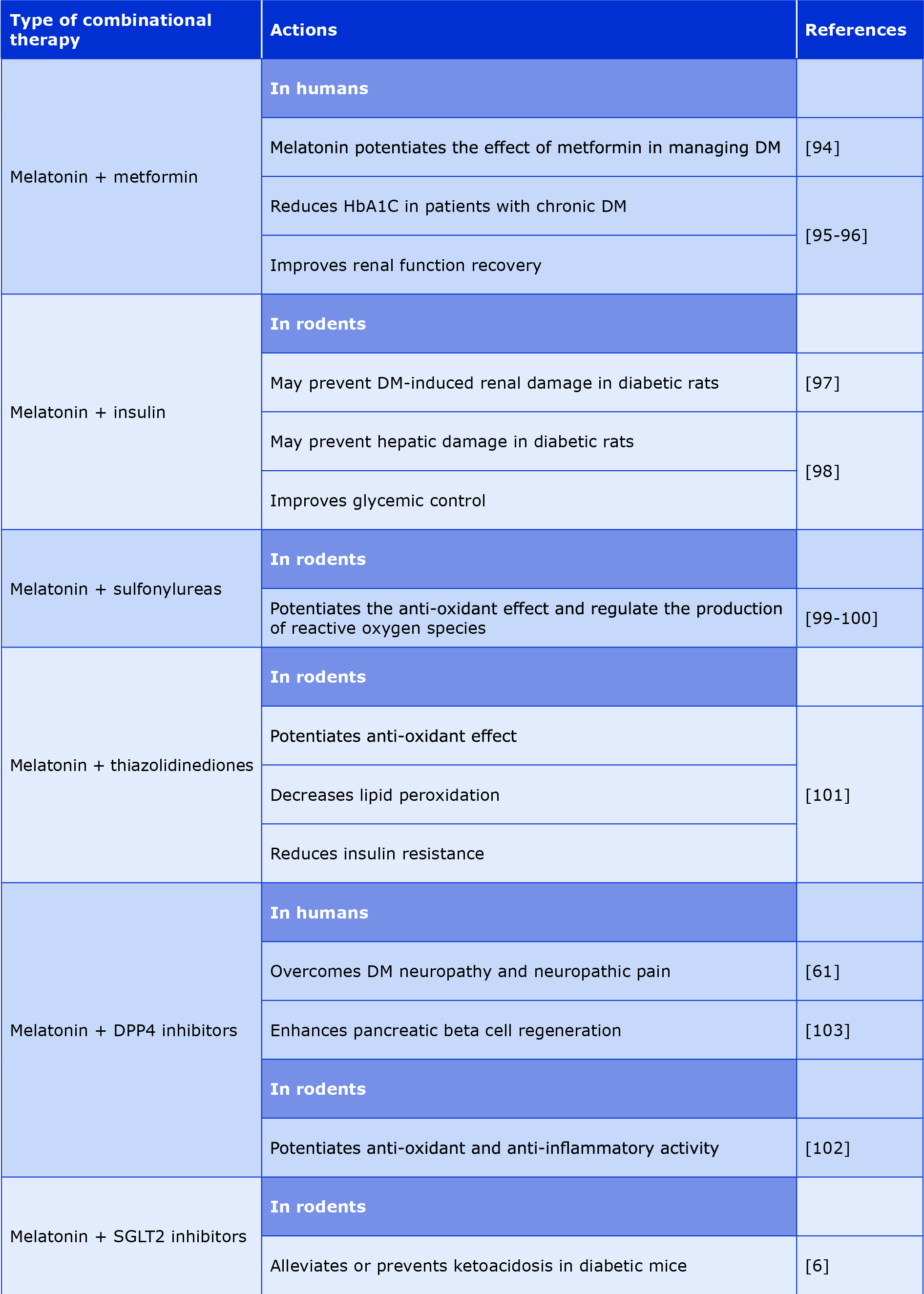
Although the effectiveness of melatonin in relieving DM with associated complications has been described in several studies, the use of melatonin remains an adjunctive therapy. In contrast to T1DM which is an auto-immune disease that is difficult to prevent, the progression of T2DM could be delayed by using certain medications [92]. Metformin is considered the first line treatment for T2DM due to its effectiveness and tolerability for patients [93]. However, metformin alone cannot control severe hyperglycemia and needs adjunctive therapies [94]. Subsequently, both metformin and melatonin have important potential to control DM and its complications, because melatonin can potentiate the effects of metformin [94]. Metformin combined with melatonin enhances renal function recovery in patients with DM who have chronic hyperglycemia. Additionally, the co-administration of melatonin-metformin improved glycemic control in T2DM patients [95-96].
The combination of melatonin and insulin can be used as effective therapeutants to prevent DN in diabetic rats. Melatonin and insulin possess nephroprotective properties by reinforcing the anti-oxidant enzymes and acting as free-radical scavengers. Renal diseases, such as fibrosis in DM, are triggered by inflammation, cell hypertrophy and kidney cell dedifferentiation. Inflammatory cytokines, e.g. TNF-α, IL-6 and monocyte chemoattractant protein-1 are linked to renal complications. In diabetic rats, elevated levels of these cytokines indicate altered innate immunity and chronic inflammation linked to insulin resistance. Combining melatonin and insulin can normalize pro-inflammatory cytokines and improve IL10 levels in circulation. Melatonin reduces pro-inflammatory cytokines by reducing free-radical damage, while insulin increases anti-inflammatory cytokines, thus preventing DN [97].
The adjunctive action of melatonin and insulin can further decrease the hepatic damage along with improving glycemic control in diabetic rats [98]. The use of melatonin along with sulfonylureas (glibenclamide) shows significant regulation of ROS formation. Several animal studies on rats showed the role of exogenous melatonin together with glibenclamide in cell damage recovery from DM and cell function regulation, particularly in the epididymis [99-100]. The combination of melatonin with thiazolidinedione will show a potential anti-oxidant effect, due to the potent anti-oxidant properties of melatonin for scavenging the ROS and it is ability to decrease lipid peroxidation, in addition to the ability of thiazolidinediones in reducing oxidative stress by reducing hyperglycemia. Therefore, this combination might be effective in minimizing insulin resistance [101].
In recent years the use of melatonin and dipeptidyl peptidase inhibitors (DPP4-in) has significant therapeutic efficacy in DM, both have potent free-radical scavenging activity and anti-inflammatory properties. Such combination could overcome DM complications, particularly DNP and neuropathic pain [61]. The co-administration of sitagliptin and melatonin could be superior to monotherapy in the management of T2DM [102]. The combination of melatonin with sodium-glucose co-transporter inhibitors (SGLT2-in) can alleviate or prevent the ketoacidosis induced by SGLT2-in in patients with DM via inhibition of lipolysis and hepatic ketogenesis [6]. Additionally, in DM mouse models the combination of melatonin and DM therapy improved DM by regenerating β-cells and enhancing insulin sensitivity. Melatonin can promote propagation of β-cells, while sitagliptin promotes neogenesis of the β-cells, therefore they can promote β-cell redevelopment in the pancreas [103]. In Table 2 we summarized the therapeutic potential of combining melatonin with DM drugs.
Table 2. The potential role of melatonin as combined therapy with anti-DM medications
Conclusions
Due to the global increase in DM mellitus incidence, it is crucial to discover new biologically active molecules that can effectively treat DM and its complications. Current treatments fall short of completely addressing insulin resistance, oxidative stress, impairment of β-cell function, inflammatory mediation and other pathological mechanisms linked to DM. Several preclinical and clinical studies demonstrated the role of melatonin as a potential DM treatment option to suppress microvascular and macrovascular DM complications. Thus, melatonin might be a promising therapeutant mainly due to its anti-oxidant and anti-inflammatory effects.
Moreover, in this review, we described the roles of exogenous melatonin (particularly when combined with DM medication) in enhancing and regulating the anti-oxidative process and preventing cell damage in the course of DM. Large-scale, randomized clinical studies are needed to elucidate the precise role of melatonin and its signaling pathways in the management of T2DM and its complications. Additionally, human clinical trials are required to assess the effectiveness of exogenous melatonin as monotherapy or combinational therapy for patients with DM.
Acknowledgments
We would like to thank our colleagues at the College of Pharmacy of the University of Mosul and at the Nineveh Health Directorate for their guidance and support.
Conflict of interest
None.
Funding
Self-funded.
_______
Image – commons.wikimedia.org; GNU Free Documentation License
References
| 1. |
Samanta S. Physiological and pharmacological perspectives of melatonin. Arch Physiol Biochem [Internet]. 2022;128(5):1346–67. Available from: https://www.tandfonline.com/doi/full/10.1080/13813455.2020.1770799.
|
| 2. |
Boutin JA, Jockers R. Melatonin controversies, an update. J Pineal Res [Internet]. 2021;70(2). Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12702.
|
| 3. |
Dhankhar S, Chauhan S, Mehta DK, Nitika, Saini K, Saini M, et al. Novel targets for potential therapeutic use in Diabetes mellitus. Diabetol Metab Syndr [Internet]. 2023;15(1):17. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-023-00983-5.
|
| 4. |
Silva ACP e, Santos MJ dos, Koike BDV, Moreira MSA, Gitai DLG, de Miranda Coelho JAP, et al. Melatonin receptor 1B −1193T>C polymorphism is associated with diurnal preference and sleep habits. Sleep Med [Internet]. 2019;53:106–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S138994571830813X.
|
| 5. |
Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol [Internet]. 2019;15(2):105–25. Available from: https://www.nature.com/articles/s41574-018-0130-1.
|
| 6. |
Park J, Seo I, Shim H, Cho H. Melatonin ameliorates SGLT2 inhibitor‐induced diabetic ketoacidosis by inhibiting lipolysis and hepatic ketogenesis in type 2 diabetic mice. J Pineal Res [Internet]. 2020;68(2). Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12623.
|
| 7. |
Ahmed GM, Abed MN, Alassaf FA. Impact of calcium channel blockers and angiotensin receptor blockers on hematological parameters in type 2 diabetic patients. Naunyn Schmiedebergs Arch Pharmacol [Internet]. 2024;397(3):1817–28. Available from: https://link.springer.com/10.1007/s00210-023-02731-y.
|
| 8. |
Ahmed G, Abed M, Alassaf F. An Overview of the Effects of Sodium-Glucose Cotransporter-2 Inhibitors on Hematological Parameters in Diabetic Patients. Iraqi J Pharm [Internet]. 2023;20(1):65–71. Available from: https://iphr.mosuljournals.com/article_178516.html.
|
| 9. |
Alassaf F, Jasim M, Alfahad M, Qazzaz M, Abed M, Thanoon I. Effects of Bee Propolis on FBG, HbA1c, and Insulin Resistance in Healthy Volunteers. Turkish J Pharm Sci [Internet]. 2021;18(4):405–9. Available from: https://turkjps.org/articles/doi/tjps.galenos.2020.50024.
|
| 10. |
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol [Internet]. 2020;16(7):349–62. Available from: https://www.nature.com/articles/s41574-020-0355-7.
|
| 11. |
Garaulet M, Qian J, Florez JC, Arendt J, Saxena R, Scheer FAJL. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol Metab [Internet]. 2020;31(3):192–204. Available from: https://linkinghub.elsevier.com/retrieve/pii/S104327601930236X.
|
| 12. |
Xia A-Y, Zhu H, Zhao Z-J, Liu H-Y, Wang P-H, Ji L-D, et al. Molecular Mechanisms of the Melatonin Receptor Pathway Linking Circadian Rhythm to Type 2 Diabetes Mellitus. Nutrients [Internet]. 2023;15(6):1406. Available from: https://www.mdpi.com/2072-6643/15/6/1406.
|
| 13. |
Ahmed GM, Abed MN, Alassaf FA. The diabetic-anemia nexus:implications for clinical practice. Mil Med Sci Lett [Internet]. 2023;92(92):1–11. Available from: http://mmsl.cz/doi/10.31482/mmsl.2023.042.html.
|
| 14. |
Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology [Internet]. 2021;2(2):36–50. Available from: https://www.mdpi.com/2673-4540/2/2/4.
|
| 15. |
Al-dabbagh BM, Abed MN, Mahmood NM, Alassaf FA, Jasim MH, Alfahad MA, et al. Anti-Inflammatory, Antioxidant and Hepatoprotective Potential of Milk Thistle in Albino Rats. Lat Am J Pharm [Internet]. 2022;41(9):1832–41. Available from: https://www.researchgate.net/profile/Mohammed-Abed-10/publication/364030570_Anti-Inflammatory_Antioxidant_and_Hepatoprotective_Potential_of_Milk_Thistle_in_Albino_Rats/links/6336ca5b9cb4fe44f3ed38a1/Anti-Inflammatory-Antioxidant-and-Hepatoprotective-Poten.
|
| 16. |
Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants [Internet]. 2021;10(5):727. Available from: https://www.mdpi.com/2076-3921/10/5/727.
|
| 17. |
Mok JX, Ooi JH, Ng KY, Koh RY, Chye SM. A new prospective on the role of melatonin in diabetes and its complications. Horm Mol Biol Clin Investig [Internet]. 2019;40(1). Available from: https://www.degruyter.com/document/doi/10.1515/hmbci-2019-0036/html.
|
| 18. |
Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neurosci Res [Internet]. 2018;96(7):1136–49. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jnr.24220.
|
| 19. |
Cardinali DP, Hardeland R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology [Internet]. 2017;104(4):382–97. Available from: https://karger.com/NEN/article/doi/10.1159/000446543.
|
| 20. |
Campos C. Chronic Hyperglycemia and Glucose Toxicity: Pathology and Clinical Sequelae. Postgrad Med [Internet]. 2012;124(6):90–7. Available from: http://www.tandfonline.com/doi/full/10.3810/pgm.2012.11.2615.
|
| 21. |
Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother [Internet]. 2018;107:306–28. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332218322406.
|
| 22. |
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm J [Internet]. 2016;24(5):547–53. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1319016415000766.
|
| 23. |
Yki‐Järvinen H, McClain DA. Glucose toxicity. In: International Textbook of Diabetes Mellitus [Internet]. Wiley; 2015. p. 413–25. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9781118387658.ch27.
|
| 24. |
Viggiano D. Mechanisms of Diabetic Nephropathy Not Mediated by Hyperglycemia. J Clin Med [Internet]. 2023;12(21):6848. Available from: https://www.mdpi.com/2077-0383/12/21/6848.
|
| 25. |
Vázquez J, González B, Sempere V, Mas A, Torija MJ, Beltran G. Melatonin Reduces Oxidative Stress Damage Induced by Hydrogen Peroxide in Saccharomyces cerevisiae. Front Microbiol [Internet]. 2017;8. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2017.01066/full.
|
| 26. |
Ferlazzo N, Andolina G, Cannata A, Costanzo MG, Rizzo V, Currò M, et al. Is Melatonin the Cornucopia of the 21st Century? Antioxidants [Internet]. 2020;9(11):1088. Available from: https://www.mdpi.com/2076-3921/9/11/1088.
|
| 27. |
Bantounou M, Plascevic J, Galley HF. Melatonin and Related Compounds: Antioxidant and Anti-Inflammatory Actions. Antioxidants [Internet]. 2022;11(3):532. Available from: https://www.mdpi.com/2076-3921/11/3/532.
|
| 28. |
Melhuish Beaupre LM, Brown GM, Gonçalves VF, Kennedy JL. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl Psychiatry [Internet]. 2021;11(1):339. Available from: https://www.nature.com/articles/s41398-021-01464-x.
|
| 29. |
Kopustinskiene DM, Bernatoniene J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics [Internet]. 2021;13(2):129. Available from: https://www.mdpi.com/1999-4923/13/2/129.
|
| 30. |
Galano A, Medina ME, Tan DX, Reiter RJ. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J Pineal Res [Internet]. 2015;58(1):107–16. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12196.
|
| 31. |
Lei X, Xu Z, Huang L, Huang Y, Tu S, Xu L, et al. The potential influence of melatonin on mitochondrial quality control: a review. Front Pharmacol [Internet]. 2024;14. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2023.1332567/full.
|
| 32. |
Hacışevki A, Baba B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. In: Melatonin - Molecular Biology, Clinical and Pharmaceutical Approaches [Internet]. IntechOpen; 2018. Available from: https://doi.org/10.5772/intechopen.79421.
|
| 33. |
Alnaser RI, Alassaf FA, Abed MN. Adulteration of hypoglycemic products: the silent threat. Rom J Med Pract [Internet]. 2023;18(4):202–5. Available from: https://rjmp.com.ro/articles/2023.4/RJMP_2023_4_Art-04.pdf.
|
| 34. |
2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care [Internet]. 2021;44(Supplement_1):S15–33. Available from: https://diabetesjournals.org/care/article/44/Supplement_1/S15/30859/2-Classification-and-Diagnosis-of-Diabetes.
|
| 35. |
Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus – A comprehensive review. J Diabetes Complications [Internet]. 2020 Aug;34(8):107613. Available from: https://linkinghub.elsevier.com/retrieve/pii/S105687272030369X.
|
| 36. |
Lotfy M, Kalasz H, Singh J. Chronic Complications of Diabetes Mellitus: A Mini Review. Chronic Complicat Diabetes Mellit A Mini Rev [Internet]. 2017;13(1):3–10. Available from: https://www.ingentaconnect.com/content/ben/cdr/2017/00000013/00000001/art00003.
|
| 37. |
Dehdashtian E, Mehrzadi S, Yousefi B, Hosseinzadeh A, Reiter RJ, Safa M, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci [Internet]. 2018;193:20–33. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0024320517306288.
|
| 38. |
Roy S, Kern TS, Song B, Stuebe C. Mechanistic Insights into Pathological Changes in the Diabetic Retina. Am J Pathol [Internet]. 2017;187(1):9–19. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944016304138.
|
| 39. |
Aranda ML, Fleitas MFG, Dieguez H, Iaquinandi A, Sande PH, Dorfman D, et al. Melatonin as a Therapeutic Resource for Inflammatory Visual Diseases. Curr Neuropharmacol [Internet]. 2017;15(7). Available from: http://www.eurekaselect.com/149254/article.
|
| 40. |
Gong Q, Wang H, Yu P, Qian T, Xu X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front Med [Internet]. 2021;8. Available from: https://www.frontiersin.org/articles/10.3389/fmed.2021.644121/full.
|
| 41. |
Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S. Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol Metab Syndr [Internet]. 2020;12(1):30. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-020-00537-z.
|
| 42. |
Wajid F, Poolacherla R, Mim FK, Bangash A, Rutkofsky IH. Therapeutic potential of melatonin as a chronobiotic and cytoprotective agent in diabetes mellitus. J Diabetes Metab Disord [Internet]. 2020;19(2):1797–825. Available from: https://link.springer.com/10.1007/s40200-020-00585-2.
|
| 43. |
Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol [Internet]. 2011 May;655. Available from: http://www.dovepress.com/alteration-of-melatonin-secretion-in-patients-with-type-2-diabetes-and-peer-reviewed-article-OPTH.
|
| 44. |
Reutrakul S, Siwasaranond N, Nimitphong H, Saetung S, Chirakalwasan N, Chailurkit L, et al. Associations between nocturnal urinary 6-sulfatoxymelatonin, obstructive sleep apnea severity and glycemic control in type 2 diabetes. Chronobiol Int [Internet]. 2017;34(3):382–92. Available from: https://www.tandfonline.com/doi/full/10.1080/07420528.2016.1278382.
|
| 45. |
Sirisreetreerux S, Sujirakul T, Nimitphong H, Pinyopodjanard S, Saetung S, Chailurkit L, et al. Sleep variability, 6-sulfatoxymelatonin, and diabetic retinopathy. Sleep Breath [Internet]. 2021;25(2):1069–74. Available from: https://link.springer.com/10.1007/s11325-020-02165-3.
|
| 46. |
Wan W-C, Long Y, Wan W-W, Liu H-Z, Zhang H-H, Zhu W. Plasma melatonin levels in patients with diabetic retinopathy secondary to type 2 diabetes. World J Diabetes [Internet]. 2021;12(2):138–48. Available from: https://www.wjgnet.com/1948-9358/full/v12/i2/138.htm.
|
| 47. |
Tang L, Zhang C, Lu L, Tian H, Liu K, Luo D, et al. Melatonin Maintains Inner Blood–Retinal Barrier by Regulating Microglia via Inhibition of PI3K/Akt/Stat3/NF-κB Signaling Pathways in Experimental Diabetic Retinopathy. Front Immunol [Internet]. 2022;13. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2022.831660/full.
|
| 48. |
Kashihara N, Haruna Y, K. Kondeti V, S. Kanwar Y. Oxidative Stress in Diabetic Nephropathy. Curr Med Chem [Internet]. 2010;17(34):4256–69. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=0929-8673&volume=17&issue=34&spage=4256.
|
| 49. |
Wu T, Ding L, Andoh V, Zhang J, Chen L. The Mechanism of Hyperglycemia-Induced Renal Cell Injury in Diabetic Nephropathy Disease: An Update. Life [Internet]. 2023;13(2):539. Available from: https://www.mdpi.com/2075-1729/13/2/539.
|
| 50. |
Guo C, He J, Deng X, Wang D, Yuan G. Potential therapeutic value of melatonin in diabetic nephropathy: improvement beyond anti-oxidative stress. Arch Physiol Biochem [Internet]. 2023;129(6):1250–61. Available from: https://www.tandfonline.com/doi/full/10.1080/13813455.2021.1933539.
|
| 51. |
Han Y-S, Yoon YM, Go G, Lee JH, Lee SH. Melatonin Protects Human Renal Proximal Tubule Epithelial Cells Against High Glucose-Mediated Fibrosis via the Cellular Prion Protein-TGF-β-Smad Signaling Axis. Int J Med Sci [Internet]. 2020;17(9):1235–45. Available from: http://www.medsci.org/v17p1235.htm.
|
| 52. |
Ostadmohammadi V, Soleimani A, Bahmani F, Aghadavod E, Ramezani R, Reiter RJ, et al. The Effects of Melatonin Supplementation on Parameters of Mental Health, Glycemic Control, Markers of Cardiometabolic Risk, and Oxidative Stress in Diabetic Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J Ren Nutr [Internet]. 2020;30(3):242–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1051227619303139.
|
| 53. |
Ishigaki S, Ohashi N, Isobe S, Tsuji N, Iwakura T, Ono M, et al. Impaired endogenous nighttime melatonin secretion relates to intrarenal renin–angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol [Internet]. 2016;20(6):878–84. Available from: http://link.springer.com/10.1007/s10157-015-1224-x.
|
| 54. |
Owino S, Sánchez‐Bretaño A, Tchio C, Cecon E, Karamitri A, Dam J, et al. Nocturnal activation of melatonin receptor type 1 signaling modulates diurnal insulin sensitivity via regulation of PI 3K activity. J Pineal Res [Internet]. 2018;64(3). Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12462.
|
| 55. |
Afsar B, Elsurer Afsar R, Sag AA, Kanbay A, Korkmaz H, Cipolla-Neto J, et al. Sweet dreams: therapeutic insights, targeting imaging and physiologic evidence linking sleep, melatonin and diabetic nephropathy. Clin Kidney J [Internet]. 2020;13(4):522–30. Available from: https://academic.oup.com/ckj/article/13/4/522/5728763.
|
| 56. |
Satari M, Bahmani F, Reiner Z, Soleimani A, Aghadavod E, Kheiripour N, et al. Metabolic and anti-inflammatory response to melatonin administration in patients with diabetic nephropathy. Iran J Kidney Dis [Internet]. 2021;1(1):22. Available from: https://www.researchgate.net/profile/Nejat-Kheiripour/publication/348809011_Metabolic_and_Anti-inflammatory_Response_to_Melatonin_Administration_in_Patients_with_Diabetic_Nephropathy/links/601b0e80a6fdcc37a8ff67fe/Metabolic-and-Anti-inflammatory-Response-.
|
| 57. |
Panah F, Ghorbanihaghjo A, Argani H, Haiaty S, Rashtchizadeh N, Hosseini L, et al. The effect of oral melatonin on renal ischemia–reperfusion injury in transplant patients: A double-blind, randomized controlled trial. Transpl Immunol [Internet]. 2019;57:101241. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0966327419300899.
|
| 58. |
Hosseini A, Abdollahi M. Diabetic Neuropathy and Oxidative Stress: Therapeutic Perspectives. Oxid Med Cell Longev [Internet]. 2013;2013:1–15. Available from: http://www.hindawi.com/journals/omcl/2013/168039/.
|
| 59. |
Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care [Internet]. 2017;40(1):136–54. Available from: https://diabetesjournals.org/care/article/40/1/136/37160/Diabetic-Neuropathy-A-Position-Statement-by-the.
|
| 60. |
Feldman EL, Nave K-A, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron [Internet]. 2017;93(6):1296–313. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0896627317300892.
|
| 61. |
Busa P, Kuthati Y, Huang N, Wong C-S. New Advances on Pathophysiology of Diabetes Neuropathy and Pain Management: Potential Role of Melatonin and DPP-4 Inhibitors. Front Pharmacol [Internet]. 2022;13. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2022.864088/full.
|
| 62. |
Onphachanh X, Lee HJ, Lim JR, Jung YH, Kim JS, Chae CW, et al. Enhancement of high glucose‐induced PINK1 expression by melatonin stimulates neuronal cell survival: Involvement of MT 2 /Akt/NF‐κB pathway. J Pineal Res [Internet]. 2017;63(2). Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12427.
|
| 63. |
Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants [Internet]. 2019;8(7):198. Available from: https://www.mdpi.com/2076-3921/8/7/198.
|
| 64. |
Song J, Kim O. Melatonin Modulates Neuronal Cell Death Induced by Endoplasmic Reticulum Stress under Insulin Resistance Condition. Nutrients [Internet]. 2017;9(6):593. Available from: https://www.mdpi.com/2072-6643/9/6/593.
|
| 65. |
Shokri M, Sajedi F, Mohammadi Y, Mehrpooya M. Adjuvant use of melatonin for relieving symptoms of painful diabetic neuropathy: results of a randomized, double-blinded, controlled trial. Eur J Clin Pharmacol [Internet]. 2021;77(11):1649–63. Available from: https://link.springer.com/10.1007/s00228-021-03170-5.
|
| 66. |
Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res Int [Internet]. 2017;2017:1–9. Available from: https://www.hindawi.com/journals/bmri/2017/7839101/.
|
| 67. |
Gurel‐Gokmen B, Ipekci H, Oktay S, Alev B, Ustundag UV, Ak E, et al. Melatonin improves hyperglycemia induced damages in rat brain. Diabetes Metab Res Rev [Internet]. 2018;34(8). Available from: https://onlinelibrary.wiley.com/doi/10.1002/dmrr.3060.
|
| 68. |
Shen S, Liao Q, Wong YK, Chen X, Yang C, Xu C, et al. The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer’s disease. Int J Biol Sci [Internet]. 2022;18(3):983–94. Available from: https://www.ijbs.com/v18p0983.htm.
|
| 69. |
Mehrpooya M, Mazdeh M, Rahmani E, Khazaie M, Ahmadimoghaddam D. Melatonin supplementation may benefit patients with acute ischemic stroke not eligible for reperfusion therapies: Results of a pilot study. J Clin Neurosci [Internet]. 2022;106:66–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0967586822004167.
|
| 70. |
Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet [Internet]. 2018;392(10159):1736–88. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618322037.
|
| 71. |
Bartnik M. The prevalence of abnormal glucose regulation in patients with coronary artery disease across EuropeThe Euro Heart Survey on diabetes and the heart. Eur Heart J [Internet]. 2004;25(21):1880–90. Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1016/j.ehj.2004.07.027.
|
| 72. |
Nielson C, Lange T, Hadjokas N. Blood Glucose and Coronary Artery Disease in Nondiabetic Patients. Diabetes Care [Internet]. 2006;29(5):998–1001. Available from: https://diabetesjournals.org/care/article/29/5/998/25316/Blood-Glucose-and-Coronary-Artery-Disease-in.
|
| 73. |
Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime Risks of Cardiovascular Disease. N Engl J Med [Internet]. 2012;366(4):321–9. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1012848.
|
| 74. |
Alfahad M, Qazzaz ME, Abed MN, Alassaf FA, Jasim MHM. Comparison of Anti-Oxidant Activity of Different Brands of Esomeprazole Available in Iraqi Pharmacies. Syst Rev Pharm [Internet]. 2020;11(05):330–4. Available from: http://www.sysrevpharm.org//index.php?fulltxt=109065&fulltxtj=196&fulltxtp=196-1590059996.pdf.
|
| 75. |
Zhang R, Ni L, Di X, Ma B, Niu S, Rong Z, et al. Potential Role of Melatonin as an Adjuvant for Atherosclerotic Carotid Arterial Stenosis. Molecules [Internet]. 2021;26(4):811. Available from: https://www.mdpi.com/1420-3049/26/4/811.
|
| 76. |
Ozkalayci F, Kocabas U, Altun BU, Pandi-Perumal S, Altun A. Relationship Between Melatonin and Cardiovascular Disease. Cureus [Internet]. 2021; Available from: https://www.cureus.com/articles/47653-relationship-between-melatonin-and-cardiovascular-disease.
|
| 77. |
Obayashi K, Saeki K, Tone N, Kurumatani N. Relationship between melatonin secretion and nighttime blood pressure in elderly individuals with and without antihypertensive treatment: a cross-sectional study of the HEIJO-KYO cohort. Hypertens Res [Internet]. 2014;37(10):908–13. Available from: https://www.nature.com/articles/hr201499.
|
| 78. |
Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. Diabetes and peripheral artery disease: A review. World J Diabetes [Internet]. 2021;12(6):827–38. Available from: https://www.wjgnet.com/1948-9358/full/v12/i6/827.htm.
|
| 79. |
Tunali T, Sener G, Yarat A, Emekli N. Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci [Internet]. 2005;76(11):1259–65. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0024320504009695.
|
| 80. |
Bekyarova G, Tancheva S, Hristova M. The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find Exp Clin Pharmacol [Internet]. 2010;32(5):299. Available from: http://access.portico.org/stable?au=pjbf78vzpw5.
|
| 81. |
Wirtz PH, Spillmann M, Bärtschi C, Ehlert U, Von Känel R. Oral melatonin reduces blood coagulation activity: a placebo‐controlled study in healthy young men. J Pineal Res [Internet]. 2008;44(2):127–33. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1600-079X.2007.00499.x.
|
| 82. |
Wirtz PH, Ehlert U, Emini L, Rüdisüli K, Groessbauer S, Gaab J, et al. Anticipatory Cognitive Stress Appraisal and the Acute Procoagulant Stress Response in Men. Psychosom Med [Internet]. 2006;68(6):851–8. Available from: http://journals.lww.com/00006842-200611000-00006.
|
| 83. |
Otamas A, Grant PJ, Ajjan RA. Diabetes and atherothrombosis: The circadian rhythm and role of melatonin in vascular protection. Diabetes Vasc Dis Res [Internet]. 2020;17(3):147916412092058. Available from: http://journals.sagepub.com/doi/10.1177/1479164120920582.
|
| 84. |
Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, et al. Exposure to Room Light before Bedtime Suppresses Melatonin Onset and Shortens Melatonin Duration in Humans. J Clin Endocrinol Metab [Internet]. 2011;96(3):E463–72. Available from: https://academic.oup.com/jcem/article/96/3/E463/2597236.
|
| 85. |
Cipolla‐Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res [Internet]. 2014;56(4):371–81. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12137.
|
| 86. |
Peschke E, Bähr I, Mühlbauer E. Experimental and clinical aspects of melatonin and clock genes in diabetes. J Pineal Res [Internet]. 2015;59(1):1–23. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jpi.12240.
|
| 87. |
Banerjee A, Chattopadhyay A, Bandyopadhyay D. Prevention of diabetic cardiomyopathy through metabolic amendments of myocardium by melatonin: a role beyond antioxidative efficiency. Melatonin Res [Internet]. 2022;5(2):133–53. Available from: http://www.melatonin-research.net/index.php/MR/article/view/186.
|
| 88. |
Nikolaev G, Robeva R, Konakchieva R. Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int J Mol Sci [Internet]. 2021;23(1):471. Available from: https://www.mdpi.com/1422-0067/23/1/471.
|
| 89. |
Rezvanfar MR, Heshmati G, Chehrei A, Haghverdi F, Rafiee F, Rezvanfar F. Effect of bedtime melatonin consumption on diabetes control and lipid profile. Int J Diabetes Dev Ctries [Internet]. 2017;37(1):74–7. Available from: http://link.springer.com/10.1007/s13410-016-0497-2.
|
| 90. |
Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr [Internet]. 2019;38(1):191–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0261561417314243.
|
| 91. |
Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L, et al. Melatonin Treatment Improves Insulin Resistance and Pigmentation in Obese Patients with Acanthosis Nigricans. Int J Endocrinol [Internet]. 2018;2018:1–7. Available from: https://www.hindawi.com/journals/ije/2018/2304746/.
|
| 92. |
Zhou T, Xu X, Du M, Zhao T, Wang J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed Pharmacother [Internet]. 2018;106(July):1227–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332218335698.
|
| 93. |
Shurrab NT, Arafa E-SA. Metformin: A review of its therapeutic efficacy and adverse effects. Obes Med [Internet]. 2020;17:100186. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2451847620300063.
|
| 94. |
Banerjee A, Chattopadhyay A, Bandyopadhyay D. Potentially synergistic effects of melatonin and metformin in alleviating hyperglycaemia: a comprehensive review. Melatonin Res [Internet]. 2021;4(4):522–50. Available from: http://www.melatonin-research.net/index.php/MR/article/view/149.
|
| 95. |
Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH, et al. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J Pineal Res [Internet]. 2006;41(2):189–93. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1600-079X.2006.00353.x.
|
| 96. |
Hussain SA, Khadim HM, Khalaf BH, Ismail SH, Hussein KI, Sahib AS. Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J [Internet]. 2006;27(10):1483–8. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/emr-80600.
|
| 97. |
Hajam YA, Rai S, Pandi-Perumal SR, Brown GM, Reiter RJ, Cardinali DP. Coadministration of Melatonin and Insulin Improves Diabetes-Induced Impairment of Rat Kidney Function. Neuroendocrinology [Internet]. 2022;112(8):807–22. Available from: https://karger.com/NEN/article/doi/10.1159/000520280.
|
| 98. |
Hajam YA, Rai S. Melatonin and insulin modulates the cellular biochemistry, histoarchitecture and receptor expression during hepatic injury in diabetic rats. Life Sci [Internet]. 2019;239:117046. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0024320519309737.
|
| 99. |
Hajam YA, Rai S, Shree S, Basheer M, Ghosh H. Retrieval of Reproductive Complications by Exogenous Melatonin Treatment in Sreptozotocin Induced Diabetic Rat Model. Res Rev J Zool Sci [Internet]. 2017;(5):96–104. Available from: https://www.academia.edu/download/81650458/retrieval-of-reproductive-complications-by-exogenous-melatonin-treatment-in-sreptozotocin-induced-diabetic-rat-model.pdf.
|
| 100. |
Zhang K, Lv Z, Jia X, Huang D. Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia [Internet]. 2012 Aug;44(4):230–6. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0272.2012.01272.x.
|
| 101. |
Ahire Y, Gandhi S, Ghaisas M, Dandawate P, Mule M. Effects of combination of thiazolidinediones with melatonin in dexamethasone-induced insulin resistance in mice. Indian J Pharm Sci [Internet]. 2011;73(6):601. Available from: http://www.ijpsonline.com/text.asp?2011/73/6/601/100232.
|
| 102. |
Patel R, Parmar N, Palit SP, Rathwa N, Begum R. A novel combination of sitagliptin and melatonin ameliorates T2D manifestations: studies on experimental diabetic models. J Endocrinol Invest [Internet]. 2023;46(8):1597–612. Available from: https://link.springer.com/10.1007/s40618-023-02014-6.
|
| 103. |
Patel R, Pramanik S, Rathwa N, Parmar N, Dhimmar H. 112-LB: Melatonin and DPP-IV Inhibitor: A Novel Combinatorial Approach for ß-Cell Regeneration. Diabetes [Internet]. 2019;68(Supplement_1). Available from: https://diabetesjournals.org/diabetes/article/68/Supplement_1/112-LB/58656/112-LB-Melatonin-and-DPP-IV-Inhibitor-A-Novel.
|










