Abstract
Due to late diagnosis, ovarian cancer is the most deadly gynecologic malignancy. Inflammation is one of the risk factors of ovarian cancer and the inflammatory response implicates all stages of tumorigenesis. The purpose of this study was to analyze the concentration of molecules, which can take part in malignant processes. We analyzed patients with ovarian cancer, with endometriosis and healthy controls. Thirty-seven analytes were measured in serum using BioPlex Pro Human Inflammation Panel. We were able to detect 28 of the proteins among the studied groups. We found a significant increase in 22 of the tested molecules (BAFF, Chitinase3-like 1, IFN-alpha2, IFN-beta, IFN-gamma, IFN-lambda2, IFN-lambda1, gp130, IL-2, IL-12 (p40), IL-11, IL-32, IL-35, MMP3, Osteocalcin, Pantraxin-3, sCD163, TNFRSF8, sIL-6Ralpha, STNF-R1, STNF-R2, and TSLP) in the ovarian cancer group in comparison to the healthy controls. Two of them (IL-20, MMP1) did not show significant differences between groups. Moreover, we identified decreased concentrations of APRIL and osteopontin in ovarian cancer vs. healthy controls. While this study is a preliminary report, we hope this will encourage a further use of multiplex analysis in ovarian cancer biomarker research.
Citation
Wyciszkiewicz A, Lach M, Kalinowska A, Michalak S, Nowakowski B. Multiplexed Immunobead-Based Cytokine Profiling in patients with ovarian cancer. Eur J Transl Clin Med. 2021;4(2):31-43Introduction
Ovarian cancer (OvCa) is often an asymptomatic disease, without reliable specific biomarkers indicating the early forms of developing cancer, leading to clinical diagnosis at an advanced stage with metastases. For these reasons, it represents the third most common gynecologic malignancy and the fifth cause of death among cancers in women [1].
To date, only the serum cancer antigen 125 (CA-125) and human epididymis secretory protein E4 (HE4/WFDC2) are used widely in OvCa diagnosis. However, it has limited specificity and sensitivity. At an early stage of the disease, the detection of CA-125 is not always possible. Moreover, the elevated level of CA-125 is also observed during endometriosis and ovarian cysts. 50% of patients with stage I OvCa, where the disease is limited to the ovaries, have a normal preoperative CA125 level [2-4].
Another marker, namely HE4, has similar limitations in detecting early and asymptomatic cancers [5-6] and, as our team in concordance with others have reported previously, it has no added diagnostic value over ultrasound examination [7]. Therefore, two algorithms, such as Risk of Malignancy Index (RMI) and Risk of Ovarian Malignancy Algorithm (ROMA), were used to enhance the essential character of these biomarkers. The RMI was proposed by Jacobs et al. [8]. It is a validated clinical algorithm used for the risk stratification of OvCa lesions. In comparison to CA-125, RMI combines three features: serum CA-125, menopausal status, and ultrasound score. This enables to obtain higher sensitivity and specificity in detecting malignant cases than using HE4 or CA-125 alone [9]. Another clinical tool is ROMA, which was developed by combining CA-125 and HE4 serum levels with patients’ menopausal status. Based on the ROMA score, a woman can be classed according to their risk of malignancy level, i.e. low or high.
Over recent decades, researchers have been evaluating additional serum proteins as potential biomarker candidates, which could support the early diagnosis of OvCa. The panels of biomarkers seem to be more accurate and provide the elevated sensitivity and specificity necessary for screening [10].
Inflammation is one of the hallmarks of many cancers, including ovarian cancer. Identification of specific serum markers related to this process could be a useful tool for screening patients with increased risk of malignant disease [11]. The local inflammatory response could be a double edge sword. On the one hand, cytokines can inflect an anti-tumoral response but on the other hand, chronic inflammation could also lead to malignancy. This phenomenon is related to the balance of pro-and anti-inflammatory cytokines and the activation state of the surrounding cells [12]. In this study, we aimed to identify serum concentration of the most common cytokines, which could be involved in malignant processes, and pinpoint the potential involvement in ovarian cancer pathobiology. Specifically, this study aimed to evaluate the screening panel of the inflammatory cytokines.
We hypothesized that the inclusion of inflammatory or immunosuppressive biomarkers present in serum may enhance or complement the identification of factors supporting ovarian cancer progression and early diagnosis aside from the current diagnostic methods including CA-125, HE4, and ROMA, and RMI. Moreover, the research on the milieu of cytokines and chemokines in patients with ovarian tumors can support the insight into pathomechanisms of such a complication as hypercoagulability.
Material and Methods
Study Group
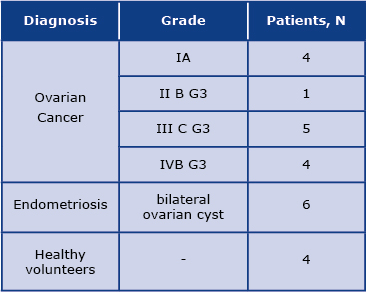
The study involved the analysis of serum obtained from 24 subjects: 14 women diagnosed with distinct stages of ovarian cancer, 6 women with endometriosis, and 4 healthy women. Detailed patient characteristics are shown in Table 1. The serum samples were collected from the Surgical, Oncological, and Endoscopic Gynecology Department of The Greater Poland Cancer. Approximately 9 mL of blood was collected into EDTA plasma tubes (Sarstedt Ltd, Leicester UK) and then centrifuged at 1200×g at 4°C for 30 minutes to obtain serum. The serum samples were then stored at –80°C until analysis. The Bioethics Committee of the Poznań University of Medical Sciences approved the study protocol (decision no. 784/13 and 1126/16). Written informed consent was obtained from all the participants. All methods were performed following the relevant guidelines and regulations. The following exclusion criteria were defined in this study: another neoplastic disease, a history of any autoimmune condition, treatment with immunomodulatory drugs, clinical manifestation or elevated markers of inflammation.
Table 1. Study population including detailed diagnosis
Analysis
The composition of cytokines was analysed using BioPlex 200 System (Bio-Rad Laboratories, Hercules CA, USA). This System allowed for the simultaneous analysis of 37 different molecules in a single well. The assay contained dyed beads conjugated with monoclonal antibodies specific for a target protein. In this study, we used Bio-Plex Pro Human Inflammation 37-plex (Bio-Rad Laboratories, Hercules CA, USA). The 50 ul of serum of each patient was used. The concentrations (pg/ml) of the analysed molecules are measured against the standard curve. The analysis was performed according to the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 6.01 (GraphPad Software, CA, USA). For the statistical analysis, the non-parametric Kruskal-Wallis test with Dunn’s post hoc multiple comparison test was performed. The differences were considered statistically significant at p < 0.05—*; p ≤ 0.01—**; p ≤ 0.001—***; p ≤ 0.0001—****.
Results
On the first view, the concentration pattern of analysed molecules in OvCa was distinct from the healthy volunteers. We found a significant difference among 22 tested molecules. The differences were also observed between patients with endometriosis and the healthy controls. The mean concentrations of detected analytes are presented in Table 2.
Table 2. The concentrations of detected analytes [pg/mL] in the studied groups. IQR= interquartile range.
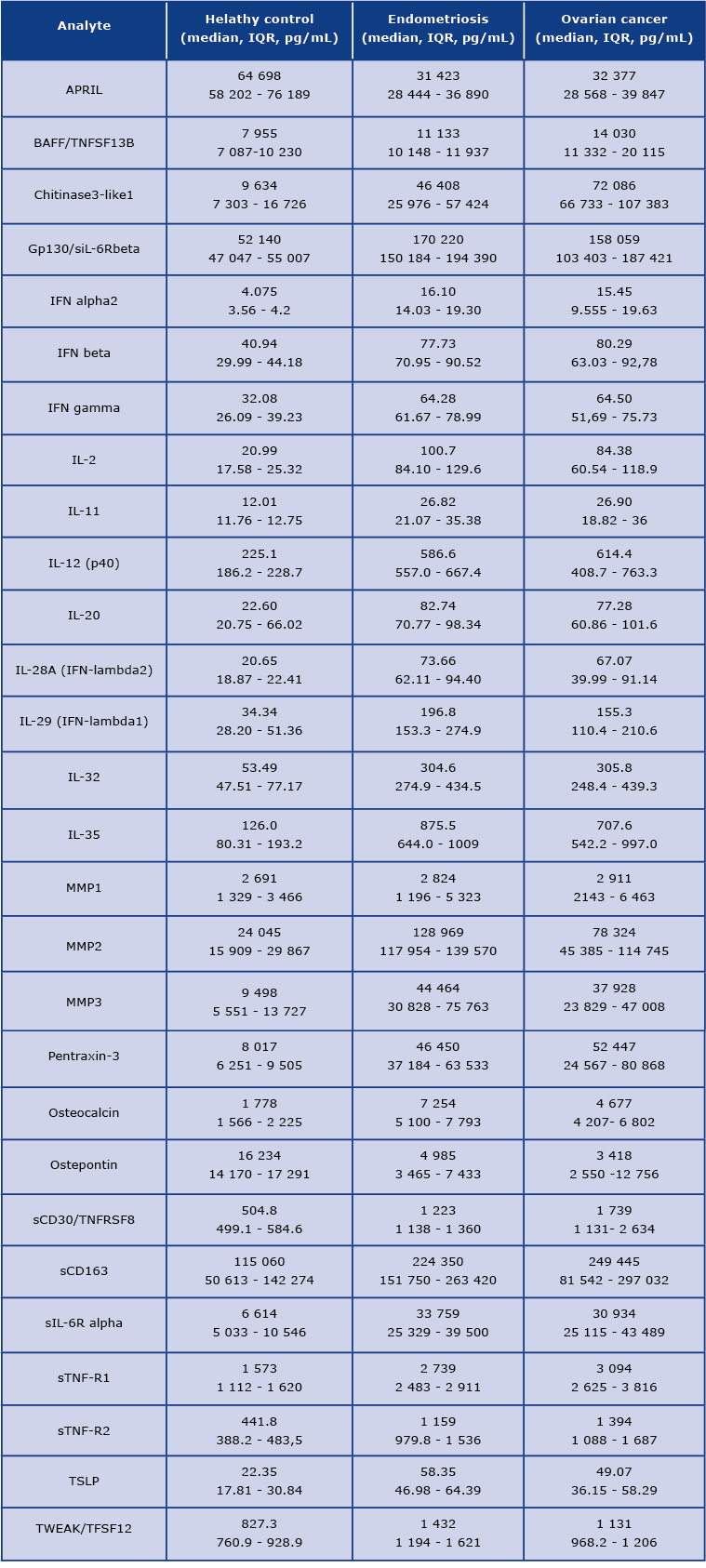
We found that the concentration of 22 analytes was significantly increased in patients with OvCa compared to the healthy controls, namely BAFF, Chitinase3-like 1, IFN-alpha2, IFN-beta, IFN-gamma, IFN-lambda2, IFN-lambda1, gp130, IL-2, IL-12 (p40), IL-11, IL-32, IL-35, MMP3, Osteocalcin, Pantraxin-3, sCD163, TNFRSF8, sIL-6Ralpha, STNF-R1, STNF-R2, and TSLP (see Figure 1 and Figure 3) In the endometriosis group, the concentration of 17 analytes was significantly higher than in the healthy controls group (see Figure 1, Figure 3, Table 2). On the other hand, both ovarian and endometriosis patients are characterized by decreased concentration of APRIL and osteopontin (see Figure 2 and Figure 3, Table 2).
The following proteins were all largely undetectable in sera of the studied groups: IL-12(p70), IL-19, IL-22, IL27, IL-34, and TNFSF14. These analytes were omitted from further analysis.
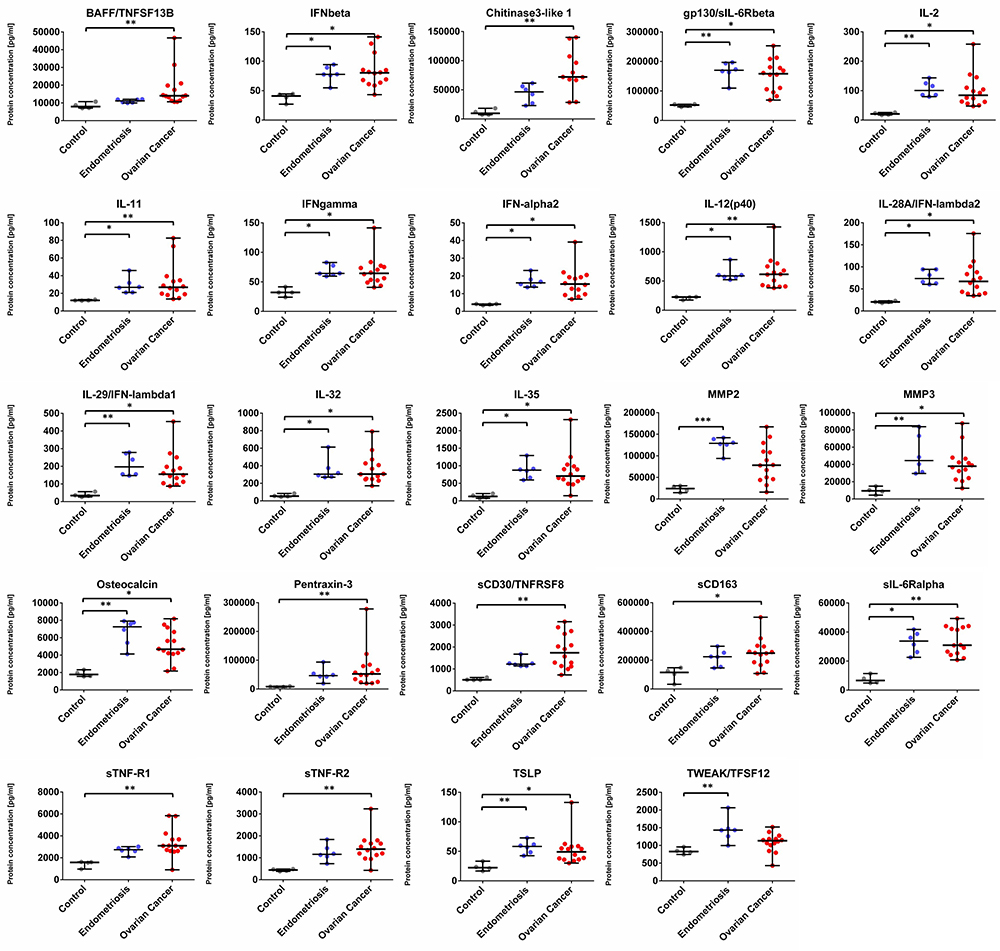
Figure 1. Dot plots (medians and interquartile range, IQR = Q1 − Q3) presenting analytes concentrations (pg/mL) within serum samples collected from patients with ovarian cancer, endometriosis, and healthy controls. * p < 0.05, ** p < 0.01, *** p < 0.001: based on Kruskal–Wallis test with Dunn’s post hoc multiple comparison test.
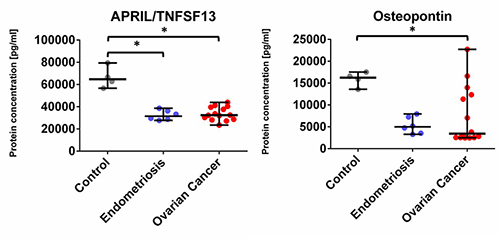
Figure 2. Dot plots (medians and interquartile range, IQR = Q1 − Q3) presenting analytes concentrations (pg/mL) within serum samples collected from patients with ovarian cancer, endometriosis, and healthy control. * p < 0.05, ** p < 0.01, *** p < 0.001: based on Kruskal–Wallis test with Dunn’s post hoc multiple comparison test.
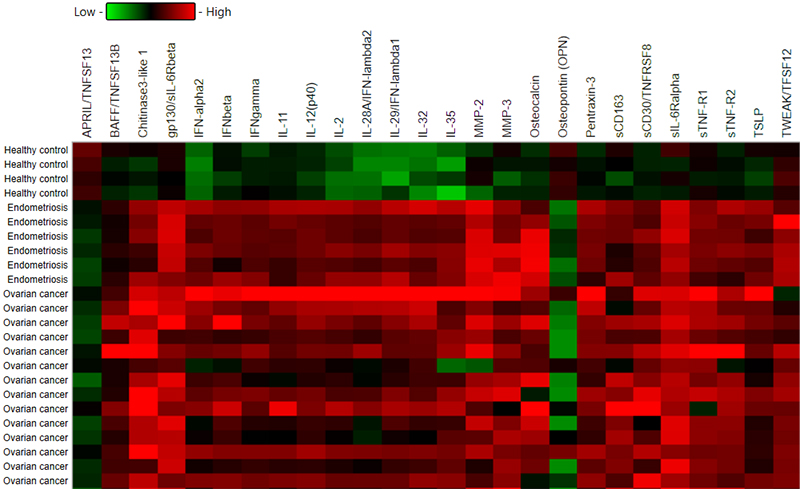
Figure 3. Heatmap representing a concentration of all analyzed molecules in patients with ovarian cancer and endometriosis group compared to healthy controls.
Discussion
OvCa is typically diagnosed at the advanced stages of the disease, therefore successful treatment challenging. The survival of patients with stages I and II of OvCa ranges from 60% to 90% shows the higher success rate of treatment depends on earlier detection of the disease. Therefore, the development and/or identification of an assay or finding an appropriate biomarker could bring significant benefits to OvCa patients. In this small, preliminary and comparative study, we conducted a multiple, quantitative analysis of 37 cytokines, chemokines, and MMPs in OvCa patients and endometriosis patients compared to the healthy volunteers. Our research confirmed that serum concentrations of analysed molecules varied between the studied groups.
Ovarian Cancer vs. Healthy Control
We divided the results into two distinct patterns of differences in the mean concentrations of specific proteins in patients with OvCa. Specifically, BAFF, Chitinase3-like 1, IFN-alpha2, IFN-beta, IFN-gamma, IFN-lambda2, IFN-lambda1, gp130, IL-2, IL-12 (p40), IL-11, IL-32, IL-35, MMP3, Osteocalcin, Pantraxin-3, sCD163, TNFRSF8, sIL-6Ralpha, STNF-R1, STNF-R2, and TSLP were all increased in OvCa patients, whereas APRIL and osteopontin concentrations were found to be decreased in OvCa as compared with the healthy controls.
We are aware that these differences in the cytokine profiles may be due to the secretion by malignant or non-malignant types of cells i.e. immune or endothelial cells, which could influence immune-mediated anti-tumor responses. Our present results are following other studies of ovarian cancers. However, to our knowledge, not all the 22 molecules have so far been analyzed and published, including single-cytokines analysis and multiplex analysis.
Consistently with our findings, significantly elevated mean concentrations of the Tumor Necrosis Factor Superfamily (TNFSF) cytokines, namely TNFSF8 (sCD30) and soluble Tumor Necrosis Factor Receptor-1 (sTNFR1) and Receptor-2 (sTNFR2), were demonstrated by Mielczarek-Palacz et al. [13- 14] and Dobrzycka et al. [15]. These proteins are responsible for the regulation of apoptosis and immune response. The above-mentioned studies suggested that these molecules could be used as early diagnostic indicators of OvCa [13-15]. Moreover, sTNFR1 and sTNFR2 are also independently related to poor prognosis [16]. Also, we found the elevated level of another member of the TNFSF family: Tumor necrosis factor-like, a weak inducer of apoptosis (TWEAK/TNFSF12). Similar data have only been published regarding prostate cancer [17] or colorectal cancer [18]. Although TWEAK was firstly identified as an inducer of apoptosis in tumor cells, it can also stimulate cell proliferation, angiogenesis, and inflammation [19-20].
Another interesting finding is the decrease of a proliferation-inducing ligand (APRIL), also known as TNFSF13, which is primarily involved in B-lymphocyte maturation, in sera OvCa patients compared to the healthy controls. According to recent studies, this cytokine is highly expressed in several tumor tissues, including breast cancer, which stimulates the growth of tumor cells [21-22]. However, we observed the opposite results, with the concentrations decreased in both, ovarian patients and endometriosis patients, compared to the healthy controls. Moreover, the retrospective studies indicated that the role of APRIL protein in ovarian, bladder, and head and neck carcinoma was not associated with the promotion of tumor development and its presence was not related to autocrine production by malignant cells, but rather derived from APRIL-producing neutrophils [23]. However, the role of neutrophils in OvCa is probably crucial in cancer progression and treatment, which was shown by the comparison of neutrophil to lymphocytes ratio (NLR), indicating the better outcome and survival of OvCa patients with decreased NLR [24]. Neutrophil extracellular traps (NETs) and their interplay with platelets contribute to paraneoplastic thrombophilia [25] thus the analysis of factors that orchestrate the dynamic interplay between tumor cells, immune system, and hemostasis seems to be crucial for monitoring and treatment decisions in OvCa patients.
The second molecule with a significantly lower level in both groups (OvCa and endometriosis vs. healthy control) was osteopontin. However, these findings are in contrast with our previous study where the concentration of osteopontin in serum was higher in OvCa patients than in benign OvCa [26], similar data showed also a team by Schorge [27]. One of the reasons for these differences might be the limited sample size in our study.
Chitinase-3-like-1 (YKL-40) is mainly responsible for tissue injury and repair,and inflammation [28]. Our results are consistent with others, which indicated the elevated level of YKL-40 in serum in patients with metastatic disease, and poor diagnosis in OvCa. High expression of YKL-40 is strongly associated with a high FIGO stage and histological type of tumor [29-30].
In our study, the concentration of two receptors of interleukin 6: siL-6R beta (Gp130) and sIL-6R alpha were also increased in ovarian patients. Both soluble receptors can bind to IL-6 and serve as the major IL-6 signalling pathways including cancer, thereby contributing to cancer progression [31-32].
The vitamin K-dependent protein, osteocalcin is a specific product of the osteoblast and is a biomarker for bone formation activity [33]. Previous studies have shown that osteocalcin is highly expressed in solid tumors, including osteosarcoma [34], breast [35], and prostate cancers [36-37]. Osteocalcin was also correlated with cancer cell transformation [38]. Our findings confirmed the elevated levels of osteocalcin in OvCa.
In terms of the metalloproteases (MMPs) family, we identified increase expression levels of two members: MMP2 and MMP3. According to previous studies, higher levels of MMP2 were significantly higher in advanced stages of OvCa compare to benign premalignant counterparts [39]. Also, it may contribute to the poor prognosis of OvCa patients [40]. Another reported role of MMP2 in OvCa is the involvement in the adhesion of OvCa cells to the peritoneal surface [41]. There are also studies showing no correlation between tumor-derived MMP2 and survival rate [42-43]. MMP3 as another member of the metalloproteases family together with miR200 can modulate OvCa invasiveness. It has been shown that overexpression of MMP3 can decrease the ability of miR200 to inhibit OvCa invasion [44]. The higher expression level of MMP3 in OvCa was also positively correlated with a poor survival rate [45-46].
In our analysis, interleukin 32 (IL-32) was overexpressed. The same findings were reported in previous studies including solid tumors, but not OvCa. As a pro-inflammatory cytokine, IL-32 is involved in the progression of different malignancies, including gastric [47], breast [48], and lung cancers [48]. IL-32 together with nuclear factor-κB (NF-κB)-mediated cytokines and metalloproteinase production support the tumor development [49-50]. In a study presented by Luo et. al. [51] on 147 patients and 337 healthy controls using the polymerase chain reaction-restriction fragment length polymorphism (RLPF), the association between IL-32 single nucleotide polymorphism (SNP) and the OvCa was found. This SNP in IL-32 gene indicates higher OvCa susceptibility and may play a crucial role as a progression marker.
Given its suppressive function, interleukin 35 (IL-35) is involved in tumor progression [52] by enhancing angiogenesis [53] and inhibition of CD8+ T cells via transforming growth factor-beta 1 (TGF-β) production [54]. This data supports our results, where we observed higher IL-35 levels in OvCa group compared to the healthy.
Interleukin-12 subunit beta (IL-12p40), which we found to be significantly overexpressed, is known as a component of the bioactive cytokines IL-12 and IL-23. No other studies have shown the presence of IL-12p40 in OvCa.
Interleukin-2 (IL-2) as a T-cell growth factor has an essential role in T cell-dependent immunity. Because of its properties, IL-2 is used for cancer therapy [55]. However, a study by Bosek et. al. [56] indicated the elevated level of IL-2 in patients with colon cancer and type 2 diabetes [56]. One of the functions of IL-2 is increased proliferation and activation of Treg Lymphocytes, which according to the study by Bosek et al. [56] could be associated with tumor progression. This may be the case in our study, as well, as we observed a significantly higher concentration of IL-2 in the OvCa group compared to the healthy controls. This was also shown in the Dutch-Wicherek group, where increased T-regulatory lymphocyte levels in peripheral blood were correlated with poorer prognosis in a serous ovarian adenocarcinoma [57].
Interferons (IFNs) are mostly described regarding viral infections. The main function of type I IFNs (IFNα, IFNβ) is the stimulation of the immune system, namely the control of dendritic cell maturation, growth of granzyme, and perforin expression in cytotoxic T-lymphocytes, which makes these cytokines essential in cancer immunosurveillance [58]. Recently, we have shown that the expression of granzyme in peripheral blood mononuclear cells (PBMC) was higher in ovarian cancer patients than in lung cancer patients [59]. Moreover, PBMCs granzyme expression was upregulated in patients with onconeural antibodies than in seronegative persons [59]. Such a phenomenon indicates the role of cytotoxicity on paraneoplastic neurological syndromes.
Type I interferons (IFNs) —IFNα and IFNβ — have been widely used for the treatment of several cancers [58]. However, IFNs are also known as inflammatory factors within the tumor microenvironment [60-61]. In our study, we observed a significant increase in both, type I and type II interferons. Interferon concentrations in OvCa and endometriosis groups are similar, which suggests that either this is a consequence of immune response to inflammation and cancer progression, or that immune response was damaged, and the high concentration could be an effect of this damage.
Endometriosis vs. Healthy Control
Aberrant production and secretion of immune mediators, including cytokines, prostaglandins, and metalloproteinases, are also observed in endometriosis patients. In our study, we analysed 6 patients with endometriosis, and we found a significant increase in 17 analytes compared to the healthy controls (Table 2 and Figure 1). Interestingly, there were three proteins (MMP2, Osteocalcin, and TWEAK), which levels were increased in the endometriosis group compared to OvCa and the healthy group, however, these differences were non-significant.
The recent studies suggest that the use of single cytokines or only traditional proinflammatory molecules could underappreciate the potential benefit of using cytokines to identify patterns of response [62]. Enzyme-linked immunosorbent assay (ELISA) is one of the popular methods in the analysis of cytokines. However, to analyse a wide range of cytokines, chemokines, and MMPs simultaneously is expensive, time-consuming, and requires a large sample volume. Therefore, in our study, we decided to use multiplex analysis of serum samples. While this study is a preliminary report, we hope this will encourage further use of multiplex analysis in ovarian cancer biomarker research.
OvCa is a complicated and heterogeneous malignancy, with multiple histological subtypes, which still determine a challenge in diagnosis. We need to acknowledge the small sample size of our study groups, which limits the ability to accurately examine the relationships between the stage of cancer and cytokine levels. Further research will require an increase in the study sample.
In conclusion, our results suggest increased levels of analytes (cytokines, chemokines, MMPs, growth factors) in serum, most spectacularly in patients with ovarian cancer, but also in patients with endometriosis compared to the healthy controls. Importantly, a similarity between the two groups of distinct pathologies emphasizes diagnostic difficulties. More studies about the use of cytokines as biomarkers are crucial for getting the essential information about changes in signalling networks which may help to understand the tumorigenesis, complications related to cancer-induced thrombophilia, and immune-mediated paraneoplastic syndromes. Although this study was not powered to provide clinical prognostic utility, the results highlight the need for further research of the patterns of cytokines in patients with ovarian cancer.
Acknowledgments
Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Poznan University of Medical Sciences (decision no. 784/13 and 1126/16).
References
| 1. |
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet [Internet]. 2014 Oct;384(9951):1376–88. Available from: https://doi.org/10.1016/S0140-6736(13)62146-7.
|
| 2. |
Jacobs I, Bast RC. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod [Internet]. 1989 Jan 1;4(1):1–12. Available from: https://doi.org/10.1093/oxfordjournals.humrep.a136832.
|
| 3. |
Kitawaki J, Ishihara H, Koshiba H, Kiyomizu M, Teramoto M, Kitaoka Y, et al. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum Reprod [Internet]. 2005 Jul 1;20(7):1999–2003. Available from: https://doi.org/10.1093/humrep/deh890.
|
| 4. |
Daoud E, Bodor G. CA-125 concentrations in malignant and nonmalignant disease. Clin Chem [Internet]. 1991 Nov 1;37(11):1968–74. Available from: https://doi.org/10.1093/clinchem/37.11.1968.
|
| 5. |
Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer [Internet]. 2011;104(5):863–70. Available from: https://doi.org/10.1038/sj.bjc.6606092.
|
| 6. |
Sarojini S, Tamir A, Lim H, Li S, Zhang S, Goy A, et al. Early Detection Biomarkers for Ovarian Cancer. Hoskins WJ, editor. J Oncol [Internet]. 2012;2012:709049. Available from: https://doi.org/10.1155/2012/709049.
|
| 7. |
Moszynski R, Szubert S, Szpurek D, Michalak S, Krygowska J, Sajdak S. Usefulness of the HE4 biomarker as a second-line test in the assessment of suspicious ovarian tumors. Arch Gynecol Obstet [Internet]. 2013;288(6):1377–83. Available from: https://doi.org/10.1007/s00404-013-2901-1.
|
| 8. |
Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG An Int J Obstet Gynaecol [Internet]. 1990 Oct 1;97(10):922–9. Available from: https://doi.org/10.1111/j.1471-0528.1990.tb02448.x.
|
| 9. |
Nossov V, Amneus M, Su F, Lang J, Janco JMT, Reddy ST, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol [Internet]. 2008;199(3):215–23. Available from: https://www.sciencedirect.com/science/article/pii/S0002937808004006.
|
| 10. |
Nice E. Biomarker discovery and validation: the tide is turning. Expert Rev Proteomics [Internet]. 2013 Dec 9;10(6):505–7. Available from: http://www.tandfonline.com/doi/full/10.1586/14789450.2013.858023.
|
| 11. |
Bast RC. Status of tumor markers in ovarian cancer screening. J Clin Oncol [Internet]. 2003;21(10 Suppl):200s-205s. Available from: http://europepmc.org/abstract/MED/12743135.
|
| 12. |
Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci [Internet]. 2011/05/21. 2011;7(5):651–8. Available from: https://pubmed.ncbi.nlm.nih.gov/21647333.
|
| 13. |
Mielczarek-Palacz A, Sikora J, Kondera-Anasz Z, Hauza G. Imbalance in serum soluble CD30/CD30L and CD40/CD40L systems are associated with ovarian tumors. Hum Immunol [Internet]. 2013;74(1):70–4. Available from: https://www.sciencedirect.com/science/article/pii/S0198885912005666.
|
| 14. |
Gorelik E. Multiplexed Immunobead-Based Cytokine Profiling for Early Detection of Ovarian Cancer. Cancer Epidemiol Biomarkers Prev [Internet]. 2005 Apr 1;14(4):981–7. Available from: http://cebp.aacrjournals.org/cgi/doi/10.1158/1055-9965.EPI-04-0404.
|
| 15. |
Dobrzycka B, Terlikowski SJ, Kowalczuk O, Kinalski M. Circulating levels of TNF-α and its soluble receptors in the plasma of patients with epithelial ovarian cancer. Eur Cytokine Netw [Internet]. 2009;20(3):131–4. Available from: https://www.researchgate.net/profile/Slawomir-Terlikowski/publication/26891134_Circulating_levels_of_TNF-a_and_its_soluble_receptors_in_the_plasma_of_patients_with_epithelial_ovarian_cancer/links/5797d1b708aed51475e6a614/Circulating-levels-of-TNF-a-and-its-soluble-receptors-in-the-plasma-of-patients-with-epithelial-ovarian-cancer.pdf.
|
| 16. |
Nomelini RS, Borges Júnior LE, de Lima CA, Chiovato AFC, Micheli DC, Tavares-Murta BM, et al. TNF-R2 in tumor microenvironment as prognostic factor in epithelial ovarian cancer. Clin Exp Med [Internet]. 2018;18(4):547–54. Available from: https://doi.org/10.1007/s10238-018-0508-3.
|
| 17. |
Sanz AB, Sanchez-Niño MD, Carrasco S, Manzarbeitia F, Ruiz-Andres O, Selgas R, et al. Inflammatory Cytokines and Survival Factors from Serum Modulate Tweak-Induced Apoptosis in PC-3 Prostate Cancer Cells. Sanchis D, editor. PLoS One [Internet]. 2012 Oct 15;7(10):e47440. Available from: https://dx.plos.org/10.1371/journal.pone.0047440.
|
| 18. |
Nakamura S, Sho M, Nomi T, Koyama F, Mukogawa T, Akahori T, et al. Significance of TWEAK/Fn-14 pathway in human colorectal cancer. Cancer Res [Internet]. 2007 May 1;67(9 Supplement):2395 LP – 2395. Available from: http://cancerres.aacrjournals.org/content/67/9_Supplement/2395.abstract.
|
| 19. |
Wang D, Fung JNT, Tuo Y, Hu L, Chen C. TWEAK/Fn14 promotes apoptosis of human endometrial cancer cells via caspase pathway. Cancer Lett [Internet]. 2010;294(1):91–100. Available from: https://www.sciencedirect.com/science/article/pii/S0304383510000534.
|
| 20. |
Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer J-L, et al. APRIL, a New Ligand of the Tumor Necrosis Factor Family, Stimulates Tumor Cell Growth . J Exp Med [Internet]. 1998 Sep 21;188(6):1185–90. Available from: https://doi.org/10.1084/jem.188.6.1185.
|
| 21. |
Ware CF. April and Baff Connect Autoimmunity and Cancer. J Exp Med [Internet]. 2000 Dec 4;192(11):F35–8. Available from: https://doi.org/10.1084/jem.192.11.F35.
|
| 22. |
Mhawech-Fauceglia P, Allal A, Odunsi K, Andrews C, Herrmann FR, Huard B. Role of the tumour necrosis family ligand APRIL in solid tumour development: Retrospective studies in bladder, ovarian and head and neck carcinomas. Eur J Cancer [Internet]. 2008;44(15):2097–100. Available from: https://www.sciencedirect.com/science/article/pii/S0959804908005418.
|
| 23. |
Chen G, Zhu L, Yang Y, Long Y, Li X, Wang Y. Prognostic Role of Neutrophil to Lymphocyte Ratio in Ovarian Cancer: A Meta-Analysis. Technol Cancer Res Treat [Internet]. 2018 Jan 1;17:1533033818791500. Available from: https://doi.org/10.1177/1533033818791500.
|
| 24. |
Pfrepper C. Paraneoplastic Thromboembolism and Thrombophilia: Significance in Visceral Medicine. Visc Med [Internet]. 2020;36(4):280–7. Available from: https://www.karger.com/DOI/10.1159/000509150.
|
| 25. |
Moszynski R, Szubert S, Szpurek D, Michalak S, Sajdak S. Role of osteopontin in differential diagnosis of ovarian tumors. J Obstet Gynaecol Res [Internet]. 2013 Nov 1;39(11):1518–25. Available from: https://doi.org/10.1111/jog.12097.
|
| 26. |
Schorge JO, Drake RD, Lee H, Skates SJ, Rajanbabu R, Miller DS, et al. Osteopontin as an Adjunct to CA125 in Detecting Recurrent Ovarian Cancer. Clin Cancer Res [Internet]. 2004 May 15;10(10):3474 LP – 3478. Available from: http://clincancerres.aacrjournals.org/content/10/10/3474.abstract.
|
| 27. |
Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther [Internet]. 2020;5(1):201. Available from: https://doi.org/10.1038/s41392-020-00303-7.
|
| 28. |
Dupont J, Tanwar MK, Thaler HT, Fleisher M, Kauff N, Hensley ML, et al. Early Detection and Prognosis of Ovarian Cancer Using Serum YKL-40. J Clin Oncol [Internet]. 2004 Aug 15;22(16):3330–9. Available from: http://ascopubs.org/doi/10.1200/JCO.2004.09.112.
|
| 29. |
Høgdall EVS, Ringsholt M, Høgdall CK, Christensen IJ, Johansen JS, Kjaer SK, et al. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer [Internet]. 2009;9(1):8. Available from: https://doi.org/10.1186/1471-2407-9-8.
|
| 30. |
Lo C-W, Chen M-W, Hsiao M, Wang S, Chen C-A, Hsiao S-M, et al. IL-6 Trans-Signaling in Formation and Progression of Malignant Ascites in Ovarian Cancer. Cancer Res [Internet]. 2011 Jan 15;71(2):424 LP – 434. Available from: http://cancerres.aacrjournals.org/content/71/2/424.abstract.
|
| 31. |
Rath KS, Funk HM, Bowling MC, Richards WE, Drew AF. Expression of soluble interleukin-6 receptor in malignant ovarian tissue. Am J Obstet Gynecol [Internet]. 2010;203(3):230.e1-230.e8. Available from: https://www.sciencedirect.com/science/article/pii/S0002937810003534.
|
| 32. |
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord [Internet]. 2015 Jun 11;16(2):93–8. Available from: https://doi.org/10.1007/s11914-015-0267-y.
|
| 33. |
Agustina H, Asyifa I, Aziz A, Hernowo BS. The Role of Osteocalcin and Alkaline Phosphatase Immunohistochemistry in Osteosarcoma Diagnosis. Tosi P, editor. Patholog Res Int [Internet]. 2018;2018:6346409. Available from: https://doi.org/10.1155/2018/6346409.
|
| 34. |
Pietschmann P, Zielinski C, Woloszczuk W. Serum osteocalcin levels in breast cancer patients. J Cancer Res Clin Oncol [Internet]. 1989;115(5):456–8. Available from: https://doi.org/10.1007/BF00393337.
|
| 35. |
Francini G, Bigazzi S, Leone V, Gennari C. Serum osteocalcin concentration in patients with prostatic cancer. Am J Clin Oncol [Internet]. 1988;11 Suppl 2:S83-7. Available from: http://europepmc.org/abstract/MED/3266542.
|
| 36. |
Koeneman KS, Kao C, Ko S-C, Yang L, Wada Y, Kallmes DF, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol [Internet]. 2000;18(2):102–10. Available from: https://doi.org/10.1007/s003450050181.
|
| 37. |
Sheu B-C, Lien H-C, Ho H-N, Lin H-H, Chow S-N, Huang S-C, et al. Increased Expression and Activation of Gelatinolytic Matrix Metalloproteinases Is Associated with the Progression and Recurrence of Human Cervical Cancer. Cancer Res [Internet]. 2003 Oct 1;63(19):6537 LP – 6542. Available from: http://cancerres.aacrjournals.org/content/63/19/6537.abstract.
|
| 38. |
Sakata K, Shigemasa K, Nagai N, Ohama K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol [Internet]. 2000 Oct 1; Available from: http://www.spandidos-publications.com/10.3892/ijo.17.4.673.
|
| 39. |
Westerlund A, Apaja-Sarkkinen M, Höyhtyä M, Puistola U, Turpeenniemi-Hujanen T. Gelatinase A-Immunoreactive Protein in Ovarian Lesions— Prognostic Value in Epithelial Ovarian Cancer. Gynecol Oncol [Internet]. 1999;75(1):91–8. Available from: https://www.sciencedirect.com/science/article/pii/S0090825899955336.
|
| 40. |
Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest [Internet]. 2008 Apr 1;118(4):1367–79. Available from: https://doi.org/10.1172/JCI33775.
|
| 41. |
Brun J-L, Cortez A, Lesieur B, Uzan S, Rouzier R, Daraï E. Expression of MMP-2, −7, −9, MT1-MMP and TIMP-1 and −2 has no prognostic relevance in patients with advanced epithelial ovarian cancer. Oncol Rep [Internet]. 2012 Apr;27(4):1049–57. Available from: https://www.spandidos-publications.com/10.3892/or.2011.1608.
|
| 42. |
Périgny M, Bairati I, Harvey I, Beauchemin M, Harel F, Plante M, et al. Role of Immunohistochemical Overexpression of Matrix Metalloproteinases MMP-2 and MMP-11 in the Prognosis of Death by Ovarian Cancer. Am J Clin Pathol [Internet]. 2008 Feb 1;129(2):226–31. Available from: https://doi.org/10.1309/49LA9XCBGWJ8F2KM.
|
| 43. |
Sun N, Zhang Q, Xu C, Zhao Q, Ma Y, Lu X, et al. Molecular regulation of ovarian cancer cell invasion. Tumour Biol [Internet]. 2014 Nov;35(11):11359–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25119590.
|
| 44. |
Wu Y-H, Chang T-H, Huang Y-F, Huang H-D, Chou C-Y. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene [Internet]. 2014;33(26):3432–40. Available from: https://doi.org/10.1038/onc.2013.307.
|
| 45. |
Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Machaliński B, Menkiszak J, Sompolska-Rzechuła A. Suitability assessment of baseline concentration of MMP3, TIMP3, HE4 and CA125 in the serum of patients with ovarian cancer. J Ovarian Res [Internet]. 2018;11(1):1. Available from: https://doi.org/10.1186/s13048-017-0373-9.
|
| 46. |
Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 Expression in the Pancreas. J Biol Chem [Internet]. 2009 Jun;284(26):17868–76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021925819820690.
|
| 47. |
Wang S, Chen F, Tang L. IL‑32 promotes breast cancer cell growth and invasiveness. Oncol Lett [Internet]. 2015;9(1):305–7. Available from: https://doi.org/10.3892/ol.2014.2641.
|
| 48. |
Han S, Yang Y. Interleukin-32: Frenemy in cancer? BMB Rep [Internet]. 2019 Mar;52(3):165–74. Available from: https://pubmed.ncbi.nlm.nih.gov/30638183.
|
| 49. |
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang L, et al. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine [Internet]. 2014;65(1):24–32. Available from: https://www.sciencedirect.com/science/article/pii/S1043466613007084.
|
| 50. |
Luo X, Bai P, Li Q, Zhang Y, Song Y, Su M, et al. Association between interleukin-32 polymorphisms and ovarian cancer in the Chinese Han population. Int J Clin Exp Pathol [Internet]. 2020 Jul 1;13(7):1733–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32782697.
|
| 51. |
Sawant D V, Hamilton K, Vignali DAA. Interleukin-35: Expanding Its Job Profile. J Interf Cytokine Res [Internet]. 2015 Apr 28;35(7):499–512. Available from: https://doi.org/10.1089/jir.2015.0015.
|
| 52. |
Wang Z, Liu J-Q, Liu Z, Shen R, Zhang G, Xu J, et al. Tumor-Derived IL-35 Promotes Tumor Growth by Enhancing Myeloid Cell Accumulation and Angiogenesis. J Immunol [Internet]. 2013 Mar 1;190(5):2415 LP – 2423. Available from: http://www.jimmunol.org/content/190/5/2415.abstract.
|
| 53. |
Friedman A, Liao K-L. The role of the cytokines IL-27 and IL-35 in cancer. Math Biosci Eng [Internet]. 2015 Aug;12(6):1203–17. Available from: http://aimsciences.org//article/id/4a0b0f03-e6e6-4b45-a9b1-684648dc41f9.
|
| 54. |
Parkinson DR. Interleukin-2 in cancer therapy. Semin Oncol [Internet]. 1988;15(6 Suppl 6):10–26. Available from: http://europepmc.org/abstract/MED/3061014.
|
| 55. |
Bosek I, Kuczerowski R, Miłek T, Rabijewski M, Kaleta B, Kniotek M, et al. The levels of interleukin-2 and interleukin-10 in patients with type 2 diabetes and colon cancer. / Stężenie interleukiny 2 i interleukiny 10 u pacjentów z cukrzycą typu 2 i rakiem okrężnicy [in Polish]. Diabetol Prakt [Internet]. 2018;4(2):121–9. Available from: https://journals.viamedica.pl/diabetologia_praktyczna/article/view/58009.
|
| 56. |
Dutsch-Wicherek M, Szubert S, Dziobek K, Wisniewski M, Lukaszewska E, Wicherek L, et al. Analysis of the treg cell population in the peripheral blood of ovarian cancer patients in relation to the long-term outcomes. Ginekol Pol [Internet]. 2019;90(4):179–84. Available from: https://doi.org/10.5603/GP.2019.0032.
|
| 57. |
Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol [Internet]. 2015;15(7):405–14. Available from: https://doi.org/10.1038/nri3845.
|
| 58. |
Zaborowski MP, Stefens-Stawna P, Osztynowicz K, Piorunek T, Batura-Gabryel H, Dyzmann-Sroka A, et al. Granzyme B in peripheral blood mononuclear cells as a measure of cell-mediated immune response in paraneoplastic neurological syndromes and malignancy. Cancer Immunol Immunother [Internet]. 2021;70(5):1277–89. Available from: https://doi.org/10.1007/s00262-020-02750-1.
|
| 59. |
Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol [Internet]. 2006;6(11):836–48. Available from: https://doi.org/10.1038/nri1961.
|
| 60. |
Snell LM, McGaha TL, Brooks DG. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol [Internet]. 2017;38(8):542–57. Available from: https://www.sciencedirect.com/science/article/pii/S1471490617300935.
|
| 61. |
Lyon DE, McCain NL, Walter J, Schubert C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs Res [Internet]. 2008;57(1):51–8. Available from: https://pubmed.ncbi.nlm.nih.gov/18091292.
|











