Precision medicine and improving the outcomes of atrial tachycardia ablation: a comprehensive review
Abstract
Atrial tachycardia (AT) is a prevalent cardiac arrhythmia characterized by rapid, abnormal electrical activity originating from the atria. It represents a significant clinical challenge due to its potential for recurrence, adverse cardiovascular outcomes and impact on quality of life. Catheter ablation has emerged as a primary therapeutic modality for AT, offering the potential for rhythm control and symptom alleviation. Despite advancements in techniques and technology, the success of AT ablation can vary widely among patients. Identifying prognostic factors associated with successful AT ablation and potential outcome improving techniques is imperative for optimizing patient care.
Citation
Młyński M, Sławiński G, Kozłowski D. Precision medicine and improving the outcomes of atrial tachycardia ablation: a comprehensive review. Eur J Transl Clin Med. 2024;7(1):5-15Introduction
Atrial tachycardia poses a considerable clinical challenge due to its propensity for recurrence and adverse cardiovascular outcomes. Focal AT represents up to 17% of supraventricular arrhythmias referred for catheter ablation treatment [1]. This treatment modality has revolutionized the management of AT, providing a cure for many patients. However, the efficacy of AT ablation is influenced by numerous factors, ranging from patient demographics to procedural intricacies and post-ablation monitoring strategies [2-9].
Advancements in predictive modeling and risk stratification algorithms enable clinicians to assess the likelihood of procedural success and arrhythmia recurrence more accurately [7, 10-14]. Incorporating clinical, electrocardiographic, imaging and genetic data into comprehensive risk assessment tools allows for personalized treatment recommendations and informed decision-making [3]. Furthermore, longitudinal data collection through large-scale registries and multicenter studies facilitates ongoing refinement of prognostic models and validation of novel biomarkers.
The identification of novel outcome predictors for successful ablation of AT is an active area of research aimed at improving procedural efficacy and patient outcomes. While traditional predictors (e.g. demographic factors, concomitant diseases) remain important [4], recent advancements have led to the exploration of additional techniques and candidate selection strategies that may enhance risk stratification and treatment planning (Figure 1).

Figure 1. Atrial tachycardia classification according to the 2019 ESC Guidelines
Precision medicine approaches
The concept of precision medicine, which emphasizes individualized treatment based on patients’ unique characteristics, is gaining traction in the field of AT ablation [10]. Tailoring treatment strategies to patients’ genetic profiles, atrial substrate characteristics and comorbidities enables more effective targeting of arrhythmogenic mechanisms and optimization of procedural outcomes. Integrating approaches such as genetic testing, biomarker analysis, advanced imaging modalities, multi-omics (i.e. the analysis of multiple data sets regarding for example genomics, metabolomics etc.) and personalized ablation strategies, into clinical practice holds promise for further improving patient care and long-term outcomes.
Multivariable risk scores incorporate demographic factors, comorbidities, electrocardiographic parameters, imaging findings and genetic biomarkers to stratify patients into low, intermediate, and high-risk categories [11-12, 15-17]. Personalized risk assessment facilitates shared decision-making, treatment planning, and optimization of post-ablation management strategies tailored to each patient’s unique profile.
Patient Characteristics
Patient-related variables play a crucial role in determining the success of AT ablation. Although age and the presence of comorbidities (e.g. coronary artery disease, cardiomyopathy, valvular heart disease) is not clearly correlated with higher arrhythmia recurrence rates in the population of patients with supraventricular tachycardias (SVT), their presence can correlate with a decreased success rate and increased occurrence of complications and major adverse cardiac events [4]. This may be attributed to age-related changes in atrial tissue characteristics and its electrical properties [18-19], however the lack of symptom improvement can be also attributed to the natural course of the primary concomitant cardiovascular diseases. Additionally, the presence of comorbidities (e.g. hypertension, diabetes, obesity) can have impact on procedural efficacy and long-term arrhythmia recurrence [5, 20].
Novel patient characteristics, including genetic variants, inflammatory profiles, autonomic modulation, comorbidity burdens and metabolic phenotypes, can play critical roles in determining the success of AT ablation [4, 6, 20-22]. Understanding the interplay between patient demographics, comorbidities and genetic factors is essential for personalized risk stratification and treatment planning in AT ablation [4-6, 23-24]. Incorporating those into risk stratification algorithms and personalized treatment approaches can optimize procedural outcomes, minimize complication risks, and improve long-term arrhythmia-free survival in patients with AT [5-6, 10, 23, 25].
Genetic variants and biomarkers
Advancements in genetic profiling and biomarker discovery have identified specific genetic variants associated with general susceptibility to SVT, arrhythmia mechanisms, and treatment response, most importantly for atrial fibrillation (AF) [6]. Genetic polymorphisms in ion channel genes, cardiac structural proteins and regulatory molecules may influence atrial electrophysiology and arrhythmogenic substrate (i.e. the cardiac tissue, where the arrhythmia originates from) remodeling [6].
Although the mechanisms for AT development have been studied most thoroughly in patients with specific genetic disorders (e.g. RAS/MAPK pathway) [26], it has also been investigated in animal models, such as the tafazzin mutation [27]. Moreover, it has been shown, that certain genetic mutations can be related to an increased risk of isolated atrial cardiomyopathy and atrial arrhythmias [28-30]. Genome-wide association studies (GWAS) and candidate gene analyses can identify polymorphisms in genes encoding ion channels (e.g. potassium channels), cardiac structural proteins, such as gap junction proteins, and regulatory molecules that modulate atrial electrophysiology and arrhythmogenesis [26-30].
Inflammatory and immune profiles
Growing evidence suggests that systemic inflammation and immune activation play a crucial role in the pathogenesis and recurrence of atrial arrhythmias. Elevated levels of pro-inflammatory cytokines, chemokine and circulating immune cells correlate with atrial remodeling, fibrosis and electrical instability [21, 25]. In the past years, it has been shown that genetic polymorphisms in the structure of proteins such as interleukin-6 are related to an increased risk of postoperative atrial fibrillation [21]. It is noteworthy that in some studies this correlation has not been confirmed for AT [25]. The existence of an inflammatory-mediated AT pathway may create a possibility for the implementation of immunomodulatory therapies targeting inflammatory pathways in order to attenuate atrial fibrosis, reduce arrhythmia burden and improve ablation outcomes [31].
Markers of atrial fibrosis, inflammation and extracellular matrix remodeling, which are consistent with atrial myopathy, have emerged as potential predictors of ablation success and long-term outcomes, documented mostly in atrial fibrillation. Serum biomarkers such as galectin-3, soluble ST2 and matrix metalloproteinases (MMPs) reflect underlying atrial structural changes and myocardial injury [23-24]. Integration of biomarker data into risk prediction algorithms may enhance risk stratification and facilitate targeted therapeutic interventions.
Autonomic modulation
Although described mostly in animal experimental models and patients with AF, the role of the autonomic nervous system (ANS) dysregulation, including heterogenic or increased autonomic innervation of the cardiac tissue, contributes to atrial arrhythmogenesis and treatment resistance [22, 32]. Assessment of stellate ganglion nerve activity and vagal nerve activity may be beneficial in the prognostic evaluation of tachyarrhythmias. Novel intervention strategies targeting sympathetic activation or vagal tone may enhance procedural success rates and reduce arrhythmia recurrence in susceptible individuals [33-35].
Individualized arrhythmia considerations
The characteristics of the AT itself profoundly influence the success of catheter ablation. AT can arise from various mechanisms (including focal ectopy, reentrant circuits, triggered activity), each requiring distinct ablation strategies for successful termination. Focal AT originates from discrete sites within the atria, often exhibiting centrifugal activation patterns and rapid ectopic firing rates [1-2]. In contrast, reentrant AT involves the formation of macro-reentrant circuits, commonly involving areas of scar or abnormal tissue conduction [1-2]. Electrocardiographic features, such as P-wave morphology, atrial activation patterns and atrial substrate characteristics, provide valuable insights into the underlying arrhythmia mechanism and guide ablation strategies [2]. Advanced imaging modalities, such as cardiac magnetic resonance imaging (MRI) and three-dimensional electroanatomic mapping systems, offer additional tools for characterizing arrhythmogenic substrates and optimizing ablation targets [1-2, 36] (Figure 2, Figure 3).
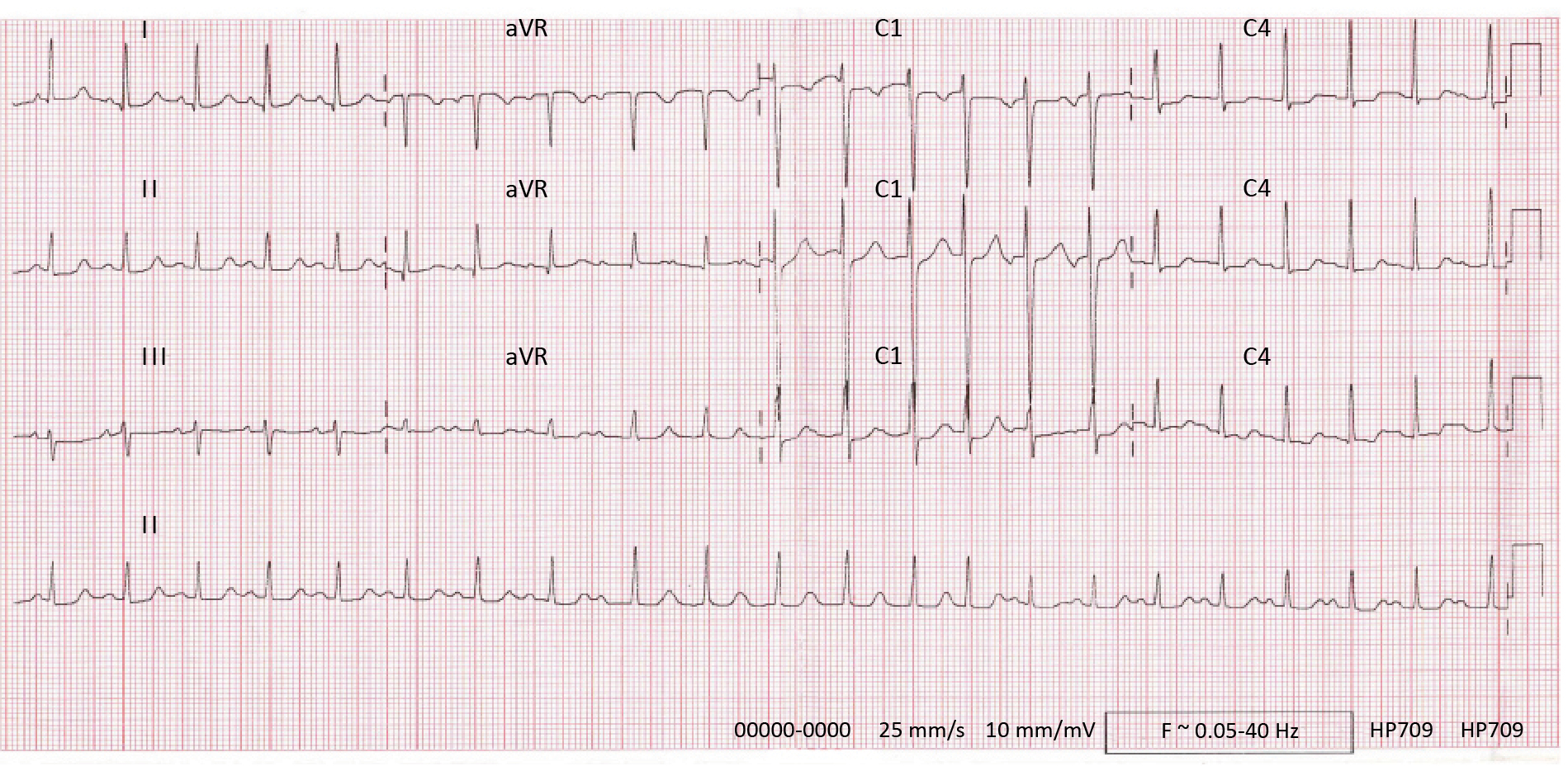
Figure 2. Electrocardiogram of focal AT
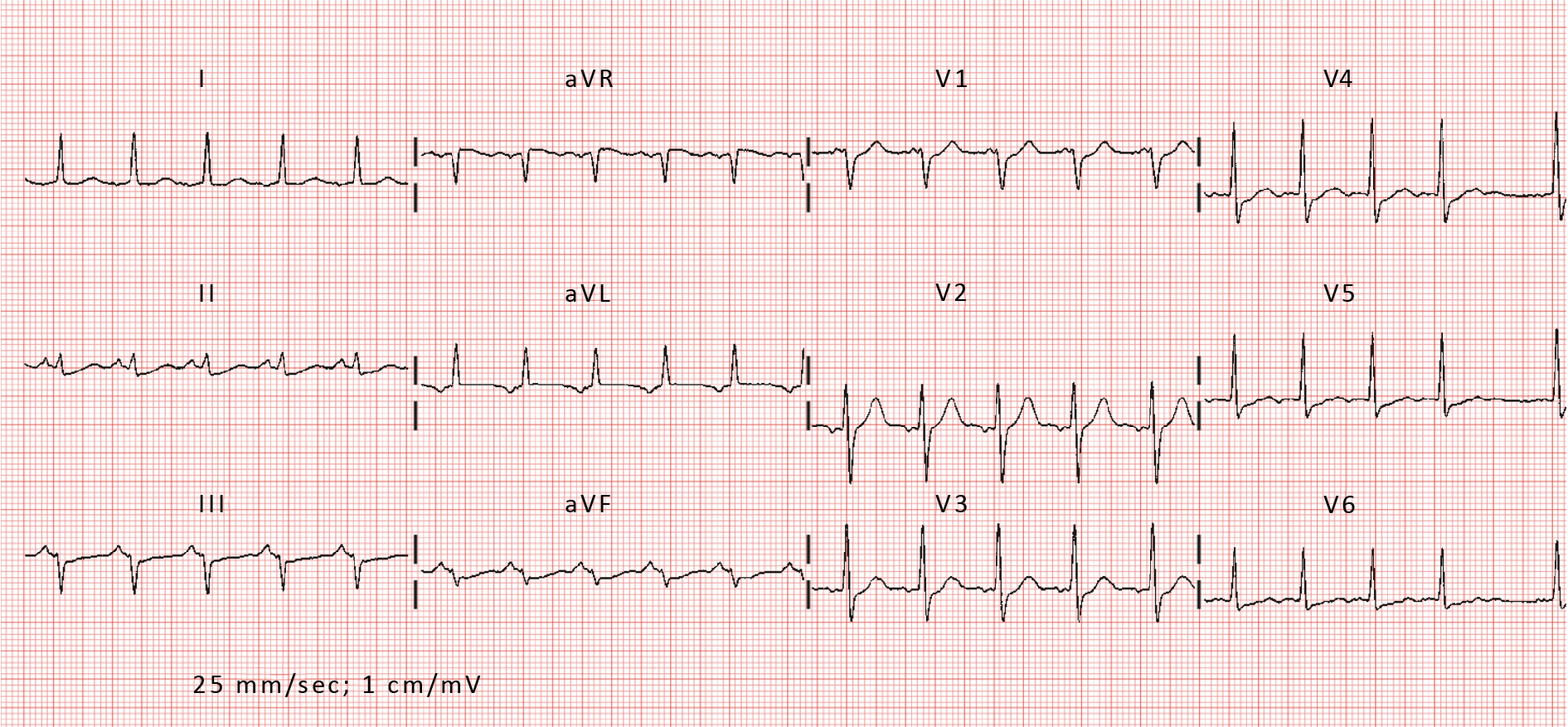
Figure 3. Electrocardiogram of reentrant AT
Substrate-focused approach
Important factors to consider are the location, origin and pathophysiology of the arrhythmogenic substrates. ATs more often emerge from foci located in the right atrium, most commonly from crista terminalis, which is associated with a good prognosis and long-term ablation success rate [1, 7, 37]. On the contrary, procedures performed in delicate regions of the atria, close to critical structures (e.g. atrioventricular node (AVN) or local nerves) require a more cautious approach and pose a greater risk of complications [1-2].
Precision medicine approaches recognize the heterogeneity of atrial substrate underlying AT and tailor ablation strategies accordingly. While procedural advancements, namely electroanatomic mapping modalities, have led to significant improvement in ablation success rate [38], the use of non-invasive imaging modalities, such as magnetic resonance is not always beneficial, when combined with standard diagnostic protocol [39]. However, in some studies, the assessment of late gadolinium enhancement (LGE) patterns in cardiac MRI – consistent with atrial fibrosis and scarring – has been shown to significantly increase procedural success rate and decrease risk in ablation of AF and AT substrates [36, 40-41]. Other imaging modalities, such as speckle-tracking echocardiography can be used to assess the risk of arrhythmia recurrence after catheter ablation [42].
Additional factors, including the origin of atrial scarring can contribute to the clinical characteristics of AT treatment. The emergence of substrates after surgical procedures involving atria is well known. However, the phenomenon of spontaneous scarring (SS), unrelated to prior surgery or significant structural heart disease can contribute to the development of atrial arrhythmia. Patients with SS have a higher prevalence of AT, with a lower ablation success rate and an increased risk of concomitant sinus node dysfunction [43-44].
Interestingly, novel techniques of patient-specific substrate modelling are being investigated, including MRI-based myocardial fiber organization. In silico this technique has shown satisfying results in both focal and rotor-based AT, when compared to procedural data such as local activation time (LAT) tracking accuracy [8].
Procedural factors
Technical aspects of the ablation procedure significantly contribute to its success. Operator experience, catheter technology, mapping methodologies and energy delivery modalities influence procedural efficacy and safety. Experienced operators with proficiency in catheter manipulation, electroanatomic mapping (EAM) interpretation and ablation lesion creation are essential for achieving optimal outcomes. Comprehensive procedural planning, including pre-procedural imaging, EAM, and intra-procedural monitoring, optimizes ablation outcomes and minimizes procedural complications (Figure 4).
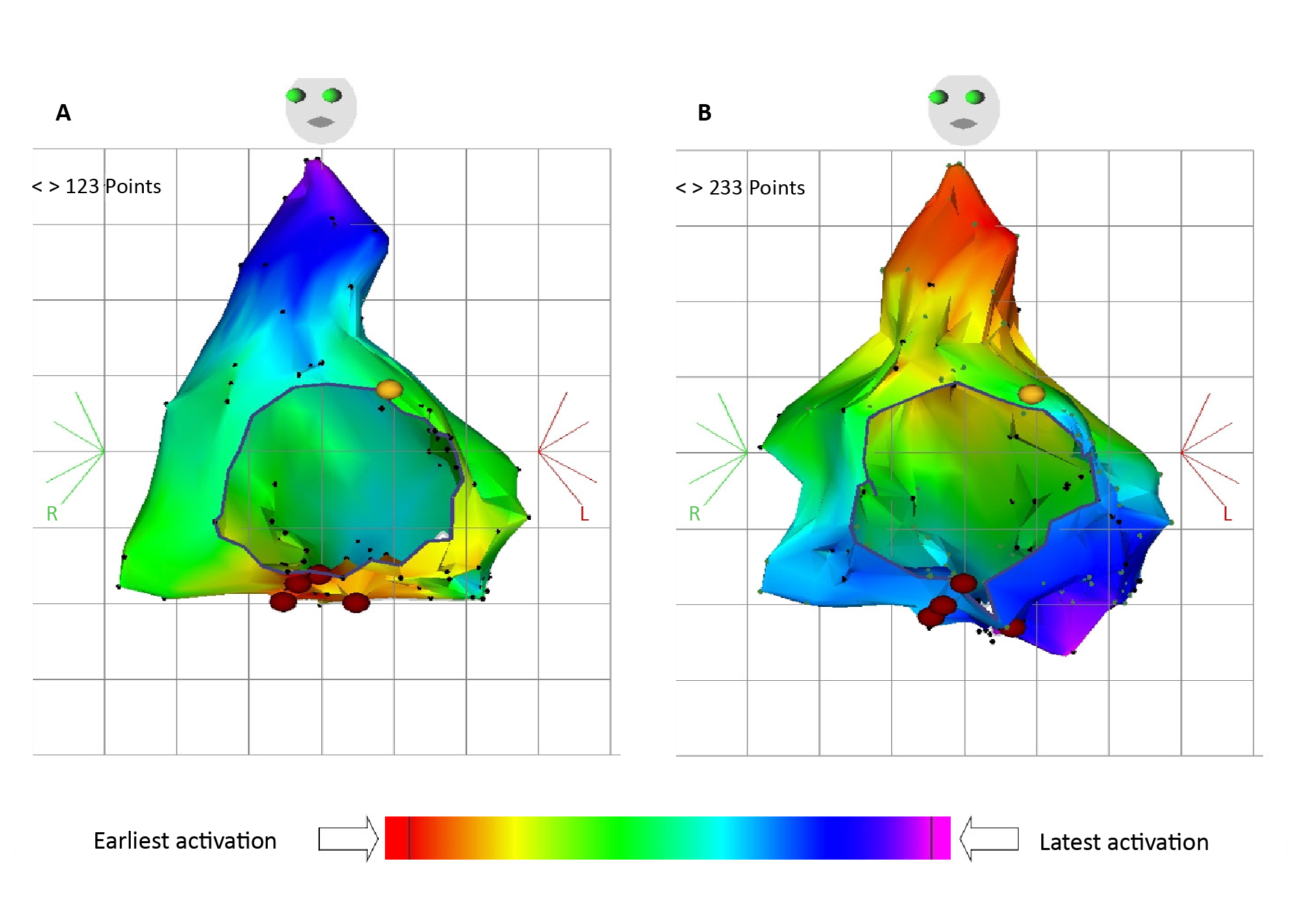
Figure 4. Three-dimensional mapping of atrial potentials during an electrophysiological study
In general, the choice of catheter type and energy source depends on the arrhythmia mechanism, atrial anatomy and operator preference. Emerging techniques, such as contact force sensing catheters, high-density mapping and robotic navigation systems, hold promise for further improving procedural success rates and reducing complication risks.
Contact force sensing catheters
Optimal catheter-tissue contact force (CF) and lesion quality are crucial for procedural precision and efficacy, leading to better patient outcomes. Maintaining optimal CF enhances lesion transmurality, reduces impedance changes and minimizes the risk of steam pops and thrombus formation. CF-sensing catheters provide real-time, quantitative feedback during ablation procedures, that is normally derived from indirect parameters, e.g. baseline impedance, tactile feedback and electrogram (EGM) amplitude. Although some studies have promising results regarding the decreased number of energy applications and total procedure time during radiofrequency (RF) ablation, the available randomized controlled trials (RCTs) have not proved the superiority of CF-sensing catheter over standard equipment [45-47].
Energy delivery
Radiofrequency (RF) energy remains the most commonly used ablation modality, delivering localized thermal energy to create transmural lesions. However, RF ablation may be limited by inadequate lesion depth, tissue charring and the risk of collateral damage to adjacent structures. In contrast, cryoablation, utilizing freezing temperatures to produce reversible tissue injury, provides more accurate and localized lesion application. Current data shows that RF ablation remains the most effective method, although cryoablation may be considered during procedures performed in regions of close proximity to vital structures (e.g. near the phrenic nerve or in para-Hisian arrhythmic substrates) [1-2, 48-49].
Modulation of ablation energy parameters, including power, duration and lesion depth, optimizes lesion formation and procedural efficacy. Novel energy delivery modalities, such as pulsed field ablation (PFA), offer precise and controlled lesion creation while minimizing collateral damage to surrounding tissues [50-51]. PFA utilizes non-thermal, rapid electrical pulses to induce cell death mediated by membrane electroporation [50]. Current data supports the possible efficiency and safety of this modality in the treatment of ATs, however no RCTs have been published, comparing its outcomes to standard treatment protocol [50-54].
Mapping technologies
Standard electrophysiologic mapping, based on local activation time (LAT) assignation can be insufficient, often upon examination of low-voltage regions within atrial scarring [9, 55]. In recent years we have seen the introduction of high-resolution mapping systems, such as high-density mapping (HDM) catheters and basket catheters, which allow for more detailed characterization of atrial substrate and precise identification of arrhythmia targets [9, 55]. These technologies enable operators to create accurate electroanatomic maps of the atria, facilitating targeted ablation and reducing procedural times [1-2, 9, 55-56]. Nevertheless, HDM has also been shown to lack accuracy, displaying local pseudo-reentrant patterns in patients with AT upon encountering electric wavefront collision or annotating noise, resulting in incorrect lesion application during ablation [56]. Other advanced methods, such as ripple mapping (RM), which serves as a three-dimensional graphic representation of electrograms, have been proven to increase the success rate of AT ablation [56-58].
Innovative pacing and activation mapping techniques, including entrainment mapping, voltage mapping, and pace-mapping algorithms, aid in the identification of arrhythmia mechanisms and critical isthmuses. Entrainment mapping confirms the participation of specific sites within the reentrant circuit, guiding targeted ablation strategies [59]. Voltage mapping identifies areas of low-voltage substrate and scar tissue, delineating regions at high risk for arrhythmia recurrence [60-61].
Furthermore, introduction of functional substrate mapping (FSM) may lead to a more accurate assessment of atrial electroanatomic remodeling or heterogeneity. This method investigates activation complexity and conduction velocity, calculating conduction delay between adjacent tissue locations. Current data supports the correlation between critical isthmus (CI) location in macro-reentrant ATs, low voltage regions and findings such as deceleration zones during isochronal late activation mapping, achieved via FSM during sinus or paced rhythm. FSM may provide additional guidance for substrate mapping and lesion placement in AT ablation [62-64].
Additionally, adjunctive strategies, such as adenosine-guided effectiveness assessment may help identify areas of incomplete isolation, facilitating targeted substrate modification and reducing arrhythmia recurrence rate [54, 65].
Imaging-guided ablation
Advanced imaging modalities can provide detailed anatomical information and substrate characterization for targeted ablation, not only in the pre-procedural planning period but also during the intervention. Intracardiac echocardiography (ICE) and rotational angiography allow real-time visualization of catheter position and can be used to assess the accuracy of catheter-tissue contact, which translates to better procedural outcomes [66]. ICE imaging has been reported to facilitate ablation of critically located substrates, as in para-Hisian ATs [66], and in cases with altered atrial anatomy, as in patients with surgically corrected congenital heart disease [67]. Intracardiac thrombus detection is another potential contribution of this imaging modality to procedural safety is attributed to [68]. Additionally, when combined with EAM techniques, ICE can serve as a substitute for fluoroscopy monitoring in transseptal puncture for ablation of left-sided arrhythmias [69].
Data science meets medicine
Computational modeling and simulation
Advanced analysis of electrogram characteristics, including signal amplitude, duration, fractionation, and voltage mapping, provides insights into atrial substrate properties and arrhythmia mechanisms. Precise delineation of atrial anatomy, scar tissue distribution and arrhythmogenic foci localization guides catheter navigation and lesion creation during ablation procedures. Novel algorithms utilizing machine learning and artificial intelligence techniques can identify subtle electrogram features associated with arrhythmogenic substrate and may eventually be able to predict ablation success [13, 70-71].
Artificial intelligence (AI) and machine learning
Artificial intelligence (AI) and machine learning algorithms integrated into electrophysiology workflows hold promise for improving procedural planning, navigation and outcomes. AI-driven algorithms analyze vast amounts of patient data, including electrocardiographic signals, imaging studies and procedural outcomes, to identify patterns and predict optimal treatment strategies. By assisting in substrate identification, ablation site selection and lesion assessment, AI-powered technologies have the potential to enhance procedural success rates and reduce complication risks. By analyzing diverse patient characteristics, clinical variables, imaging parameters, and electrophysiological data, machine learning algorithms identify complex relationships and patterns that inform individualized treatment decisions [14, 72-73]. Integration of real-time data streams and continuous learning frameworks enhances the adaptability and accuracy of predictive models over time [14, 72-74].
Coordination of multidisciplinary care
Effective implementation of precision medicine in AT ablation requires close collaboration among multidisciplinary teams, including electrophysiologists, imaging specialists, genetic counselors and clinical pharmacologists [75]. Multidisciplinary care coordination can provide comprehensive evaluation, personalized treatment planning, and integrated follow-up care to optimize patient outcomes [75]. As in all fields of contemporary medicine, shared decision-making principles can be effective in empowering patients to actively participate in treatment decisions, contribute to their care plans, and engage in lifestyle modifications that support long-term rhythm control and cardiovascular health (Figure 5).
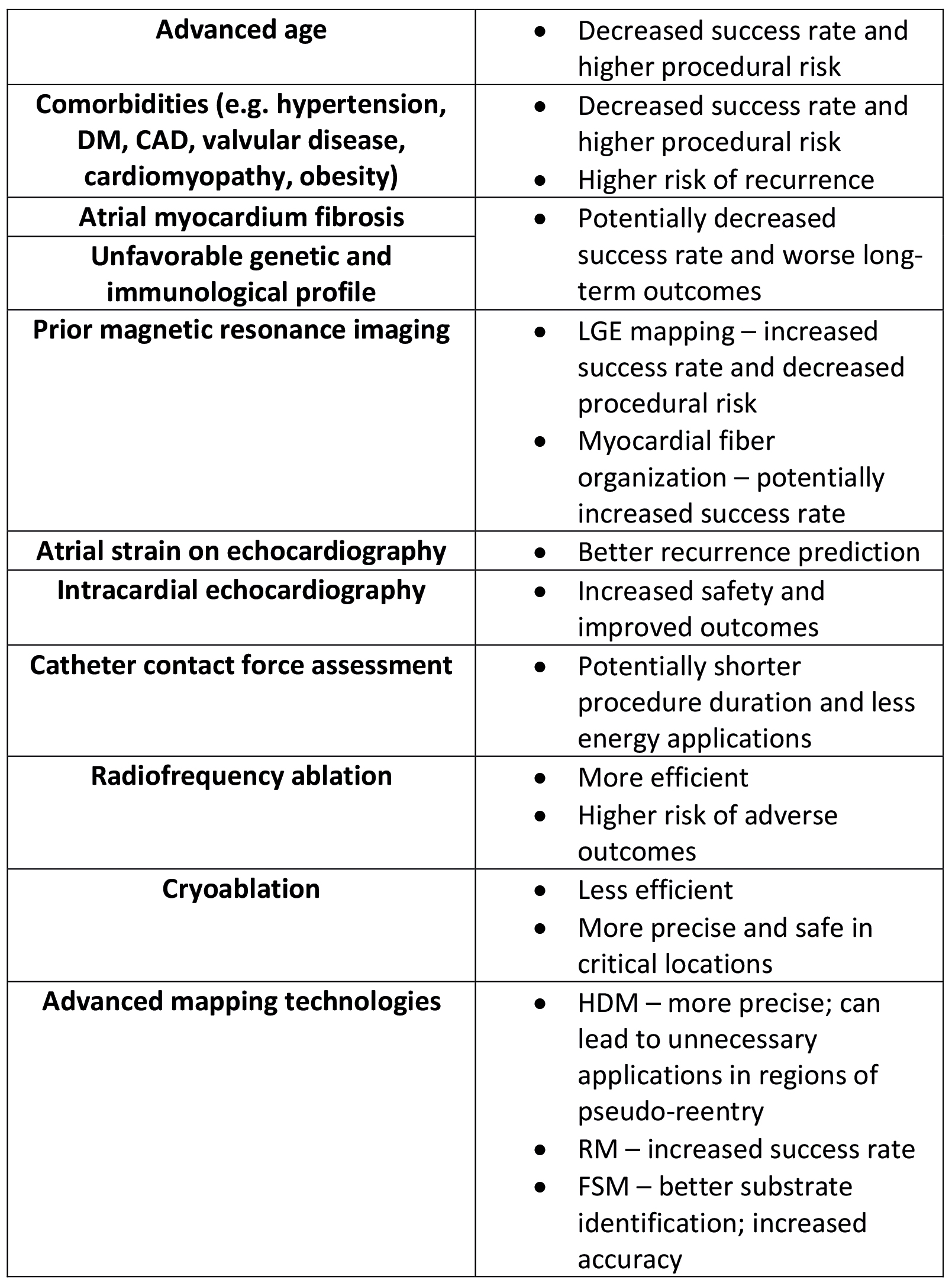
Figure 5. Factors impacting the AT ablation success and prognosis
CAD – coronary artery disease; DM – diabetes mellitus; FSM – functional substrate mapping; HDM – high density mapping; LGE – late gadolinium enhancement; RM – ripple mapping
Conclusion
Successful ablation of AT requires a multidimensional approach, incorporating patient-specific factors, arrhythmia characteristics, procedural techniques and long-term monitoring strategies. Continued research efforts aimed at elucidating novel prognostic markers, refining ablation strategies and leveraging digital health technologies are essential for advancing the field of AT ablation and improving patient care in the future. Precision medicine applications hold promise for improving the success and safety of atrial tachycardia ablation by individualizing treatment strategies based on patient-specific characteristics.
Conflict of interest
None.
Funding
None.
References
| 1. |
Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2016;134(11):e234-5]. Circulation. 2016;133(14):e506-e574. Available from: https://doi.org/10.1161/CIR.0000000000000311.
|
| 2. |
Markowitz SM, Thomas G, Liu CF, Cheung JW, Ip JE, Lerman BB. Atrial Tachycardias and Atypical Atrial Flutters: Mechanisms and Approaches to Ablation. Arrhythm Electrophysiol Rev. 2019;8(2):131-137. Available from: https://doi.org/10.15420/aer.2019.17.2.
|
| 3. |
Roberts-Thomson KC, Kistler PM, Kalman JM. Atrial tachycardia: mechanisms, diagnosis, and management. Curr Probl Cardiol. 2005;30(10):529-573. Available from: https://doi.org/10.1016/j.cpcardiol.2005.06.004.
|
| 4. |
Eitel C, Ince H, Brachmann J, et al. Catheter ablation of supraventricular tachycardia in patients with and without structural heart disease: insights from the German ablation registry. Clin Res Cardiol. 2022;111(5):522-529. Available from: https://doi.org/10.1007/s00392-021-01878-z.
|
| 5. |
Bamimore A, Mounsey P. Ablation of atrial tachycardia and atrial flutter in heart failure. Heart Fail Clin. 2013;9(4):501-514. Available from: https://doi.org/10.1016/j.hfc.2013.07.002.
|
| 6. |
Shoemaker MB, Husser D, Roselli C, et al. Genetic Susceptibility for Atrial Fibrillation in Patients Undergoing Atrial Fibrillation Ablation. Circ Arrhythm Electrophysiol. 2020;13(3):e007676. Available from: https://doi.org/10.1161/CIRCEP.119.007676.
|
| 7. |
Lee G, Sanders P, Kalman JM. Catheter ablation of atrial arrhythmias: state of the art. Lancet. 2012;380(9852):1509-1519. Available from: https://doi.org/10.1016/S0140-6736(12)61463-9.
|
| 8. |
He J, Pertsov A, Bullinga J, Mangharam R. Individualization of Atrial Tachycardia Models for Clinical Applications: Performance of Fiber-Independent Model. IEEE Trans Biomed Eng. 2024;71(1):258-269. Available from: https://doi.org/10.1109/TBME.2023.3298003.
|
| 9. |
Luther V, Linton NW, Koa-Wing M, et al. A Prospective Study of Ripple Mapping in Atrial Tachycardias: A Novel Approach to Interpreting Activation in Low-Voltage Areas. Circ Arrhythm Electrophysiol. 2016;9(1):e003582. Available from: https://doi.org/10.1161/CIRCEP.115.003582.
|
| 10. |
Giudicessi JR, Ackerman MJ, Fatkin D, Kovacic JC. Precision Medicine Approaches to Cardiac Arrhythmias: JACC Focus Seminar 4/5 [published correction appears in J Am Coll Cardiol. 2021;78(1):93. Available from: https://doi.org/10.1016/j.jacc.2021.05.032]. J Am Coll Cardiol. 2021;77(20):2573-2591. Available from: https://doi.org/10.1016/j.jacc.2021.03.325.
|
| 11. |
Compagnucci P, Dello Russo A, Bergonti M, et al. Ablation Index Predicts Successful Ablation of Focal Atrial Tachycardia: Results of a Multicenter Study. J Clin Med. 2022;11(7):1802. Available from: https://doi.org/10.3390/jcm11071802.
|
| 12. |
Mulder MJ, Kemme MJB, Hopman LHGA, et al. Comparison of the predictive value of ten risk scores for outcomes of atrial fibrillation patients undergoing radiofrequency pulmonary vein isolation. Int J Cardiol. 2021;344:103-110. Available from: https://doi.org/10.1016/j.ijcard.2021.09.029.
|
| 13. |
Choi Y, Lim B, Yang SY, et al. Clinical Usefulness of Virtual Ablation Guided Catheter Ablation of Atrial Fibrillation Targeting Restitution Parameter-Guided Catheter Ablation: CUVIA-REGAB Prospective Randomized Study. Korean Circ J. 2022;52(9):699-711. Available from: https://doi.org/10.4070/kcj.2022.0113.
|
| 14. |
Sau A, Ibrahim S, Ahmed A, et al. Artificial intelligence-enabled electrocardiogram to distinguish cavotricuspid isthmus dependence from other atrial tachycardia mechanisms. Eur Heart J Digit Health. 2022;3(3):405-414. Available from: https://doi.org/10.1093/ehjdh/ztac042.
|
| 15. |
Vergara P, Tzou WS, Tung R, et al. Predictive Score for Identifying Survival and Recurrence Risk Profiles in Patients Undergoing Ventricular Tachycardia Ablation: The I-VT Score. Circ Arrhythm Electrophysiol. 2018;11(12):e006730. Available from: https://doi.org/10.1161/CIRCEP.118.006730.
|
| 16. |
Al-Kaisey A, Wong G, Young P, et al. Polygenic risk scores identify atrial electrophysiological substrate abnormalities and predict atrial fibrillation recurrence following catheter ablation. Heart, Lung and Circulation. 2022;31:S52. Available from: https://doi.org/10.1016/j.hlc.2022.06.027.
|
| 17. |
Zado ES, Garg L, Tschabrunn C, et al. Risk of atrial arrhythmias in patients with ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2024;21(2):133-140. Available from: https://doi.org/10.1016/j.hrthm.2023.11.005.
|
| 18. |
van der Does WFB, Houck CA, Heida A, et al. Atrial electrophysiological characteristics of aging. J Cardiovasc Electrophysiol. 2021;32(4):903-912. Available from: https://doi.org/10.1111/jce.14978.
|
| 19. |
Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44(1):109-116. Available from: https://doi.org/10.1016/j.jacc.2004.03.044.
|
| 20. |
Zhang J, Yang J, Liu L, et al. Significant abnormal glycemic variability increased the risk for arrhythmias in elderly type 2 diabetic patients. BMC Endocr Disord. 2021;21(1):83. Available from: https://doi.org/10.1186/s12902-021-00753-2.
|
| 21. |
Gaudino M, Andreotti F, Zamparelli R, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication?. Circulation. 2003;108 Suppl 1:II195-II199. Available from: https://doi.org/10.1161/01.cir.0000087441.48566.0d.
|
| 22. |
Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500-1515. Available from: https://doi.org/10.1161/CIRCRESAHA.114.303772.
|
| 23. |
Wu XY, Li SN, Wen SN, et al. Plasma galectin-3 predicts clinical outcomes after catheter ablation in persistent atrial fibrillation patients without structural heart disease. Europace. 2015;17(10):1541-1547. Available from: https://doi.org/10.1093/europace/euv045.
|
| 24. |
Sandeep B, Ding W, Huang X, et al. Mechanism and Prevention of Atrial Remodeling and Their Related Genes in Cardiovascular Disorders [published correction appears in Curr Probl Cardiol. 2023;48(2):101514]. Curr Probl Cardiol. 2023;48(1):101414. Available from: https://doi.org/10.1016/j.cpcardiol.2022.101414.
|
| 25. |
O'Connor AM, Smith AH, Crum K, Edwards TL, Kannankeril PJ. Analysis of clinical and candidate genetic risk factors for postoperative atrial tachycardia after congenital heart surgery in infants. Am Heart J. 2018;202:1-4. Available from: https://doi.org/10.1016/j.ahj.2018.04.014.
|
| 26. |
Levin MD, Saitta SC, Gripp KW, et al. Nonreentrant atrial tachycardia occurs independently of hypertrophic cardiomyopathy in RASopathy patients. Am J Med Genet A. 2018;176(8):1711-1722. Available from: https://doi.org/10.1002/ajmg.a.38854.
|
| 27. |
Liu X, Wang S, Guo X, et al. Increased Reactive Oxygen Species-Mediated Ca2+/Calmodulin-Dependent Protein Kinase II Activation Contributes to Calcium Handling Abnormalities and Impaired Contraction in Barth Syndrome. Circulation. 2021;143(19):1894-1911. Available from: https://doi.org/10.1161/CIRCULATIONAHA.120.048698.
|
| 28. |
Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251-254. Available from: https://doi.org/10.1126/science.1077771.
|
| 29. |
Disertori M, Masè M, Marini M, et al. Electroanatomic mapping and late gadolinium enhancement MRI in a genetic model of arrhythmogenic atrial cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25(9):964-970. Available from: https://doi.org/10.1111/jce.12440.
|
| 30. |
Barefield DY, Yamakawa S, Tahtah I, et al. Partial and complete loss of myosin binding protein H-like cause cardiac conduction defects. J Mol Cell Cardiol. 2022;169:28-40. Available from: https://doi.org/10.1016/j.yjmcc.2022.04.012.
|
| 31. |
Yao Y, Yang M, Liu D, Zhao Q. Immune remodeling and atrial fibrillation. Front Physiol. 2022;13:927221. Available from: https://doi.org/10.3389/fphys.2022.927221.
|
| 32. |
Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118(9):916-925. Available from: https://doi.org/10.1161/CIRCULATIONAHA.108.776203.
|
| 33. |
Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62(24):2318-2325. Available from: https://doi.org/10.1016/j.jacc.2013.06.053.
|
| 34. |
Rossi P, Ricci A, De Paulis R, et al. Epicardial ganglionated plexus stimulation decreases postoperative inflammatory response in humans. Heart Rhythm. 2012;9(6):943-950. Available from: https://doi.org/10.1016/j.hrthm.2012.01.025.
|
| 35. |
Ogawa M, Tan AY, Song J, et al. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2009;6(12):1772-1779. Available from: https://doi.org/10.1016/j.hrthm.2009.08.011.
|
| 36. |
Fochler F, Yamaguchi T, Kheirkahan M, Kholmovski EG, Morris AK, Marrouche NF. Late Gadolinium Enhancement Magnetic Resonance Imaging Guided Treatment of Post-Atrial Fibrillation Ablation Recurrent Arrhythmia. Circ Arrhythm Electrophysiol. 2019;12(8):e007174. Available from: https://doi.org/10.1161/CIRCEP.119.007174.
|
| 37. |
Morris GM, Segan L, Wong G, et al. Atrial Tachycardia Arising From the Crista Terminalis, Detailed Electrophysiological Features and Long-Term Ablation Outcomes. JACC Clin Electrophysiol. 2019;5(4):448-458. Available from: https://doi.org/10.1016/j.jacep.2019.01.014.
|
| 38. |
Szegedi N, Zima E, Clemens M, et al. Radiofrequency ablation of focal atrial tachycardia: Benefit of electroanatomical mapping over conventional mapping. Acta Physiol Hung. 2015;102(3):252-262. Available from: https://doi.org/10.1556/036.102.2015.3.3.
|
| 39. |
Harrison JL, Sohns C, Linton NW, et al. Repeat left atrial catheter ablation: cardiac magnetic resonance prediction of endocardial voltage and gaps in ablation lesion sets. Circ Arrhythm Electrophysiol. 2015;8(2):270-278. Available from: https://doi.org/10.1161/CIRCEP.114.002066.
|
| 40. |
Quinto L, Cozzari J, Benito E, et al. Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case-control study. Europace. 2020;22(12):1805-1811. Available from: https://doi.org/10.1093/europace/euaa252.
|
| 41. |
Nakasone K, Kiuchi K, Takami M, Izawa Y, Fukuzawa K, Hirata KI. Successful catheter ablation of postoperative atrial tachycardia with conduction disturbances: Assessment by late-gadolinium enhancement magnetic resonance imaging and high-resolution electro-anatomical mapping. Clin Case Rep. 2021;9(6):e04198. Available from: https://doi.org/10.1002/ccr3.4198.
|
| 42. |
Nielsen AB, Skaarup KG, Djernæs K, et al. Left atrial contractile strain predicts recurrence of atrial tachyarrhythmia after catheter ablation. Int J Cardiol. 2022;358:51-57. Available from: https://doi.org/10.1016/j.ijcard.2022.04.056.
|
| 43. |
Wang J, Li S, Liang M, et al. Characteristics and Ablation Outcomes of Atrial Tachycardia in Patients with Prior Cardiac Surgery vs. Spontaneous Scars: Where Are the Differences?. J Clin Med. 2022;11(18):5407. Available from: https://doi.org/10.3390/jcm11185407.
|
| 44. |
Ju W, Li M, Wang DW, et al. Idiopathic isolated fibrotic atrial cardiomyopathy underlies unexplained scar-related atrial tachycardia in younger patients. Europace. 2018;20(10):1657-1665. Available from: https://doi.org/10.1093/europace/eux340.
|
| 45. |
Geczy T, Ramdat Misier NL, Szili-Torok T. Contact-Force-Sensing-Based Radiofrequency Catheter Ablation in Paroxysmal Supraventricular Tachycardias (COBRA-PATH): a randomized controlled trial. Trials. 2020;21(1):321. Available from: https://doi.org/10.1186/s13063-020-4219-1.
|
| 46. |
Ariyarathna N, Kumar S, Thomas SP, Stevenson WG, Michaud GF. Role of Contact Force Sensing in Catheter Ablation of Cardiac Arrhythmias: Evolution or History Repeating Itself?. JACC Clin Electrophysiol. 2018;4(6):707-723. Available from: https://doi.org/10.1016/j.jacep.2018.03.014.
|
| 47. |
Santoro F, Metzner A, Brunetti ND, et al. Left atrial anterior line ablation using ablation index and inter-lesion distance measurement. Clin Res Cardiol. 2019;108(9):1009-1016. Available from: https://doi.org/10.1007/s00392-019-01428-8.
|
| 48. |
Hanninen M, Yeung-Lai-Wah N, Massel D, et al. Cryoablation versus RF ablation for AVNRT: A meta-analysis and systematic review. J Cardiovasc Electrophysiol. 2013;24(12):1354-1360. Available from: https://doi.org/10.1111/jce.12247.
|
| 49. |
Okishige K, Shigeta T, Nishimura T, et al. Cryofreezing catheter ablation of adenosine triphosphate sensitive atrial tachycardia. J Cardiovasc Electrophysiol. 2019;30(4):528-537. Available from: https://doi.org/10.1111/jce.13844.
|
| 50. |
Turagam MK, Neuzil P, Schmidt B, et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation. 2023;148(1):35-46. Available from: https://doi.org/10.1161/CIRCULATIONAHA.123.064959.
|
| 51. |
Gunawardene MA, Schaeffer BN, Jularic M, et al. Pulsed field ablation in patients with complex consecutive atrial tachycardia in conjunction with ultra-high density mapping: Proof of concept. J Cardiovasc Electrophysiol. 2022;33(12):2431-2443. Available from: https://doi.org/10.1111/jce.15713.
|
| 52. |
Kueffer T, Seiler J, Madaffari A, et al. Pulsed-field ablation for the treatment of left atrial reentry tachycardia. J Interv Card Electrophysiol. 2023;66(6):1431-1440. Available from: https://doi.org/10.1007/s10840-022-01436-1.
|
| 53. |
Rostock T, Benz AP, Spittler R. Left atrial field isolation with pulsed field ablation: A new option for challenging left atrial tachycardias?. J Cardiovasc Electrophysiol. 2022;33(12):2444-2446. Available from: https://doi.org/10.1111/jce.15712.
|
| 54. |
Weyand S, Adam V, Biehler P, et al. Focal Pulsed Field Ablation for Atrial Arrhythmias: Efficacy and Safety under Deep Sedation. J Clin Med. 2024;13(2):576. Available from: https://doi.org/10.3390/jcm13020576.
|
| 55. |
Takigawa M, Derval N, Frontera A, et al. Revisiting anatomic macroreentrant tachycardia after atrial fibrillation ablation using ultrahigh-resolution mapping: Implications for ablation. Heart Rhythm. 2018;15(3):326-333. Available from: https://doi.org/10.1016/j.hrthm.2017.10.029.
|
| 56. |
Luther V, Sikkel M, Bennett N, et al. Visualizing Localized Reentry With Ultra-High Density Mapping in Iatrogenic Atrial Tachycardia: Beware Pseudo-Reentry. Circ Arrhythm Electrophysiol. 2017;10(4):e004724. Available from: https://doi.org/10.1161/CIRCEP.116.004724.
|
| 57. |
Luther V, Cortez-Dias N, Carpinteiro L, et al. Ripple mapping: Initial multicenter experience of an intuitive approach to overcoming the limitations of 3D activation mapping. J Cardiovasc Electrophysiol. 2017;28(11):1285-1294. Available from: https://doi.org/10.1111/jce.13308.
|
| 58. |
Jamil-Copley S, Linton N, Koa-Wing M, et al. Application of ripple mapping with an electroanatomic mapping system for diagnosis of atrial tachycardias. J Cardiovasc Electrophysiol. 2013;24(12):1361-1369. Available from: https://doi.org/10.1111/jce.12259.
|
| 59. |
Strisciuglio T, Vandersickel N, Lorenzo G, et al. Prospective evaluation of entrainment mapping as an adjunct to new-generation high-density activation mapping systems of left atrial tachycardias. Heart Rhythm. 2020;17(2):211-219. Available from: https://doi.org/10.1016/j.hrthm.2019.09.014.
|
| 60. |
Blandino A, Bianchi F, Grossi S, et al. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol. 2017;40(2):199-212. Available from: https://doi.org/10.1111/pace.13015.
|
| 61. |
Vlachos K, Efremidis M, Derval N, et al. Use of high-density activation and voltage mapping in combination with entrainment to delineate gap-related atrial tachycardias post atrial fibrillation ablation. Europace. 2021;23(7):1052-1062. Available from: https://doi.org/10.1093/europace/euaa394.
|
| 62. |
Yorgun H, Coteli C, Kılıç GS, Aytemir K. Functional substrate mapping of atrium in patients with atrial scar: A novel method to predict critical isthmus of atrial tachycardia. Pacing Clin Electrophysiol. 2024;47(5):653-660. Available from: https://doi.org/10.1111/pace.14981.
|
| 63. |
Vicera JJB, Lin YJ, Lee PT, et al. Identification of critical isthmus using coherent mapping in patients with scar-related atrial tachycardia. J Cardiovasc Electrophysiol. 2020;31(6):1436-1447. Available from: https://doi.org/10.1111/jce.14457.
|
| 64. |
Yorgun H, Çöteli C, Kılıç GS, et al. Functional substrate mapping characteristics during sinus rhythm predicts critical isthmus of reentrant atrial tachycardia. J Cardiovasc Electrophysiol. 2023;34(7):1539-1548. Available from: https://doi.org/10.1111/jce.15961.
|
| 65. |
Gupta A, Lokhandwala Y, Rai N, Malviya A. Adenosine-A drug with myriad utility in the diagnosis and treatment of arrhythmias. J Arrhythm. 2020;37(1):103-112. Available from: https://doi.org/10.1002/joa3.12453.
|
| 66. |
Campbell T, Bennett RG, Kumar S. Intracardiac Echocardiography to Guide the Ablation of Parahisian Arrhythmias. Card Electrophysiol Clin. 2021;13(2S):e1-e16. Available from: https://doi.org/10.1016/j.ccep.2022.01.001.
|
| 67. |
Peichl P, Kautzner J, Gebauer R. Ablation of atrial tachycardias after correction of complex congenital heart diseases: utility of intracardiac echocardiography. Europace. 2009;11(1):48-53. Available from: https://doi.org/10.1093/europace/eun316.
|
| 68. |
Tonegawa-Kuji R, Yamagata K, Suzuki S, Miyazaki Y, Ueda N, Kusano K. Prompt recognition and successful aspiration of a left atrial thrombus under intracardiac echocardiography guidance during radiofrequency catheter ablation for atrial tachycardia. Europace. 2021;23(10):1527. Available from: https://doi.org/10.1093/europace/euab210.
|
| 69. |
Žižek D, Antolič B, Prolič Kalinšek T, et al. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J Interv Card Electrophysiol. 2021;61(3):595-602. Available from: https://doi.org/10.1007/s10840-020-00858-z.
|
| 70. |
Vila M, Rivolta MW, Barrios Espinosa CA, et al. Recommender system for ablation lines to treat complex atrial tachycardia. Comput Methods Programs Biomed. 2023;231:107406. Available from: https://doi.org/10.1016/j.cmpb.2023.107406.
|
| 71. |
Shi R, Zaman JAB, Chen Z, et al. Novel aggregated multiposition noncontact mapping of atrial tachycardia in humans: From computational modeling to clinical validation. Heart Rhythm. 2022;19(1):61-69. Available from: https://doi.org/10.1016/j.hrthm.2021.09.025.
|
| 72. |
Lee JH, Kwon OS, Shim J, et al. Left Atrial Wall Stress and the Long-Term Outcome of Catheter Ablation of Atrial Fibrillation: An Artificial Intelligence-Based Prediction of Atrial Wall Stress. Front Physiol. 2021;12:686507. Available from: https://doi.org/10.3389/fphys.2021.686507.
|
| 73. |
Bahlke F, Englert F, Popa M, et al. First clinical data on artificial intelligence-guided catheter ablation in long-standing persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2024;35(3):406-414. Available from: https://doi.org/10.1111/jce.16184.
|
| 74. |
Piccini JP, Russo AM, Sharma PS, et al. Advances in Cardiac Electrophysiology. Circ Arrhythm Electrophysiol. 2022;15(12):e009911. Available from: https://doi.org/10.1161/CIRCEP.121.009911.
|
| 75. |
Khan A, Cereda A, Walther C, Aslam A. Multidisciplinary Integrated Care in Atrial Fibrillation (MICAF): A Systematic Review and Meta-Analysis. Clin Med Res. 2022;20(4):219-230. Available from: https://doi.org/10.3121/cmr.2022.1702.
|












