Comorbidities and clinical outcomes of a lung cancer screening trial participants with chronic obstructive pulmonary disease in three-year follow-up
Abstract
Background: To improve the effectiveness of lung cancer screening using low-dose computed tomography (LDCT), the presence of smoking-related comorbidities that may significantly affect mortality in this group should be taken into account.
Material and methods: A questionnaire survey and spirometry tests were conducted in a group of 730 respondents as part of a lung cancer screening study between 2016 and 2018. People diagnosed with COPD underwent a three-year follow-up to assess the incidence of medical events.
Results: Our study confirmed that cardiovascular diseases (CVDs) were the most common comorbidities in patients who were diagnosed with COPD and participated in LDCT lung cancer screening. Among the CVDs, the most common were arterial hypertension (45.8%) and coronary artery disease (12.5%). Tobacco-related diseases (e.g. CVD, lung cancer, and exacerbations of COPD) were the leading causes of emergency department visits and hospitalizations. The number of visits due to COPD in specialized clinics more than doubled in the observed period.
Conclusions: Properly planned screening tests allow not only for the detection of the disease for which they were designed but also for the assessment of comorbidities. In patients undergoing lung cancer screening, it is justified to extend the diagnostics to include spirometry.
Citation
Undrunas A, Bąk A, Kasprzyk P, Dziedzic R, Dziurda D, Rzyman W, Gierlotka M, Kuziemski K. Comorbidities and clinical outcomes of a lung cancer screening trial participants with chronic obstructive pulmonary disease in three-year follow-up. Eur J Transl Clin Med. 2022;5(2):24-30Introduction
Chronic obstructive pulmonary disease (COPD) is a major health problem worldwide [1]. According to the World Health Organization (WHO), it is the third leading cause of death after coronary artery disease and stroke [2]. The most significant risk factor for COPD is exposure to tobacco smoke [3]. Changes caused by harmful gases and particles lead not only to local inflammatory processes in the airways, but also to systemic inflammation responsible for the considerable comorbidities found in COPD patients [3-5]. According to available data, individuals with COPD have a five-fold higher risk of developing cardiovascular diseases and a two-fold risk of lung cancer compared with smokers without COPD [6-8].
Data from randomized trials targeting the smoking population shows that low-dose computed tomographic (LDCT) screening, through secondary prevention, reduces lung cancer mortality in this group [9-11]. To improve the effectiveness of LDCT screening, trials assessing the potential benefits of combined oncological-pulmonary screening have been conducted in many countries [12-14]. In Poland, there is still not enough epidemiological data about the prevalence of COPD [4]. There is a significant need to assess the impact of lung cancer comobribidities on LDCT screening effectiveness. Therefore, after lung canceer screning programmes, observational studies on medical outcomes of participants are conducted. In this article, we present the data from a three-year follow-up of Polish LDCT screening trial participants in whom complete spirometry was performed to detect COPD.
Patients and methods
To establish the prevalence of main tobacco-related comorbidities among participants of the lung cancer screening program “MOLTEST-BIS: Validation of molecular signatures of early lung cancer in the high-risk group”, started in 2016 [15], we conducted an additional investigation to assess the prevalence of COPD. The eligibility criteria were based on the lung cancer screening trial criteria were as follows: aged 50 to 79 years, citizens of Pomeranian Voivodeship, with a smoking history of over 30 pack-years, and either current or former smokers (but only those who had quit smoking no more than 15 years before the screening). Patients provided written informed consent to participate in the study. The study was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (approval No NKBBN/173/2016).
Standardized questionnaire surveys that included questions about the patients’ medical histories, previous diseases, medications, smoking histories and respiratory symptoms were distributed. All participants then underwent physical examination and spirometry with a bronchodilator reversibility test We established the prevalence and staging of COPD according to the Global Intiative for Chronic Obstructive Lung Disease (GOLD) criteria (3). We also assessed the impact of the disease on quality of life and published that analysis separately [16]. All participants diagnosed with COPD were informed about their spirometry results and the necessity of specialized pulmonary care to implement appropriate treatment. They underwent a three-year follow-up observational study.
In cooperation with the Agency for Health Technology Assessment and Tariff System (AOTMiT) and according to the agreement with the Medical University of Gdańsk, we performed an assessment of participants’ main causes of death, hospitalizations and number of COPD-related visits in specialized healthcare centers until the end of 2020. The analyses were performed based on the International Classification of Diseases, Tenth Revision (ICD-10) coded data from Statistics Poland (Główny Urząd Statystyczny).
Patients were identified in the National Health Fund database, which is implemented in the AOTMiT’s data archive system. Episodes of hospitalizations, outpatient specialist care and basic medical care (with the main ICD-10 codes) from the period of two years before and three years after the date of examination in the screening trial were arranged chronologically. Then, episodes of visits and hospitalizations by main disease were summarized at six-month intervals, starting from the date of examination. As a result, the number of visits and hospitalizations was presented in the six-month interval periods before and after the date of the study. A different scenario was applied to summarize the number of patients, which was counted incrementally starting from the date of examination in the screening trial. A period of six months was applied, thus the number of patients was presented in six-month follow-up intervals as well as in six-month period intervals before the date of the study. Descriptive statistics were used to summarize and present the data.
Results
The records of 730 participants (335 women and 395 men) from the lung cancer screening program MOLTEST-BIS were analyzed. The mean age of the men and women participating in the study was similar (63.5 vs 63). Based on spirometry results before and after bronchodilator administration, 144 COPD cases (19.7%) were diagnosed (86 men and 58 women). Most cases detected were in the mild stage of the disease, according to the GOLD classification. These results are presented more precisely in a separate article [16].
Table 1 presents the data on the main comorbidities among COPD patients at the beginging of the programme. Comorbidities were recorded based on the questionnaire data. The most common chronic disease reported by the participants was hypertension (45.8%), followed by coronary artery disease (12.5%), diabetes (11.8%) and atrial fibrillation (10.5%). Asthma was reported by 9.7% of the respondents, which required a differential diagnosis with COPD.
Table 1. Illnesses coexisting with COPD
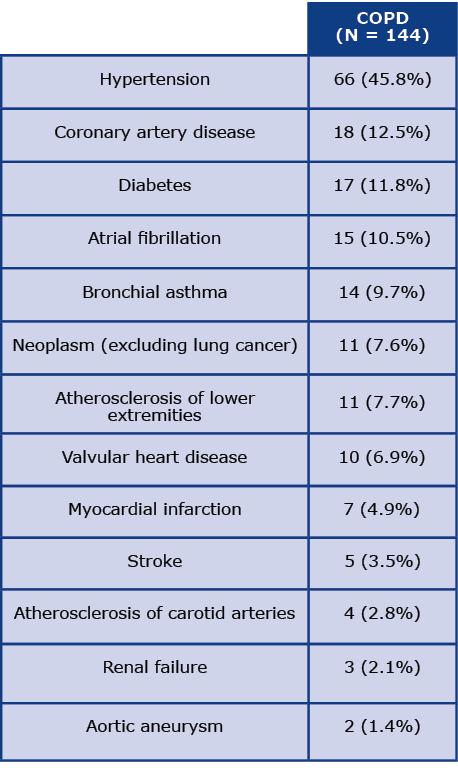
The mean duration of follow-up after the screening was 44 months (SD 8.3). We assessed the main causes of death, hospitalizations and visits to specialized healthcare centers among all respondents with COPD diagnoses. Five deaths were reported during the follow-up period. Causes of death according to the ICD-10 code and the most common causes of visits to the Emergency Departments (ED) during threeyear observation are presented in Tables 2 and 3, respectively. Most commonly, patients were admitted to the ED due to cardiovascular diseases (CVD), particularly atrial fibrillation (I48) and hypertension (I10). The next most common causes of patients’ visits to the ED were lung cancer (C34) and COPD (J44). Additionally, one hospitalization was coded as dyspnoea (R06) and another as a different respiratory disorder (J98). Other causes of visits to the ED were reported very rarely.
Table 2. Causes of death according to ICD-10
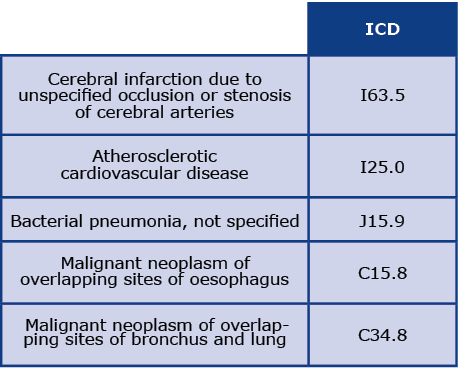
Table 3. Causes of visits to the Emergency Department (ED)
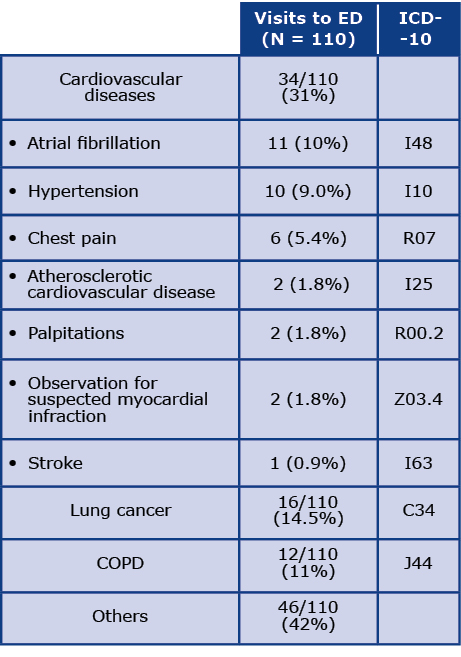
Table 4 presents the most common causes of hospital admissions. The most common cause of hospitalization was cataracts (17.6%, ICD codes H25 and H26). Additionally, 12.2% of hospitalizations were due to CVD, 7.5% were due to lung cancer and almost 5% were related to COPD. Other causes of hospitalization occurred sporadically and thus have not been analyzed.
Table 4. Causes of hospital admissions
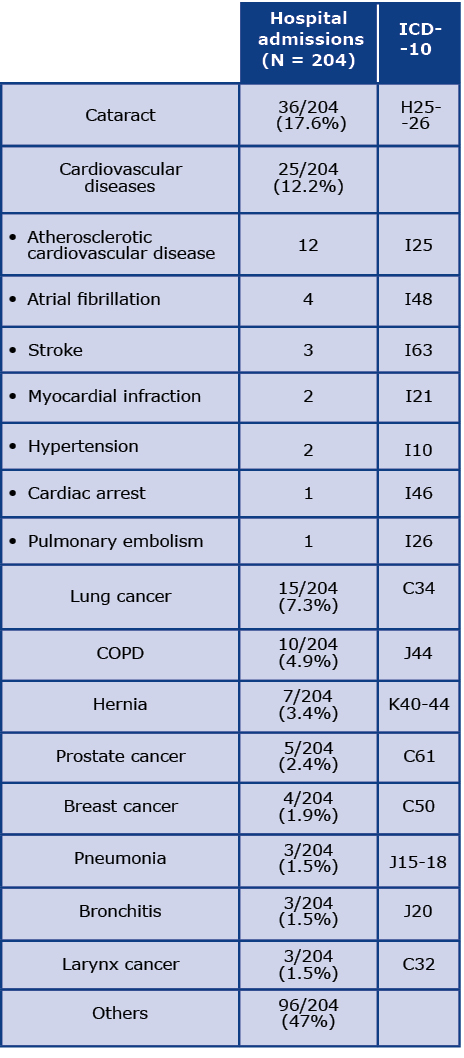
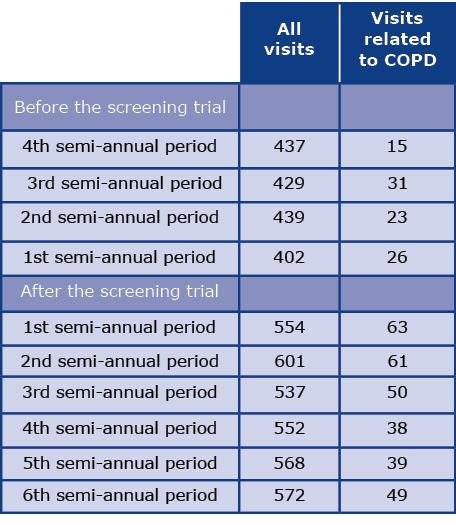
Table 5 shows the number of visits to specialized healthcare centers related to COPD before and after the screening program in semi-annual periods. The total number of visits within three years of follow-up was 3384. A two-fold increase in the number of visits related to COPD was found after the screening. There were 95 visits during the two-year pre-screening observation, compared with 212 visits in two years of follow-up and 300 in three years of follow-up.
Table 5. Number of visits to specialized healthcare centres
Discussion
In many research centers, additional attempts have been made to assess the prevalence of COPD during lung cancer screening programs. These data are heterogeneous and depend on the diagnostic criteria and individual characteristics of the studied population [16]. The prevalence of COPD among participants of lung cancer screening is reported to be as high as 60% [17-18]. In the Polish lung cancer screening program, MOLTEST-BIS, the prevalence of COPD was almost 20% [27]. According to National Lung Screening Trial (NLST) data, mortality from lung cancer, cardiovascular diseases and respiratory failure among patients with COPD in USA increases with the increasing severity of the disease [19]. COPD is a known risk factor for CVD and lung cancer, independent of smoking status [7]. Moreover, patients with COPD tend to have a longer duration of hospitalization and an increased risk of 30-day mortality after myocardial infarction, exacerbations of heart failure and cardiac or surgical procedures [7, 20-21]. To achieve the highest effectiveness of lung cancer screening, it is recommended to pay special attention to participants with coexisting COPD [12, 19, 22-23]. According to epidemiological studies conducted in Europe, CVD is the most common comorbidity associated with COPD, it concerns about 20% patients with COPD and about 9% without COPD [7, 25-26]. According to the GOLD guidelines, in mild and moderate COPD the main causes of death are lung cancer and CVD. Depending on the methodology, they constitute from 12% to 37% causes of death [27].
Our analysis confirmed that the most common comorbidities among participants with COPD were CVDs and the most common among them was hypertension [7, 24-25]. A comorbidity that required particular attention in the differential diagnosis of COPD was asthma, which was reported by 14 participants (9.7%). Due to significant tobacco exposure among these participants, the history of respiratory symptoms and data on asthma diagnosis had to be more specific to exclude the asthma-COPD overlap [3].
Patients were most often admitted to the ED due to smoking-related diseases, such as CVD, lung cancer, and COPD exacerbations, which together accounted for majority of the hospitalizations. Whereas the leading single cause of hospital admission were cataracts and we hypothesize that those could have been due to planned surgical procedures. We recorded a lower number of hospitalizations due to COPD exacerbations compared with other epidemiological studies [26]. This may be related to the early detection of COPD and the provision of feedback information to participants about the diagnosis and the necessity of the immediate start of appropriate treatment.
The effectiveness of the addition of spirometry to the lung cancer screening program was confirmed by a two-fold increase in COPD-related visits to specialized healthcare centers during follow-up (Table 5). Data obtained during the MOLTEST-BIS program indicate that modification of screening programs by implementation of new diagnostic procedures and comorbidity assessment is necessary and improves their effectiveness.
Conclusions
Precisely planned screening programs not only allow us to diagnose a disease that they were designed for but also give us the opportunity to assess patients’ comorbidities. Lung cancer screening trials are particularly important in this regard. Among the group of patients with a high burden of active and passive smoking, it is important to broaden the screening to assess the presence of COPD and comorbidities. This would allow those patients to be referred to specialized healthcare centers and receive appropriate treatment, which could prevent the progression of the disease and minimize the risk of future respiratory failure. Further studies are needed to assess the effectiveness of diagnosing and preventing COPD in this group and the possible correlation of our results with the assessment of the severity of emphysema and cardiac calcium scoring using LDCT.
Acknowledgements
We thank Prof. Tomasz Zdrojewski MD PhD, Roman Topór-Mądry MD PhD and Katarzyna Leoszkiewicz for supervising and coordinating cooperation between the Medical University of Gdańsk and the Agency for Health Technology Assessment and Tariff System.
Funding
None.
Conflicts of interests
None.
References
| 1. |
Maselli DJ, Bhatt SP, Anzueto A, Bowler RP, DeMeo DL, Diaz AA, et al. Clinical Epidemiology of COPD. Chest [Internet]. 2019 Aug;156(2):228-238. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0012369219311286.
|
| 2. |
The top 10 causes of death, 2000-2016 [Internet]. WHO. 2016 [cited 2018 May 1]. p. 1-9. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
|
| 3. |
GOLD. Global Strategy for Prevention, Diagnosis and Management of COPD:2022 Report [Internet]. Global Initiative for Chronic Obstructive Lung Disease. 2022. p. 177. Available from: https://goldcopd.org/2022-gold-reports-2/.
|
| 4. |
Śliwiński P, Górecka D, Jassem E, Pierzchała W. Polish Respiratory Society Guidelines for Chronic Obstructive Pulmonary Disease [in Polish]. Adv Respir Med [Internet]. 2014 Apr 30;82(3):227-63. Available from: https://www.mdpi.com/2543-6031/82/3/227.
|
| 5. |
Putcha N, Puhan MA, Hansel NN, Drummond MB, Boyd CM. Impact of co-morbidities on self-rated health in self-reported COPD: An analysis of NHANES 2001–2008. COPD J Chronic Obstr Pulm Dis [Internet]. 2013 Jun 28;10(3):324-32. Available from: http://www.tandfonline.com/doi/full/10.3109/15412555.2012.744963.
|
| 6. |
Ho C-H, Chen Y-C, Wang J-J, Liao K-M. Incidence and relative risk for developing cancer among patients with COPD: a nationwide cohort study in Taiwan. BMJ Open [Internet]. 2017 Mar 9;7(3):e013195. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2016-013195.
|
| 7. |
Rabe KF, Wedzicha JA, Wouters EFM, editors. COPD and Comorbidity [Internet]. European Respiratory Society; 2013. 240 p. Available from: http://erspublications.com/lookup/doi/10.1183/1025448x.erm5913.
|
| 8. |
Mouronte-Roibás C, Leiro-Fernández V, Fernández-Villar A, Botana-Rial M, Ramos-Hernández C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett [Internet]. 2016 Nov;382(2):240-4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304383516305444.
|
| 9. |
O’Dowd EL, Baldwin DR. Lung cancer screening – low dose CT for lung cancer screening: recent trial results and next steps. Br J Radiol [Internet]. 2018 Oct;91(1090):20170460. Available from: https://www.birpublications.org/doi/10.1259/bjr.20170460.
|
| 10. |
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med [Internet]. 2020 Feb 6;382(6):503-13. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1911793.
|
| 11. |
Usman Ali M, Miller J, Peirson L, Fitzpatrick-Lewis D, Kenny M, Sherifali D, et al. Screening for lung cancer: A systematic review and meta-analysis. Prev Med (Baltim) [Internet]. 2016 Aug;89:301-14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091743516300706.
|
| 12. |
Regan EA, Lowe KE, Make BJ, Lynch DA, Kinney GL, Budoff MJ, et al. Identifying Smoking-Related Disease on Lung Cancer Screening CT Scans: Increasing the Value. Chronic Obstr Pulm Dis J COPD Found [Internet]. 2019;6(3):233-45. Available from: https://journal.copdfoundation.org/jcopdf/id/1236/Identifying-Smoking-Related-Disease-on-Lung-Cancer-Screening-CT-Scans-Increasing-the-Value.
|
| 13. |
Rivera MP, Tanner NT, Silvestri GA, Detterbeck FC, Tammemägi MC, Young RP, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med [Internet]. 2018 Jul 15;198(2):e3-13. Available from: https://www.atsjournals.org/doi/10.1164/rccm.201805-0986ST.
|
| 14. |
Heuvelmans MA, Vonder M, Rook M, Groen HJM, De Bock GH, Xie X, et al. Screening for Early Lung Cancer, Chronic Obstructive Pulmonary Disease, and Cardiovascular Disease (the Big-3) Using Low-dose Chest Computed Tomography. J Thorac Imaging [Internet]. 2019 May;34(3):160-9. Available from: https://journals.lww.com/00005382-201905000-00004.
|
| 15. |
Ostrowski M, Bińczyk F, Marjański T, Dziedzic R, Pisiak S, Małgorzewicz S, et al. Performance of various risk prediction models in a large lung cancer screening cohort in Gdańsk, Poland – a comparative study. Transl Lung Cancer Res [Internet]. 2021 Feb;10(2):1083-90. Available from: https://tlcr.amegroups.com/article/view/41797/html.
|
| 16. |
Undrunas A, Kasprzyk P, Rajca A, Kuziemski K, Rzyman W, Zdrojewski T. Prevalence, symptom burden and under-diagnosis of chronic obstructive pulmonary disease in Polish lung cancer screening population: a cohort observational study. BMJ Open [Internet]. 2022 Apr 11;12(4):e055007. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2021-055007.
|
| 17. |
Iaccarino JM, Steiling KA, Wiener RS. Lung Cancer Screening in a Safety-Net Hospital: Implications of Screening a Real-World Population versus the National Lung Screening Trial. Ann Am Thorac Soc [Internet]. 2018 Dec;15(12):1493-5. Available from: https://www.atsjournals.org/doi/10.1513/AnnalsATS.201806-389RL.
|
| 18. |
Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer [Internet]. 2016 Dec 20;16(1):281. Available from: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2316-z.
|
| 19. |
Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, et al. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med [Internet]. 2015 Nov 1;192(9):1060-7. Available from: https://www.atsjournals.org/doi/10.1164/rccm.201505-0894OC.
|
| 20. |
Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res [Internet]. 2018 Jun;7(3):347-60. Available from: http://tlcr.amegroups.com/article/view/21631/16760.
|
| 21. |
Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-Based Risk for Complications After Transthoracic Needle Lung Biopsy of a Pulmonary Nodule: An Analysis of Discharge Records. Ann Intern Med [Internet]. 2011 Aug 2;155(3):137. Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-155-3-201108020-00003.
|
| 22. |
Lowry KP, Gazelle GS, Gilmore ME, Johanson C, Munshi V, Choi SE, et al. Personalizing annual lung cancer screening for patients with chronic obstructive pulmonary disease: A decision analysis. Cancer [Internet]. 2015/02/03. 2015 May 15;121(10):1556-62. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cncr.29225.
|
| 23. |
Seijo LM, Zulueta JJ. Understanding the Links Between Lung Cancer, COPD, and Emphysema: A Key to More Effective Treatment and Screening. Oncology (Williston Park) [Internet]. 2017;31(2):93-102. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28205188.
|
| 24. |
Du Y, Li Q, Sidorenkov G, Vonder M, Cai J, de Bock GH, et al. Computed Tomography Screening for Early Lung Cancer, COPD and Cardiovascular Disease in Shanghai: Rationale and Design of a Population-based Comparative Study. Acad Radiol [Internet]. 2021 Jan;28(1):36-45. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1076633220300489.
|
| 25. |
Mapel DW, Hurley JS, Frost FJ, Petersen H V, Picchi MA, Coultas DB. Health Care Utilization in Chronic Obstructive Pulmonary Disease. Arch Intern Med [Internet]. 2000 Sep 25;160(17):2653. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.160.17.2653.
|
| 26. |
Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res [Internet]. 2010 Dec 10;11(1):122. Available from: http://respiratory-research.biomedcentral.com/articles/10.1186/1465-9921-11-122.
|
| 27. |
GOLD. Global Initiative for Chronic Obstructive Lung Disease [Internet]. 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-11Nov20_WMV.pdf.
|
| 28. |
Barr RG, Celli BR, Mannino DM, Petty T, Rennard SI, Sciurba FC, et al. Comorbidities, Patient Knowledge, and Disease Management in a National Sample of Patients with COPD. Am J Med [Internet]. 2009 Apr;122(4):348-55. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002934308010723.
|
| 29. |
Franssen FME, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev [Internet]. 2014 Mar 1;23(131):131-41. Available from: http://err.ersjournals.com/cgi/doi/10.1183/09059180.00007613.
|
| 30. |
Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, et al. Comorbidity, Hospitalization, and Mortality in COPD: Results from a Longitudinal Study. Lung [Internet]. 2010 Aug 12;188(4):321-9. Available from: http://link.springer.com/10.1007/s00408-009-9222-y.
|











