Abstract
The increasing prevalence of diabetic kidney disease (DKD), a common complication of type 1 and type 2 diabetes, is becoming a leading risk factor of developing end stage renal disease (ESRD). The multiple mechanisms involved in renal tissue damage are a challenge for effective targeted therapy. Urolithins are metabolites generated by gut microbiota upon dietary intake of plant-derived ellagitannins. Multidirectional effects of these compounds include their anti-inflammatory, antioxidant, anti-proliferatory, anti-migratory and antiglycative properties that are mediated by modulation of signaling pathways and gene expression. Biochemical properties of urolithins indicate their capacity to regulate numerous mechanisms responsible for developing the hyperglycemia-induced tissue injury. The potentially beneficial effects of urolithins on podocytes, the most vulnerable renal cells should be particularly considered. The purpose of this review is to provide the evidence from the in vivo and in vitro studies showing that urolithin-based therapy could be a useful tool for protecting the kidneys from damage in diabetes.
Citation
Kotewicz M, Lewko B. Urolithins and their possible implications for diabetic kidney. Eur J Transl Clin Med. 2022;5(1):53-63Introduction
Urolithins (hydroxylated dibenzo [b,d]-pyran-6-one derivatives) are a family of bioactive compounds which were first isolated from beaver scent glands in 1949 [1]. Their presence in human and animal intestines is a result of bacterial metabolism in gastrointestinal tract of dietary ellagitannins (ETs) and their constituent, ellagic acid (EA) [2]. ETs and EA are naturally occurring polyphenols found in numerous fruits and vegetables, e.g. pomegranates, raspberries and nuts [3]. Their beneficial health properties including anti-inflammatory, antioxidant and antiproliferative effects have been proven in both animal and human models. Various studies demonstrated the protective effects of ETs and EA against chronic diseases of the cardiovascular system, neurodegenerative diseases, diabetes and cancer. However, ETs and EA have poor bioavailability and their biological activity is associated with urolithins that are more easily absorbed in gut [4-8]. The synthesis of urolithins depends on the composition in the gut of specific bacteria that varies between the individuals. The identification of the microorganisms responsible for the complete transformation of EA into the final urolithins is still under research. According to the recent findings, different numbers of Synergistetes phylum and members of Coriobacteriaceae (genus Gordonibacter urolithinfaciens and Gordonibacter pamelaeae) and Lachnospiraceae families can be used to discriminate between individuals producing certain urolithin forms [9- 10]. Thus, after absorption and passage through the liver, different urolithins can be found in human body fluids and tissues at nanomolar to micromolar concentrations. Absorbed urolithins undergo phase I and phase II metabolism, resulting in glucuronide, sulfate and methylated derivatives, while small amounts can be found in the form of free aglycones [2, 11-12].
Both the conjugated and unconjugated forms of urolithins can be detected in human plasma and urine even 48 hours after consumption of ET-rich food [6, 13-14]. It is clear therefore that bioactive urolithins directly contact renal tissue during the passage through the nephron. It seems plausible that the cells constituting the glomerular filter, as well as epithelial cells lining the urinary tract may be affected by these compounds. Antioxidant, anti-inflammatory and antimicrobial properties of these compounds could be beneficial in treating several renal diseases, including diabetic kidney disease (DKD) [15-16]. Nevertheless, so far there are not many data on the effects of urolithins on the renal tissue. The purpose of this review is to provide the evidence from the in vivo and in vitro studies showing that urolithin-based therapy could be a useful tool for protecting the kidneys from damage in diabetes.
Material and methods
We searched the Medline, Scopus and Science Direct databases for articles published from 2011 to April 2021 using the following keywords: kidney disease, urolithins, ellagic acid, elagitannins, podocytes, polyphenols, diabetes, nephropathy, transforming growth factor and relevant abbreviations (e.g. TGFβ, CKD, DN). Older articles describe pathomechanisms and serve as context for the presented new information. The inclusion criteria were: full-text article, on-topic. Case reports and letters to the Editor were excluded from the review.
Results
Bioavailability and metabolism
Similarly to other phytochemicals, ETs are poorly absorbed in the gut. However, following consumption the ETs undergo spontaneous hydrolysis into EA in the upper gastrointestinal tract. Microbes residing in the intestine further transform EA yielding a series of bioactive compounds including urolithins that are characterized by a dibenzopyranone structure and a decreasing number of phenolic hydroxyl groups (Figure 1) [4, 17-18]. Due to their high lipophilicity, urolithins are much more readily absorbed than the original polyphenols.

Figure 1. Dietary elagitannins and ellagic acid are converted by the gut microbiota to urolithins that are readily absorbed to the bloodstream. Most of circulating urolithins undergo II phase metabolism in liver and the conjugates as well as free aglycones reach various peripheral tissues. Urolithins from filtered plasma pass the nephron to be excreted in urine. Some urolithin metabolites are also excreted in faeces [18, 75]
After absorption in the gut, urolithins rapidly undergo metabolism by phase II enzymes in enterocytes [8, 19]. Once in the bloodstream, conjugated and unconjugated metabolites reach liver via portal circulation and are further subjected to phase II metabolism in hepatocytes. Due to enterohepatic recirculation, part of urolithin conjugates can be secreted with bile back to the small intestine [5, 20]. Analyses of human and animal blood and urine samples indicate that urolithins A and B and their glucuronide and sulfate conjugates are predominant urolithin isoforms. In particular, Uro A is considered to be a major metabolite in humans. However, depending on the study design, the measured final plasma concentrations vary substantially reaching 0.1–35 µM [4,8,15]. Human and animal studies have shown that urolithin metabolites accumulate in the gall bladder and urinary bladder [21], prostate gland [22-23], colon and intestinal tissues [22], whereas no accumulation in other tissues (e.g. muscles, adipose tissue, heart, liver, kidney) was observed [21].
Individual variability in the composition of gut microbiome results in significant differences in ET metabolism and urolithin type production, which is defined as different metabotypes [8]. Specific gut microbiota profile or illnesses generating dysbacteriosis may contribute to different bacteria composition and levels, consequently leading to various potential health effects [24-26]. Therefore, it is essential to consider the influence of factors such as age, gender, race, health condition and geographic origins on the polyphenol profile after consumption of ETs-rich products. Based on the amount and type of urolithins excreted in the urine from healthy volunteers, three different urolithin metabotypes have been described: metabotype A (only Uro A metabolites excreted), metabotype B (Uro B and/ or Uro B metabolites excreted in addition to Uro A and isourolithin A) and urolithin metabotype 0 (no urolithins/ urolithin metabolites excreted) [13]. Significant interindividual variability was also reported in the first pharmacokinetic study, that showed for the first time that EA bioavailability was not increased after intake of a high free EA dose. It was concluded that factors such as pH and food protein content have a strong impact on EA bioavailability [19].
Biological activity
The direct biological effects of urolithins have been examined in different cell models, such as various cancer cell lines, fibroblasts, immune, endothelial and epithelial cells [4, 27-28].
The studies on the chemopreventive potential of EA and its metabolites Uro A and Uro B revealed that their anti-tumor properties, including the influence on cancer cell apoptosis and proliferation involve alterations in the expression of genes involved in signaling (MAPK) pathways, oncogenes (K-Ras, c-Myc), suppressors (DASP6, Fos), p53 protein, growth factor receptors (FGFR2, EGFR) and multiple genes involved in cell cycle [11, 29-33]. EA, as well as urolithins (and principally Uro A) exhibit proapoptotic activity via caspase-dependent pathways, in which activation of caspases 3, 8 and 9 has been reported [34-35]. Inhibition of cancer growth is mediated by suppression by Uro A and Uro B of Wnt/ β-catenin signaling [36-37].
Table 1. Summarizes diverse biological effects exerted by urolithins
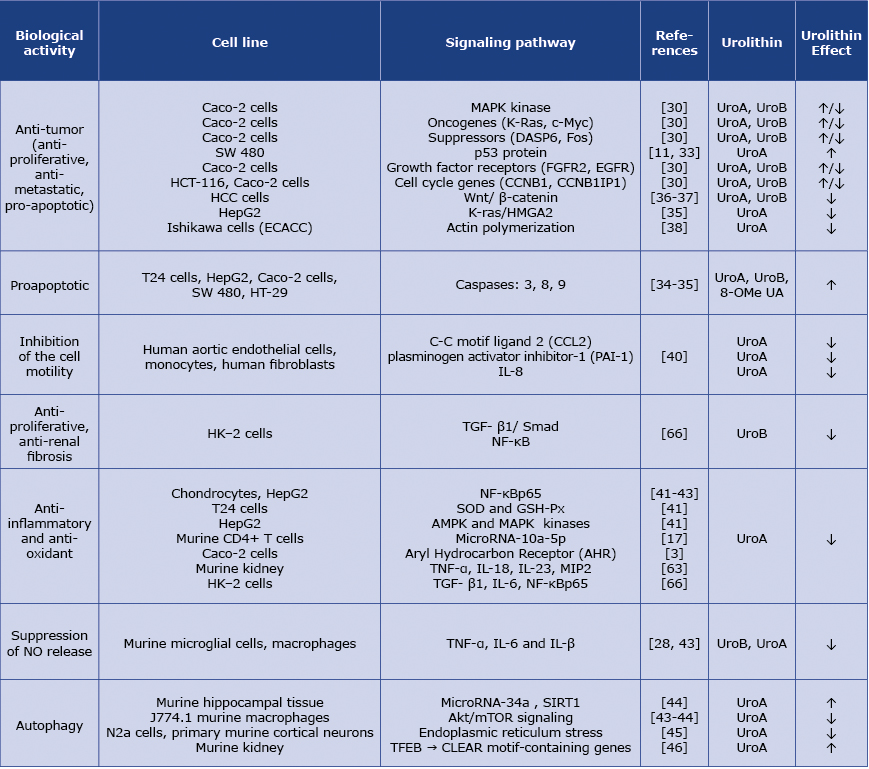
Moreover, anti-tumor and anti-metastatic effects of urolithins include inhibition of migration of cancer cells by diverse mechanisms such as suppressing the K-ras/HMGA2 expression or by decreasing actin polymerization [38-39]. Suppression by urolithins of cell motility accounts also for the mechanisms of their anti-inflammatory activity. By moderate down-regulation of chemokine C-C motif ligand 2 (CCL2), plasminogen activator inhibitor-1 (PAI-1) and decreased expression of IL-8, Uro-A and Uro-A glucuronide inhibited induced by TNFα monocyte adhesion and human fibroblast and aortic endothelial cell migration [40]. The anti-inflammatory and antioxidant activities of Uro A and Uro B involve inhibition of the nuclear factor kappa-B (NF-κB) pathway and modulation of phosphorylation of diverse kinases such as AMPK and MAPK pathway members [41]. In the IL‒β1 stimulated rat chondrocytes and in LPS-stimulated macrophages, Uro A pre-treatment inhibited NF-κBp65 translocation into the nucleus [42-43], while in the HepG2 hepatic carcinomas cell line, Uro A decreased p65 expression and increased the activity of intracellular antioxidant enzymes SOD and GSH-Px [41]. Suppression of NO production, decrease of proinflammatory molecules such as TNF-α, IL-6 and IL-β at the mRNA and protein levels was observed in the presence of Uro B in the LPS-activated mouse microglial cells and in J774 murine macrophages stimulated with LPS [28,43]. Another study demonstrated that Uro A, by regulating miR-10a-5p, reduced the proliferation of murine CD4+ T cells, that are a trigger to immune response [17]. Furthermore, Uro A exhibited anti-inflammatory effects by directly binding the aryl hydrocarbon receptor (AHR). Acting as a selective AHR antagonist, Uro A inhibited its transcriptional activity which resulted in attenuating cytokine-induced inflammatory signaling in Caco-2 cells [3].
It has been demonstrated that Uro-A exhibits protective properties against brain aging [44] and against ischemic neuronal and ischemia reperfusion renal injury (IRI) [45-46]. These anti-inflammatory and cytoprotective effects include induction by Uro A of autophagic flux, which was confirmed by observed expression of the autophagic markers LC3-II and p62 [43-45]. Some recent studies show that Uro A activates autophagy by upregulating Sirtuin 1 (SIRT1) signaling [44] and by impairing Akt/mTOR signaling [43-44]. Within the kidney, Uro A attenuated IRI by inducing autophagy through activation of transcription factor EB (TFEB) followed by regulation of target genes of Coordinated Lysosomal Expression and Regulation (CLEAR) network [46].
Safety of urolithin administration
Considering that Uro A is the most representative urolithin form and potential therapeutic agent, safety profile of this compound was evaluated in several studies. A comprehensive study by Heilman et al [47] indicated that in both 28-day and 90-day observations in rats, orally administered synthetic Uro A did not modify any clinical and blood parameters, did not disrupt homeostasis and did not indicate any specific toxic mechanisms. The 4-week clinical trial in which up to 2000 mg oral Uro A doses were administered to elderly volunteers confirmed that the treatment had no adverse health effects. The observed 31 unfavorable effects were determined to be unrelated to the compound tested [48]. On the basis of the above findings, the US Food and Drug Administration already issued a favorable review for using Uro A as a food ingredient [49].
Urolithins in diabetes
Pathophysiology of diabetes is strictly linked to metabolic changes and chronic inflammation. Overproduction of multiple pro-inflammatory cytokines, growth factors and reactive oxygen species (ROS) account for the diabetes- related damage of tissues and organs [50-51]. Thus, antioxidant and anti-inflammatory properties of urolithins may exert a protective role in diabetic state [52]. It was reported recently that while the in vivo occurring extensive conjugation severly hampers the activity of urolithins, systemic inflammation triggers tissue deconjugation of Uro A glucuronide, yielding free aglycone with remarkably higher biological activity [53]. Indeed, the in vivo, as well as the in vitro studies confirmed the beneficial effects of unconjugated Uro A and Uro B administration to diabetic rats. Urolithin injections prevented the early cardiac inflammatory response as well as the occurrence of cardiac dysfunction in the streptozocin-induced (STZ) type 1 diabetes rats [54]. In cardiomyocytes cultured in the presence of 25mM glucose, Uro B significantly reduced the glucose-induced high levels of monocyte chemoattractant protein-1 (MCP-1), the pro-inflammatory cytokine fractalkine and vascular endothelial growth factor (VEGF). In fibroblasts exposed to high glucose, expression of fractalkine was reduced by Uro A, Uro B, Uro C and Uro D [55]. In diabetes, hyperglycemia is also a causative factor for neurodegeneration and development of Alzheimer’s disease. Uro A injections in a STZ- induced diabetic mouse prevented mitochondrial ROS accumulation, amyloidogenesis and neuronal cell death, which indicates that Uro A-based therapy may be useful in prevention and treatment of diabetes-associated neuronal impairment [56]. Viability of neuronal cells exposed to oxidative stress was significantly increased in the presence of Uro A and Uro B [57]. In addition, via facilitating L‐type Ca2+ channel opening, Uro A and Uro C have been shown to enhance insulin secretion in cultured INS-1 beta-cells and isolated rat islets of Langerhans [58].
One of the hallmarks of diabetes mellitus (DM) is spontaneous non-enzymatic glycation of proteins, lipids and nucleic acids. Dependent on the level and duration of hyperglycemia this process leads to formation of advanced glycation end products (AGEs) that permanently modify protein structures and functions, contributing to oxidative stress and development of chronic diabetic complications [57, 59-60]. Anti glycative properties of Uro A and Uro B were shown in several in vitro experiments in which glycation of the bovine serum albumin (BSA) was strongly hampered by these compounds. The effects were concentration-dependent and the mechanisms included urolithins scavenging for reactive carbonyl species [57, 61].
Urolithins in diabetic and non-diabetic kidney disease
Although urolithins do not accumulate in the kidney, the circulating urolithin-rich plasma has continuous contact with renal structures [2, 6, 14], possibly affecting the renal tissue. Indeed, in the experimental rat model based on cisplatin-induced nephrotoxity, Guada et al. revealed that Uro A effectively attenuated kidney damage by inhibiting the inflammatory cascade and apoptosis pathway. Furthermore, anti-inflammatory cytokine IL-10 was markedly increased in the kidneys of Uro A-treated animals [62]. Similarly, in the cisplatin-induced acute kidney injury (AKI) mouse model, orally given nanoparticle-encapsulated Uro A not only reduced mortality but also protected the kidneys from oxidative stress and cytotoxic injury including necrosis, tubular atrophy and glomerular hypertrophy [16]. Also, in another experiment, Uro A pretreatment of mice receiving cisplatin for 3 days not only improved renal parameters but also attenuated oxidative and nitrative stress and downregulated the expression of pro-inflammatory cytokines and chemokines TNFα, IL-23, IL-18 and MIP2 [63] Beneficial properties of Uro A were also documented in the kidney ischemia reperfusion injury (IRI) in mice. Attenuation of renal injury was associated with Uro A – dependent reduction of pro-inflammatory cytokines TNFα, IL1β, MIP1α and promotion of autophagy [46]. Subcutaneously administered Uro A also increased activity of antioxidant enzymes and attenuated expression of pro-inflammatory cytokines in the kidneys of aging mice [64].
Modulation of the renal TGFβ system
The transforming growth factor beta (TGFβ) family of multipotential cytokines controls numerous physiological and pathological events such as embryogenesis, carcinogenesis and the immune response [15, 65]. Regulation of cell proliferation, differentiation, migration and apoptosis involves ability of TGFβ to affect the transcription and translation processes. The hyperglycemia and inflammation associated with diabetes strongly activate the TGFβ system, resulting in undesirable changes within tissues. In diabetic kidney disease (DKD), overactive TGFβ plays a prominent role in promoting renal cell hypertrophy, fibrosis and stimulating extracellular matrix (ECM) accumulation. So far, urolithins have been shown to counteract the TGFβ-dependent effects in unilateral ureteral obstruction (UUO) rats and in cultured renal epithelial cells. In the UUO model, Uro B treatment abolished renal damage and fibrosis, maintained tubular and glomerular structure and reduced inflammatory cell infiltration. Moreover, expression levels of TGFβ1, NF-κB p65, angiotensin II, collagen IV and several pro-inflammatory factors was significantly reduced. In cultured proximal tubular HK-2 cells, Uro B inhibited stimulated by TGFβ cell proliferation and restored cell morphology. It was demonstrated that in the in vivo, as well as in the in vitro experiments, protective effects of Uro B were related to the down-regulation of TGF-β1/Smad, most likely via inhibition of the NF-κB signaling [66]. Overproduction of the plasminogen activator inhibitor (PAI-1) results in accumulation of ECM in acute and chronic kidney diseases, including diabetic nephropathy. In stimulated by TGFβ renal epithelial NRK‐52e cell line significant increase of PAI-1 release was inhibited by Uro A in a dose-dependent manner [67]. The positive feedback loop between TGFβ and PAI-1 has also been documented in diabetic kidney [68]. Hence, targeting the TGFβ- and PAI-1-related pathways by urolithins might be an effective aproach in treating renal fibrosis and inflammation, particularly in the DKD.
Potential effects of urolithins on podocytes Podocytes are terminally-differentiated, highly specialized cells of epithelial origin covering the outer aspect of glomerular capillaries. Due to their inability to replenish in mature kidney, podocyte loss is believed to initiate irreversible impairment of the glomerular filter [69-70]. Clinical and experimental data suggest a key role of podocytes in the development of diabetic nephropathy (DN) [71]. Podocyte depletion is considered to be the first indicator of glomerular destruction in diabetic patients, even before the appearance of proteinuria [72]. For preventing detachment, podocytes rearrange their structure and migrate to seek attachment in other sites of glomerular basement membrane However, increased migration may disrupt the slit diaphragms between neighboring cells and the tightness of glomerular filter resulting in proteinuria [73]. Recently performed in our laboratory experiments showed that Uro A effectively inhibited induced by high (30 mM) glucose motility of mouse podocytes (unpublished data), which could be beneficial in the diabetic kidney.
Diabetic milieu induces multiple mechanisms in podocytes that directly affect functions and viability of these cells. In addition to AGEs and direct cytotoxic effects of hyperglycemia, primarily via increased production of ROS, deleterious for podocytes is up-regulation of their local renin-angiotensin and TGFβ systems and increased synthesis of VEGF. Moreover, angiotensin II, TGFβ and VEGF reciprocally modulate their production, this way perpetuating podocyte and glomerular impairment [74]. So far, our knowledge on urolithin-mediated effects on the podocytes is very limited. However, it seems likely that similarly to other cells, urolithins in podocytes may regulate the activities of TGFβ, VEGF, antioxidant systems and multiple signaling pathways and kinases that are sensitive to urolithins in other cell types. The urolithins’ ability to attenuate the harmful hyperglycemia-induced effects could protect these vulnerable cells from injury.
Conclusions
A growing body of evidence suggests that urolithins are potent multifunctional compounds capable of regulating a variety of cellular processes. Since they do not act through specific receptors, urolithins may affect various cell and tissue types. Not all body tissues accumulate urolithins but all urolithins present in plasma pass the glomerular filter, thus directly contacting kidney cells. However, so far relatively little is known about the effects of urolithins on kidney function and data concerning action of urolithins on glomerular cells is particularly sparse. Yet, the available reports definitely show that urolithin administration prevents inflammation and diabetes-induced changes in renal tissue. Figure 2 summarizes the current state of knowledge on urolithin-dependent protective effects in the kidney. The research on urolithins is growing and it can be expected that in the near future the beneficial effects of urolithins in kidney disease will be supported by ample evidence.
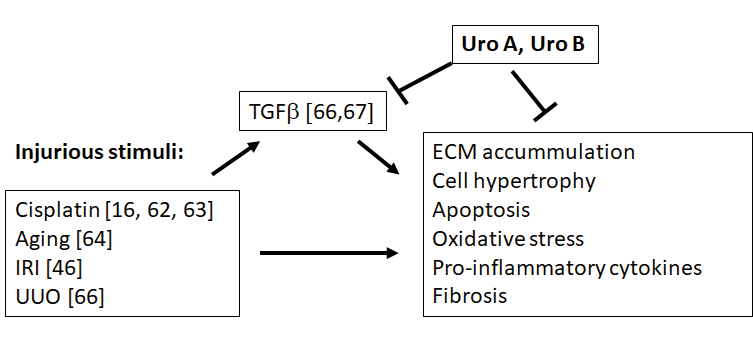
Figure 2. Protective effects of urolithins in the kidney. IRI -ischemia-reperfusion injury. UUO – unilateral ureteral obstruction [66]
Funding
None.
Conflicts of interest
None.
References
| 1. |
Lederer E. Chemistry and biochemistry of some mammalian secretions and excretions. J Chem Soc [Internet]. 1949;(0):2115. Available from: http://xlink.rsc.org/?DOI=jr9490002115.
|
| 2. |
García-Villalba R, Selma M V, Espín JC, Tomás-Barberán FA. Identification of Novel Urolithin Metabolites in Human Feces and Urine after the Intake of a Pomegranate Extract. J Agric Food Chem [Internet]. 2019 Oct 9;67(40):11099-107. Available from: https://pubs.acs.org/doi/10.1021/acs.jafc.9b04435.
|
| 3. |
Muku G, Murray I, Espín J, Perdew G. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites [Internet]. 2018 Nov 29;8(4):86. Available from: http://www.mdpi.com/2218-1989/8/4/86.
|
| 4. |
Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Heber D, editor. Evidence-Based Complement Altern Med [Internet]. 2013;2013:1-15. Available from: http://www.hindawi.com/journals/ecam/2013/270418/.
|
| 5. |
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid Redox Signal [Internet]. 2013 May 10;18(14):1818-92. Available from: http://www.liebertpub.com/doi/10.1089/ars.2012.4581.
|
| 6. |
Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate Juice Ellagitannin Metabolites Are Present in Human Plasma and Some Persist in Urine for Up to 48 Hours. J Nutr [Internet]. 2006 Oct 1;136(10):2481-5. Available from: https://academic.oup.com/jn/article/136/10/2481/4746683.
|
| 7. |
Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med [Internet]. 2010 Dec;31(6):513-39. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0098299710000749.
|
| 8. |
Tomás-Barberán FA, González-Sarrías A, García-Villalba R, Núñez-Sánchez MA, Selma M V, García-Conesa MT, et al. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res [Internet]. 2017 Jan;61(1):1500901. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201500901.
|
| 9. |
Cortés-Martín A, Romo-Vaquero M, García-Mantrana I, Rodríguez-Varela A, Collado MC, Espín JC, et al. Urolithin Metabotypes can Anticipate the Different Restoration of the Gut Microbiota and Anthropometric Profiles during the First Year Postpartum. Nutrients [Internet]. 2019 Sep 3;11(9):2079. Available from: https://www.mdpi.com/2072-6643/11/9/2079.
|
| 10. |
Romo-Vaquero M, Cortés-Martín A, Loria-Kohen V, Ramírez-de-Molina A, García-Mantrana I, Collado MC, et al. Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications. Mol Nutr Food Res [Internet]. 2019 Feb;63(4):1800958. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201800958.
|
| 11. |
Narayanan BA, Re GG. IGF-II down regulation associated cell cycle arrest in colon cancer cells exposed to phenolic antioxidant ellagic acid. Anticancer Res [Internet]. 2001;21(1A):359-64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11299762.
|
| 12. |
LOSSO J, BANSODE R, TRAPPEYII A, BAWADI H, TRUAX R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem [Internet]. 2004 Nov;15(11):672-8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0955286304001226.
|
| 13. |
Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma M V, Espín JC. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J Agric Food Chem [Internet]. 2014 Jul 16;62(28):6535-8. Available from: https://pubs.acs.org/doi/10.1021/jf5024615.
|
| 14. |
Piwowarski JP, Stanisławska I, Granica S, Stefańska J, Kiss AK. Phase II Conjugates of Urolithins Isolated from Human Urine and Potential Role of β -Glucuronidases in Their Disposition. Drug Metab Dispos [Internet]. 2017 Jun;45(6):657-65. Available from: http://dmd.aspetjournals.org/lookup/doi/10.1124/dmd.117.075200.
|
| 15. |
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β – an excellent servant but a bad master. J Transl Med [Internet]. 2012 Dec 3;10(1):183. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-10-183.
|
| 16. |
Zou D, Ganugula R, Arora M, Nabity MB, Sheikh-Hamad D, Kumar MNVR. Oral delivery of nanoparticle urolithin A normalizes cellular stress and improves survival in mouse model of cisplatin-induced AKI. Am J Physiol Physiol [Internet]. 2019 Nov 1;317(5):F1255-64. Available from: https://www.physiology.org/doi/10.1152/ajprenal.00346.2019.
|
| 17. |
Zhang S, Al-Maghout T, Cao H, Pelzl L, Salker MS, Veldhoen M, et al. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca2+ Entry in T Cells by Regulating miR-10a-5p. Front Immunol [Internet]. 2019 Jul 31;10:1737. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.01737/full.
|
| 18. |
Kang I, Buckner T, Shay NF, Gu L, Chung S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv Nutr [Internet]. 2016 Sep 1;7(5):961-72. Available from: https://academic.oup.com/advances/article/7/5/961/4616729.
|
| 19. |
González-Sarrías A, García-Villalba R, Núñez-Sánchez MÁ, Tomé-Carneiro J, Zafrilla P, Mulero J, et al. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J Funct Foods [Internet]. 2015 Dec;19:225-35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464615004363.
|
| 20. |
Mena P, Dall’Asta M, Calani L, Brighenti F, Del Rio D. Gastrointestinal stability of urolithins: an in vitro approach. Eur J Nutr [Internet]. 2017 Feb 6;56(1):99-106. Available from: http://link.springer.com/10.1007/s00394-015-1061-4.
|
| 21. |
Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA. Iberian Pig as a Model To Clarify Obscure Points in the Bioavailability and Metabolism of Ellagitannins in Humans. J Agric Food Chem [Internet]. 2007 Dec 1;55(25):10476-85. Available from: https://pubs.acs.org/doi/10.1021/jf0723864.
|
| 22. |
Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee R, et al. Pomegranate Ellagitannin-Derived Metabolites Inhibit Prostate Cancer Growth and Localize to the Mouse Prostate Gland. J Agric Food Chem [Internet]. 2007 Sep 1;55(19):7732-7. Available from: https://pubs.acs.org/doi/10.1021/jf071303g.
|
| 23. |
González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, Gómez-Sánchez MB, García-Talavera N V, Gil-Izquierdo A, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res [Internet]. 2010 Mar;54(3):311-22. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.200900152.
|
| 24. |
Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera N V, Gómez-Sánchez MB, Sánchez-Álvarez C, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res [Internet]. 2014 Jun;58(6):1199-211. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201300931.
|
| 25. |
Cerdá B, Periago P, Espín JC, Tomás-Barberán FA. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J Agric Food Chem [Internet]. 2005 Jul 1;53(14):5571-6. Available from: https://pubs.acs.org/doi/10.1021/jf050384i.
|
| 26. |
García-Villalba R, Beltrán D, Espín JC, Selma MV, Tomás-Barberán FA. Time Course Production of Urolithins from Ellagic Acid by Human Gut Microbiota. J Agric Food Chem [Internet]. 2013 Sep 18;61(37):8797-806. Available from: https://pubs.acs.org/doi/10.1021/jf402498b.
|
| 27. |
Bobowska A, Granica S, Filipek A, Melzig MF, Moeslinger T, Zentek J, et al. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur J Nutr [Internet]. 2021 Jun 22;60(4):1957-72. Available from: https://link.springer.com/10.1007/s00394-020-02386-y.
|
| 28. |
Lee G, Park J-S, Lee E-J, Ahn J-H, Kim H-S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine [Internet]. 2019 Mar;55:50–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0944711318302204.
|
| 29. |
Ceci C, Lacal P, Tentori L, De Martino M, Miano R, Graziani G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients [Internet]. 2018 Nov 14;10(11):1756. Available from: http://www.mdpi.com/2072-6643/10/11/1756.
|
| 30. |
González-Sarrías A, Espín J-C, Tomás-Barberán FA, García-Conesa M-T. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol Nutr Food Res [Internet]. 2009 Jun;53(6):686-98. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.200800150.
|
| 31. |
Cho H, Jung H, Lee H, Yi HC, Kwak H, Hwang KT. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct [Internet]. 2015;6(5):1675-83. Available from: http://xlink.rsc.org/?DOI=C5FO00274E.
|
| 32. |
Ríos J-L, Giner R, Marín M, Recio M. A Pharmacological Update of Ellagic Acid. Planta Med [Internet]. 30.05.2018. 2018 Oct 30;84(15):1068-93. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/a-0633-9492.
|
| 33. |
Mohammed Saleem YI, Albassam H, Selim M. Urolithin A induces prostate cancer cell death in p53-dependent and in p53-independent manner. Eur J Nutr [Internet]. 2020 Jun 8;59(4):1607-18. Available from: http://link.springer.com/10.1007/s00394-019-02016-2.
|
| 34. |
Qiu Z, Zhou B, Jin L, Yu H, Liu L, Liu Y, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol [Internet]. 2013 Sep;59:428-37. Available from: https://linkinghub.elsevier.com/retrieve/pii/S027869151300402X.
|
| 35. |
Qiu Z, Zhou J, Zhang C, Cheng Y, Hu J, Zheng G. Antiproliferative effect of urolithin A, the ellagic acid-derived colonic metabolite, on hepatocellular carcinoma HepG2.2.15 cells by targeting Lin28a/let-7a axis. Brazilian J Med Biol Res [Internet]. 2018/05/07. 2018;51(7):e7220-e7220. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2018000700602&tlng=en.
|
| 36. |
Lv M, Shi C, Pan F, Shao J, Feng L, Chen G, et al. Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β‐catenin signaling. J Cell Biochem [Internet]. 2019 Oct 19;120(10):17273-82. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jcb.28989.
|
| 37. |
Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram NP. Effects of Fruit Ellagitannin Extracts, Ellagic Acid, and Their Colonic Metabolite, Urolithin A, on Wnt Signaling. J Agric Food Chem [Internet]. 2010 Apr 14;58(7):3965-9. Available from: https://pubs.acs.org/doi/10.1021/jf902857v.
|
| 38. |
Alauddin M, Okumura T, Rajaxavier J, Khozooei S, Pöschel S, Takeda S, et al. Gut Bacterial Metabolite Urolithin A Decreases Actin Polymerization and Migration in Cancer Cells. Mol Nutr Food Res [Internet]. 2020 Apr;64(7):1900390. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201900390.
|
| 39. |
Zhao W, Shi F, Guo Z, Zhao J, Song X, Yang H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol Carcinog [Internet]. 2018 Feb;57(2):193-200. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mc.22746.
|
| 40. |
Giménez-Bastida JA, González-Sarrías A, Larrosa M, Tomás-Barberán F, Espín JC, García-Conesa M-T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol Nutr Food Res [Internet]. 2012 May;56(5):784-96. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201100677.
|
| 41. |
Wang Y, Qiu Z, Zhou B, Liu C, Ruan J, Yan Q, et al. In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicol Vitr [Internet]. 2015 Aug;29(5):1107-15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S088723331500079X.
|
| 42. |
Ding S-L, Pang Z-Y, Chen X-M, Li Z, Liu X-X, Zhai Q-L, et al. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J Inflamm [Internet]. 2020 Dec 24;17(1):13. Available from: https://journal-inflammation.biomedcentral.com/articles/10.1186/s12950-020-00242-8.
|
| 43. |
Boakye YD, Groyer L, Heiss EH. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. Biochim Biophys Acta - Gen Subj [Internet]. 2018 Jan;1862(1):61-70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304416517303276.
|
| 44. |
Chen P, Chen F, Lei J, Li Q, Zhou B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates d-Galactose-Induced Brain Aging in Mice. Neurotherapeutics [Internet]. 2019 Oct 1;16(4):1269-82. Available from: http://link.springer.com/10.1007/s13311-019-00753-0.
|
| 45. |
Ahsan A, Zheng Y-R, Wu X-L, Tang W-D, Liu M-R, Ma S-J, et al. Urolithin A‐activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci Ther [Internet]. 2019 Sep 11;25(9):976-86. Available from: https://onlinelibrary.wiley.com/doi/10.1111/cns.13136.
|
| 46. |
Heilman J, Andreux P, Tran N, Rinsch C, Blanco-Bose W. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem Toxicol [Internet]. 2017 Oct;108:289-97. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0278691519303801.
|
| 47. |
Heilman J, Andreux P, Tran N, Rinsch C, Blanco-Bose W. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem Toxicol. 2017;.
|
| 48. |
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab [Internet]. 2019 Jun 14;1(6):595-603. Available from: http://www.nature.com/articles/s42255-019-0073-4.
|
| 49. |
Djedjibegovic J, Marjanovic A, Panieri E, Saso L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Morroni F, editor. Oxid Med Cell Longev [Internet]. 2020 Jul 28;2020:1-15. Available from: https://www.hindawi.com/journals/omcl/2020/5194508/.
|
| 50. |
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-A, Vogiatzi G, Papaioannou S, et al. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol Rev [Internet]. 2019 Apr 30;14(1):50-9. Available from: https://www.ecrjournal.com/articles/role-inflammation-diabetes-current-concepts-and-future-perspectives.
|
| 51. |
Vinod PB. Pathophysiology of diabetic nephropathy. Clin Queries Nephrol [Internet]. 2012 Apr;1(2):121-6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211947712700055.
|
| 52. |
Roopchand DE, Kuhn P, Rojo LE, Lila MA, Raskin I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res [Internet]. 2013 Feb;68(1):59-67. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1043661812002290.
|
| 53. |
Ávila-Gálvez MA, Giménez-Bastida JA, González-Sarrías A, Espín JC. Tissue deconjugation of urolithin A glucuronide to free urolithin A in systemic inflammation. Food Funct [Internet]. 2019;10(6):3135-41. Available from: http://xlink.rsc.org/?DOI=C9FO00298G.
|
| 54. |
Savi M, Bocchi L, Mena P, Dall’Asta M, Crozier A, Brighenti F, et al. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol [Internet]. 2017 Dec 6;16(1):80. Available from: http://cardiab.biomedcentral.com/articles/10.1186/s12933-017-0561-3.
|
| 55. |
Sala R, Mena P, Savi M, Brighenti F, Crozier A, Miragoli M, et al. Urolithins at physiological concentrations affect the levels of pro-inflammatory cytokines and growth factor in cultured cardiac cells in hyperglucidic conditions. J Funct Foods [Internet]. 2015 May;15:97-105. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464615001279.
|
| 56. |
Lee HJ, Jung YH, Choi GE, Kim JS, Chae CW, Lim JR, et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ [Internet]. 2021 Jan 23;28(1):184-202. Available from: https://www.nature.com/articles/s41418-020-0593-1.
|
| 57. |
Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res [Internet]. 2011 May;55(S1):S35-43. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201000525.
|
| 58. |
Bayle M, Neasta J, Dall’Asta M, Gautheron G, Virsolvy A, Quignard J-F, et al. The ellagitannin metabolite urolithin C is a glucose‐dependent regulator of insulin secretion through activation of L‐type calcium channels. Br J Pharmacol [Internet]. 2019/10/10. 2019 Oct 10;176(20):4065-78. Available from: https://onlinelibrary.wiley.com/doi/10.1111/bph.14821.
|
| 59. |
Wautier J, Guillausseau P. Advanced glycation end products, their receptors and diabetic angiopathy. J Peripher Nerv Syst [Internet]. 2002 Jun 20;7(2):138-138. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1529-8027.2002.02011_16.x.
|
| 60. |
Bondeva T, Wojciech S, Wolf G. Advanced glycation end products inhibit adhesion ability of differentiated podocytes in a neuropilin-1-dependent manner. Am J Physiol Physiol [Internet]. 2011 Oct;301(4):F852-70. Available from: https://www.physiology.org/doi/10.1152/ajprenal.00575.2010.
|
| 61. |
Liu W, Ma H, Frost L, Yuan T, Dain JA, Seeram NP. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct [Internet]. 2014 Nov;5(11):2996-3004. Available from: http://xlink.rsc.org/?DOI=C4FO00538D.
|
| 62. |
Guada M, Ganugula R, Vadhanam M, Ravi Kumar MNV. Urolithin A Mitigates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation and Apoptosis in an Experimental Rat Model. J Pharmacol Exp Ther [Internet]. 2017 Oct;363(1):58-65. Available from: http://jpet.aspetjournals.org/lookup/doi/10.1124/jpet.117.242420.
|
| 63. |
Jing T, Liao J, Shen K, Chen X, Xu Z, Tian W, et al. Protective effect of urolithin a on cisplatin-induced nephrotoxicity in mice via modulation of inflammation and oxidative stress. Food Chem Toxicol [Internet]. 2019 Jul;129:108-14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0278691519302303.
|
| 64. |
Chen P, Lei J, Chen F, Zhou B. Ameliorative effect of urolithin A on D-gal-induced liver and kidney damage in aging mice via its antioxidative, anti-inflammatory and antiapoptotic properties. RSC Adv [Internet]. 2020;10(14):8027-38. Available from: http://xlink.rsc.org/?DOI=D0RA00774A.
|
| 65. |
Li MO, Flavell RA. TGF-β: A Master of All T Cell Trades. Cell [Internet]. 2008 Aug;134(3):392-404. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867408009458.
|
| 66. |
Li Q, Li K, Chen Z, Zhou B. Anti-renal fibrosis and anti-inflammation effect of urolithin B, ellagitannin-gut microbial-derived metabolites in unilateral ureteral obstruction rats. J Funct Foods [Internet]. 2020 Feb;65:103748. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464619306723.
|
| 67. |
Chappell MC, Pingue G, Pirro N, Tallant A, Gallagher P. The Microbiome Product Urolithin A Abolishes TGFβ‐Dependent Stimulation of PAI‐1 in Renal Epithelial Cells. FASEB J [Internet]. 2019 Apr;33(S1):1-9. Available from: https://onlinelibrary.wiley.com/doi/10.1096/fasebj.2019.33.1_supplement.lb530.
|
| 68. |
Seo JY, Park J, Yu MR, Kim YS, Ha H, Lee HB. Positive Feedback Loop between Plasminogen Activator Inhibitor-1 and Transforming Growth Factor-Beta1 during Renal Fibrosis in Diabetes. Am J Nephrol [Internet]. 2009;30(6):481-90. Available from: https://www.karger.com/Article/FullText/242477.
|
| 69. |
Shankland SJ. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int [Internet]. 2006 Jun;69(12):2131-47. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0085253815514403.
|
| 70. |
Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al. Podocyte Depletion Causes Glomerulosclerosis: Diphtheria Toxin–Induced Podocyte Depletion in Rats Expressing Human Diphtheria Toxin Receptor Transgene. J Am Soc Nephrol [Internet]. 2005 Oct;16(10):2941-52. Available from: https://jasn.asnjournals.org/lookup/doi/10.1681/ASN.2005010055.
|
| 71. |
White K. Glomerular Structure in Diabetes - Can It Predict the Future? Am J Nephrol [Internet]. 2015;41(4–5):275–6. Available from: https://www.karger.com/Article/FullText/430850.
|
| 72. |
Kostovska I, Trajkovska KT, Cekovska S, Spasovski G, Labudovic D. Nephrin and Podocalyxin - New Podocyte Proteins for Early Detection of Secondary Nephropathies. BANTAO J [Internet]. 2016 Jun 27;14(1):11-6. Available from: https://www.sciendo.com/article/10.1515/bj-2016-0003.
|
| 73. |
Kriz W, Shirato I, Nagata M, LeHir M, Lemley K V. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Physiol [Internet]. 2013 Feb 15;304(4):F333-47. Available from: https://www.physiology.org/doi/10.1152/ajprenal.00478.2012.
|
| 74. |
Lin JS, Susztak K. Podocytes: the Weakest Link in Diabetic Kidney Disease? Curr Diab Rep [Internet]. 2016 May 6;16(5):45. Available from: http://link.springer.com/10.1007/s11892-016-0735-5.
|










