Abstract
Background
We can observe an increase in acute pancreatitis (AP) incidence in the recent years.
Material and methods
Retrospective clinical data analysis of 370 patients with AP, hospitalized between 2007 and 2016 at our Department.
Results
AP was diagnosed during 406 hospitalisation in 370 patients [average age 52.15 (21-93), 237(64.05%) male]. AP of high clinical severity was diagnosed in 60/370 (16.22%) patients. Average time of hospitalisation was 16.13 (1-121) days. Mortality was 12/406 (2.96%). The after effect of AP in form of parapancreatic fluid reservoirs was diagnosed in 202/406 (54.59%) cases. Comparing the early phase of the study (2007-2011) and the later one (2012-2016) a shorter time of hospitalisation was proven and a lower mortality of the patients in the later phase of the study. Analysis of patients' blood tests revealed that patients with severe AP have significantly elevated levels of inflammatory parameters and amylase comparing to group with mild and moderate AP, during the first days of hospitalisation.
Conclusions
The development of conservative treatment options for AP, especially in early stages of the illness, has significantly shortened the duration of hospitalisation of patients with AP at our Department.
Citation
Jagielski M, Kijowski F, Fruczek M, Sternau-Kuklińska M, Janiak M, Adrych K. Clinical and epidemiological aspects of acute pancreatitis ‒ 10 years of single-center experience. Eur J Transl Clin Med. 2018;1(2):41-52Introduction
Acute pancreatitis (AP) is an acute inflammatory state of the pancreas, often involving peripancreatic tissues and organs during progression of the illness [1-2]. Pathogenesis of AP involves premature activation of pancreatic proenzymes, which cause damage to the gland [1-4]. The diagnosis of AP is based on fulfilling two out of three criteria: abdominal pain of characteristic location, blood levels of pancreatic enzyme activity elevated threefold over the norm and a typical image of pancreas in radiological examinations [1-2]. According to the 2012 Revision of Atlanta classification there are 3 clinical forms of AP (mild, moderate, severe) diagnosed depending on the occurrence and duration of organ failure, which is classified by modified Marshall scale [1-2]. The 2012 Atlanta classification also suggests a division of AP: interstitial edematous AP and necrotizing AP, which are diagnosed based on imaging (mainly abdominal contrast-enhanced computed tomography [CECT]) [1-2]. Usually cases of mild and moderate AP are described by pathomorphologists as interstitial edematous, while necrotizing AP is often clinically classified as severe.
The incidence of AP is rising worldwide, which greatly increases the costs of hospitalization and treatment of the patients [5-8]. AP is the most common pancreatic illness and one of the most common acute conditions in gastroenterology [7]. The few existing epidemiological studies of AP in Poland suggest that Poland is among the countries with the highest rates of incidence of AP in the world which is 72/100000/ year (worldwide 33.74/100000/year) [5-9]. The total annual cost of AP patients’ treatment depends on etiology, severity, treatment in intensive care units and infection complications [8].
The aim of our study was retrospectively analyze the trends in clinical data of patients with AP, who were hospitalized at our Department in the years 2007-2016.
Materials and Methods
A retrospective analysis of all patients treated at our department between 2007 and 2016 with AP was conducted. Patients who began treatment at another hospital and were referred to our department in order to treat the complications of AP were excluded from the study. Patients with a prior diagnosis of chronic pancreatitis were also excluded.
All the definitions contained herein are based on the revised Atlanta classification from 2012 [1-2]. AP was diagnosed based on revised Atlanta 2012 classification if two out of three criteria were met: (1) amylase and/or lipase levels ≥ three times above the norm, (2) typical abdominal pain radiating from front to back, (3) typical appearance on imaging [1-2]. All patients had blood tests conducted [blood morphology, amylase and lipase activity level, liver parameters (AST, ALT), bilirubin, cholestasis parameters (GGTP, ALP), C-reactive proteins, arterial blood gas]. All patients had an abdominal ultrasound upon admission. Abdominal CECT was conducted, when the diagnosis of AP was doubtful.
After diagnosing AP in the first day of hospitalization, a prognosis was made based on relevant scales. In the majority of cases the Bedside Index for Severity in Acute Pancreatitis (BISAP) score was used [1-2]. Furthermore, the clinical state during hospitalization was assessed continuously with particular attention being paid to the amount of intravenous fluids administered in the first 72 hours from admission. The type and duration of organ failure was assessed using Marshall scale [1-2]. Organ failure was deemed as transient (under 48 hours) and persistent (over 48 hours) [1-2].
Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy and gallstone removal with prosthesis introduction and/or nasal drain introduction was performed in all cases with AP and coexisting acute cholangitis within the first 24 hours. Furthermore, in cases of acute biliary pancreatitis with clinical symptoms of persistent biliary blockage ERCP was also conducted within the first 72 hours from admission.
In order to find out the etiology of AP, after excluding alcohol, biliary, trauma, drugs and iatrogenic cause, blood tests involving calcium and triglycerides concentration were done. If hypercalcemia and hypertriglicerydemia were ruled out as AP cause there were two pathways. Once the inflammatory process has seized, CECT and additional blood tests of carcinoembryonic antigen (CEA) and cancer antigen 19-9 (Ca19-9) were conducted in all patients over 40 years of age in order to rule out a tumor. If the patient was under 40 years old, endoscopic ultrasonography (EUS) or magnetic resonance cholangiopancreatography (MRCP) was made. Only after these conditions were met we looked for genetic and autoimmune causes of AP. If all the above steps were completed and the etiology was not established, then the case was diagnosed as idiopathic AP.
The standards of conservative treatment of AP at our Department are not significantly different from the international guidelines [10-11]. Nutritional therapyis the basis of treatment of patients with AP. In case of mild and moderate AP, transient starvation diet and oral fat-restricted diet was introduced after gastric syndromes such as: nausea, vomiting, abdominal pain were alleviated. Enteral nutrition via flocare wasused in severe form of AP. Some of the patients had to be fed intravenously. An intensive liquid therapy isapplied together with analgesic treatment of AP. Additionaltreatment was applied in patients with organ failure if deemed necessary.
In accordance with international guidelines [10-11] a prophylactic antibiotic therapy was not administered in patients with necrotizing AP or severe form of AP. Intravenous antibiotic therapy was administered to patients with infected necrosis in AP, proven by culture growth from necrotic tissue. Intravenous antibiotic therapy was also administered to patients in whom radiological imaging revealed signs of infection such as: gas bubble external to gastrointestinal tract imaged in CECT scans or likelihood of infection shown as appearance of new symptoms of systemic inflammatory response after a minimum of 7 days since the inception of the illness. Intravenous antibiotic therapy was also used in patients who presented symptoms of infection localized externally to the pancreas. We used the following antibiotics: ceftriaxone with metronidazole, ciprofloxacin with metronidazole, tazobactam with piperacillin or imipenem.
Abdominal CECT was performed in every patient with severe AP, with suspicion of necrosis infection or when there was no improvement within the first 48 hours of treatment. CECT scans were evaluated in accordance with computed tomography severity index (CTSI) [1-2].
The majority of AP cases (medical documentation and radiological imaging) were discussed in detail during interdisciplinary weekly clinical meetings. The professionals consisted of radiologists, gastroenterologistsand surgeons. Treatment decisions were mostly dictated by the outcomes of these meetings.
All statistical calculations were performed using the Statistica software (v 13.0 StatSoft Inc. Tulsa, USA). Quantitative variables were characterized by arithmetic means and standard deviation, along with minimal and maximal values (range). Qualitative data are presented as means of numbers and percentage. Raw data were checked for normality using the Shapiro-Wilk test. Multivariate comparisons were performed using a t-test/Mann-Whitney test for quantitative variables. Comparisons between percentage values in groups were conducted using Chi Square test for Percentage values. Two-tailed tests were carried out after setting a significance level of p ≤ 0.05.
Results
AP was diagnosed in 406 hospitalizations and in 370 consecutive patients [133 (35.95%) females, 237 (64.05%) males]. The average age was 52.15 (21-93) years. Detailed characteristics of patients with AP are presented in Table 1. The most common etiology of AP was alcohol 182/406 (44.83%), followed by biliary 135/406 (33.25%). The other etiologies were: idiopathic 45/406 (11.08%), iatrogenic 12/406 (2.96%), hyperlipidemia 11/406 (2.71%), pancreatic cancer 10/406 (2.46%), hypercalcemia 5/406 (1.23%), anatomical variant 3/406 (0.74%), drugs 2/406 (0.49%), trauma 1/406 (0.25%). The average duration of hospitalization of AP patients was 16.13 (1-121) days. Death was noted in 12/406 (2.96%) patients. The number of re-hospitalizations due to AP was 36/406 (8.87%). Only 23/370 (6.22%) patients were admitted ≥2 (2-5) times due to AP. The most common reason for readmission was alcohol 32/36 (88.89%).
Table 1. The characteristics of all hospitalized patients with acute pancreatitis
Of the 135 patients with biliary AP, 89 (65.93%) underwent ERCP with gallstone removal. Acute cholangitis was diagnosed in 42/135 (31.11%) patients. CECT was performed in 242/406 (59.61%) cases. The average CTSI in a group of 242 subjects was 6.17 (1-10). Necrotizing AP was diagnosed in 71/370 (19.19%) patients in 73/406 (17.98%) hospitalizations. In other cases 299/370 (80.81%) (333/406, 82.02% of hospitalizations) interstitial edematous AP was diagnosed.
Mild case of AP was diagnosed in 235/370 (63.51%) patients [average age 51.58±16.43 (21-92) years, 154 males] during 269/406 (66.26%) hospitalizations. Whereas moderate AP was diagnosed in 75/370 (20.27%) patients [average age 53.75±16.88 (21-93) years, 39 males] during 77/406 (18.97%) hospitalizations. The most common transient organ failure (< 48 hours) with moderate cases of AP was kidney failure, diagnosed in 48/77 (62.34%) hospitalizations. The second most common organ failure was respiratory insufficiency (23/77, 29.87% hospitalizations).
Severe AP was diagnosed in 60/370 (16.22%) patients [average age 52.95±15.14 (25-90) years, 44 males] during 60/406 (14.77%) hospitalizations. In thisgroup the most common persistent (>48 hours) organfailure was also kidney failure in 51/60 (85%) patients.All patients with severe AP had morphologically classified as necrotizing AP based on radiological imagingevidence. Fatal complications were only recorded inthe severe AP patient group 12/60 (20%). Clinical data of all patients with AP in regards to their age were presented in table 2.
Table 2. Clinical characteristics of < 60 and ≥ 60 HCM patients
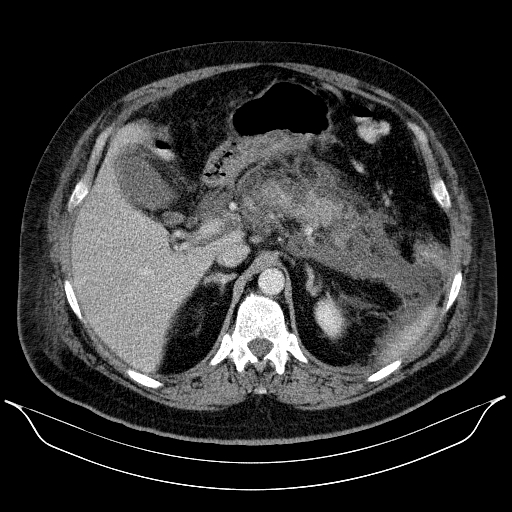
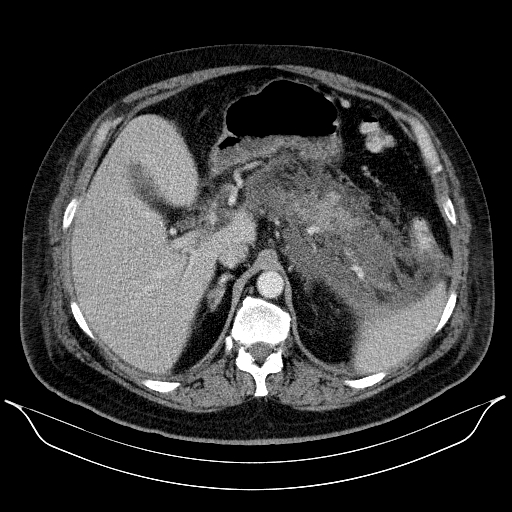
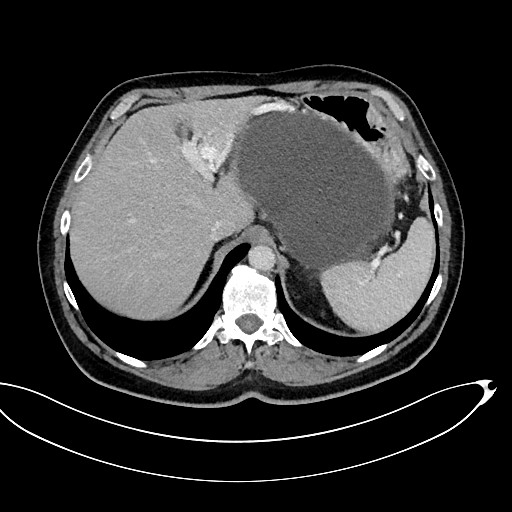
The complications of AP taking form in pancreatic and peripancreatic fluid collections (PFCs) in early phase of disease were noted in 202/406 (54.59%) of cases. In the early phases of AP (<4 weeks since the onset of symptoms) 59 (29.21%) patients were diagnosed with acute necrotic collection (ANC) (Figure 1a-c.). Acute peripancreatic fluid collection (APFC) was documented in remaining 143 (70.79%) patients. In thegroup with ANC, 40/59 (67.8%) of the patients were later diagnosed (after 4 weeks of illness) with walledoff pancreatic necrosis (WOPN) (Figure 2A-C.). Alternativelythe patients with APFC, only 24/143 (16.78%)of the patients were diagnosed with a persistent formof fluid collection, namely a pancreatic pseudocyst. 46/202 (22.77%) of patients with PFCs due to AP were requiring interventional treatment. Moreover, 51/370 (13.78%) of patients treated for AP for the first time went on to develop chronic pancreatitis.
|
Figure 1 A
|
Figure 1 B
|
Figure 1 c
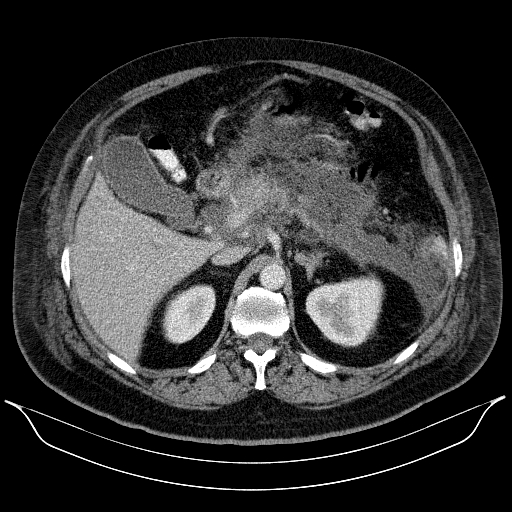
Figure 1 A-C. A 53-year-old patient with necrotizing AP. An acute necrotic collection was visible in the abdominal CECT performed on the 13rd day of illness
| Figure 2 A | Figure 2 B |
 |
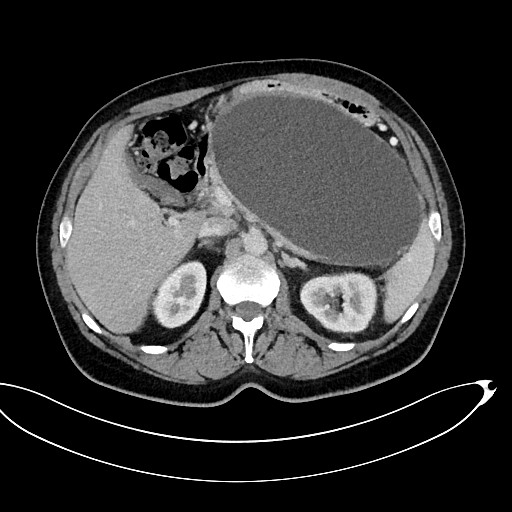 |
Figure 2 C
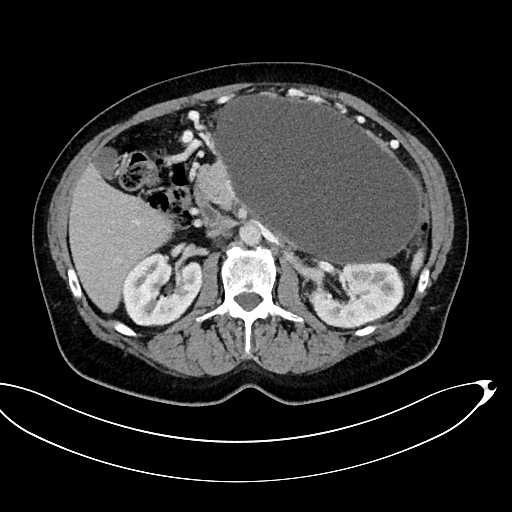
Figure 2 A-C. A 53-year-old patient with ANP. A walled-off pancreatic necrosis collection 145x220x180 mm in size, which was pressing upon the lumen of the gastrointestinal tract, was subsequently identified by abdominal CECT
Comparing the early phase of the study (2007-2011) with the late phase (2012-2016) (Table 3) a shorter time of hospitalization (20.8 days vs 10.7 days, p < 0.05)and a lower mortality rate (4.1% vs 1.6%, p < 0.05) weredocumented in the later phase of the study.
Table 3. The characteristics of all hospitalized patients depending on the study period
Furthermore, the laboratory blood test results revealed that patients with severe AP have a significantly higher levels of CRP (124.18 ± 95.93 mg/l vs 45.22 ± 58.73 mg/l, p < 0.05) and amylase (3996.6 ± 15267.03 U/l vs 1206.84 ± 2523.43 U/l, p<0.05) in comparison to patients with mild and moderate AP within the first days of hospitalization (Table 4).
Table 4. Comparison of patients with mild and moderate acute pancreatitis versus severe acute pancreatitis
Discussion
In recent years major changes occurred in the treatment strategy of patients with AP. Due to lack of large scale population studies it is difficult to accurately and scrupulously obtain viable data of incidence of AP in the Polish population. This is mainly caused by a [5]. There are 2 epidemiological studies containing clinical data of patients with AP in Poland [6, 9]. Bogdan et al. presented results of analysis of 441 hospitalizations in 298 patients admitted to a single hospital in the years 2005-2010 [9]. Whereas Głuszek et al. conducted a prospective observation of 1044 hospitalized patients with AP in all departments of surgery of the Świętokrzyskie province [6]. The results of both of the above studies suggest an average AP incidence in Poland of 64.4-99.96/100000 [6, 9].
The second problem connected with hardships in determining the realistic incidence of AP is a lack of proper diagnosis. In a 2016 article it was proven, that in a group of 694 patients with AP, 143 (20.6%) were previously admitted to the hospital for that illness without an accurate diagnosis or treatment [12]. In the same study it was determined that nearly half of the cases the cause was gallstones (46.5%) whereas in our study it accounted for just 33.35% of cases [12]. It is noteworthy that biliary AP was also a significant reason for re-hospitalization due to AP [40/132 (30.3%) cases] [12]. In our study the situation is completely opposite as alcohol was the major cause for re-hospitalization. It needs to be pointed out that the above-mentioned study was conducted in United Kingdom (excluding Scotland) [12]. As most authors highlight in different values regarding incidence and other clinical and epidemiological data and most importantly etiology differs significantly depending on the population of the country or region [5-6, 9, 12-13]. Furthermore, even within the same population, the incidence of AP differs depending on the time of year (e.g. the incidence often rises around the New Year’s Day) [13].
The most common reasons for AP are alcohol and gallstones [6, 9, 13-17]. The other etiologies are much less frequent [6, 9, 13, 16-18]. Biliary AP is the most common cause in the Mediterranean countries (Italy, Greece, Spain) [5, 17]. Whereas alcohol AP is dominant in the Northern and Eastern Europe and Russia [5, 17]. In our sample the alcohol etiology of AP was defined in 44.83% of cases and biliary etiology in 33.25%. In other Polish studies there are discrepancies regarding the dominating etiology [6, 9] According to Bogdan et al. the most common etiology was alcohol (49% cases),whereas biliary AP was diagnosed in 80/298 (27%) patients [9]. Whereas according to Głuszek et al. the mostcommon etiology was gallstones (30.1%) and alcoholwas second (24.1%) [6]. In the same article a coexistence of gallstones and alcohol abuse was diagnosedin 2.9% patients [6]. Similar results have been reported in a Swedish study, which also highlights that alcohol was also the dominant reason for AP recurrences [19].In the conclusion of the Głuszek et al. [6] we find thatalcohol is the most prevailing reason for AP in young Poles (mainly males), while biliary disease is dominantamong older females. It is worth mentioning that the mechanism of AP in the two dominating causes of AP has not been thoroughly explained.
In the majority of cases AP is mild and without organ failure or local complications [1-2, 5-6, 9]. We do however observe an increase in incidence of severe AP, which is strongly associated with a higher mortality rate [17]. In our study, severe AP was diagnosed in60/370 (16.22%) patients with 60/406 (14.77%) hospitalizations. The overall mortality was 12/406 (2.96%). Fatalities were noted exclusively in severe AP, which accounted for 12/60 (20%) patients. Similar results were reported by Bogdan et al., where total mortality due to AP stands at 3%, and in severe AP 15% [9]. Głuszek et al. declares severe AP in 7% cases with total mortality at 3.9% [6]. Mortality in severe AP was rated at 52.9% patients [6]. Within the same study 80.7% of AP cases were mild [6].
The recent years brought developments in the conservative treatment methods of the early phase of AP [20-27], particularly in the first week of illness, when the mortality is mostly connected to organ failure [20, 25, 28]. In our study, comparing the early phase of the study (2007-2011) with the late phase (2012-2016) a shorter duration of hospitalization and a lower mortality rate were documented in the later phase of the study. At our Department we have also noticed an improvement in the effectiveness of conservative treatment of early phase AP, which has led to significantly shorter duration of hospitalization with AP alongside with a decline in mortality rates. Differences between the populations (sex and etiology of AP) were noticed between the two phases of this study. Above-mentioned differences make it difficult to compare both groups. However, we noted an improvement in the effectiveness of conservative treatment of early phase AP, which has led to significantly shorter durations of hospitalization at our Department.
AP usually leads to a local inflammatory response, which involves many cytokines and inflammatory cells partake [3-4]. The inflammatory reaction can be so strong, that it can lead to a systemic inflammatory response syndrome (SIRS) and multi-organ failure [4, 28-29]. Depending on where will the organ failure manifest itself and depending on its duration, we can differentiate between three types of clinical AP (mild, moderate, severe) [1-2]. We demonstrated that in the first days since admission the inflammatory state parameters are statistically significantly higher in patients with severe AP than in those with moderate and mild AP. This seems to suggest a direct correlation with the severity of inflammation which is significantly increased in severe AP. Furthermore, in the severe AP all of the patients were diagnosed with necrotic pancreatitis which had an exacerbating effect on the inflammation in that group of patients.
One of the criteria to correctly diagnose AP is an increase of pancreatic enzymes (amylase, lipase) activity in the blood [1-2]. While it is widely thought that the direct levels of enzyme activity in the blood do nothave a prognostic value, we have noted that amylase levels do correlate with a clinical type of AP [30-34]. In the first days of illness, the amylase activity levels are significantly higher in severe AP than in mild or moderate AP.
According to the 2012 Revision of Atlanta classification, depending on the morphological type we can differentiate four types of pancreatic fluid structures in the progression of AP, all of which are complications of AP [1-2]. In interstitial edematous AP, an acute peripancreatic fluid collection (APFC) may form within the first four weeks, which c an then e volve into a pancreatic pseudocyst [1-2, 35-37]. In the necrotizing form of AP, the pancreatic fluid collections within the first four weeks are called acute necrotic collection (ANC), whereas after four weeks we are dealing with a walled-off pancreatic necrosis (WOPN) [1-2, 35-37]. According to multiple authors, the natural progression of AP varies [1-2, 35-37]. We have noted that pancreatic fluid collections were diagnosed in 54.59% of hospitalizations due to AP. Within four weeks of the illness, ANC was diagnosed in 29.21% patients with AP. In 70.79% of patients an APFC was diagnosed. Most of ANC (67.8%) transformed into WOPN. In contrast, the majority of APFC were resorbed (83.22%) and a persistent fluid collection in the form of a pancreatic pseudocyst was formed in as little as 16.78% patients. Sarathi Patra et al. presented that in all of the patients (100%) with necrotic AP an ANC was diagnosed, whereas 48.75% of cases transformed into WOPN in the latter phases of illness [35]. In the same study the authors have declared a presence of APFC in 37.93% of interstitial edematous AP and it is important to note that all of the APFC spontaneously regressed and no pseudocysts were found [35]. In a study by Manrai et al. [36] ANC was diagnosed in 93.4% cases of necrotizing AP, of which 58.74% transformed into WOPN. 22.22% of patients with interstitial edematous AP an AFPC was diagnosed. In 2.77% a pseudocyst was diagnosed [36].
The main condition that must be met to start interventional treatment of AP complications in the form of pancreatic and peripancreatic fluid collection is an infection of that collection [10-11, 38-40]. Interventional treatment is also reserved for patients with clinical symptoms of the presence of fluid collection [10-11, 38-39]. They are compression symptoms such as obstructive jaundice or ileus [10-11, 39-40]. Patients with pancreatic fluid collections without clinical symptoms should not be treated interventionally [10- 11, 41], because as we have presented in our study, these collections are susceptible to spontaneous regression during the hospitalization.
In the last decades we can observe an intensive development of minimally invasive treatment methods of consequences of AP [22-24, 26-27, 38-45]. The application of interventional methods of treatment was presented in our previous publications [38-42]. In the presented sample interventional treatment was needed in 46/202 (22.77%) patients with pancreatic and peripancreatic fluid collections, because of persistent symptoms related with fluid collection.
Summarizing, in this study a large group of cases were analyzed and the natural progression of acute pancreatitis was studied, including its complications, mainly pancreatic and peripancreatic fluid collections, with strong emphasis being placed on clinical and epidemiological data. In this study we demonstrated that intensive conservative treatment in the early phase of acute pancreatitis and delaying interventional treatment of acute pancreatitis complications significantly improves the treatment results and decreases mortality.
Diagram 1. The distribution of sex in the two age groups (under 50 and over 50). Each one of the four bars represents one sex in one age group. The first group of two bars is age group under 50 and the second group is over 50. Blue is assigned to males and the red to females
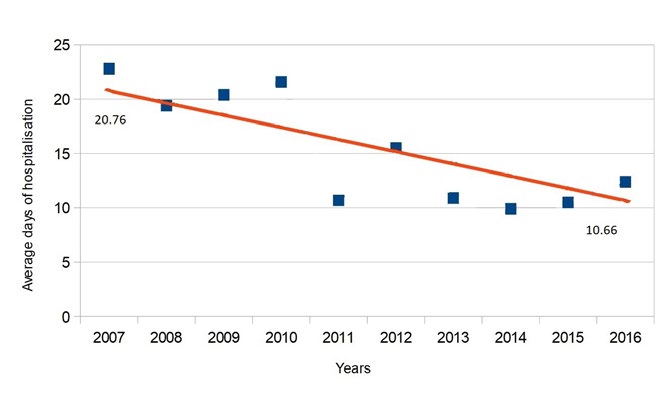
Diagram 2. The decreasing number of days of hospitalization trend in the years 2007 to 2016. The line follows a trend representing a decrease of average number of days of patient hospitalization. Values at either end represent the average number of days of hospitalization in two phases of the study (2007-2011 and 2012-2016). Each point represents the average number of days of hospitalization in that specific year
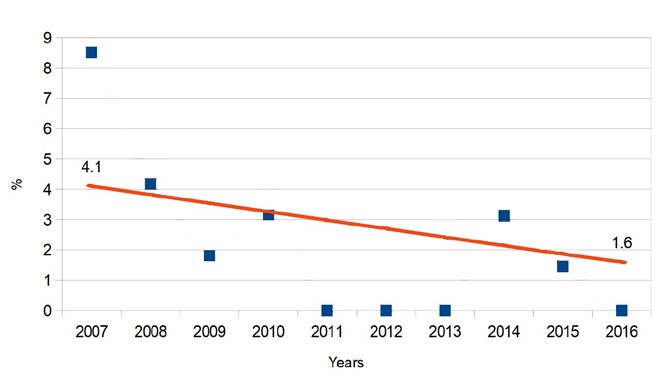
Diagram 3. The decreasing mortality trend in our Department in the years 2007-2016. The line follows a trend of decreasing mortality with values at either end representing the average percentage of mortalities in two phases of the study (2007-2011 and 2012-2016). Each point in every year represents the mortality percentage that specific year
References
| 1. |
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis − 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-11.
|
| 2. |
Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3): 751-64.
|
| 3. |
Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9(4):401-10.
|
| 4. |
Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190(2):117-25.
|
| 5. |
Kozieł D, Głuszek S. Epidemiology of acute pancreatitis in Poland – selected problems. Med Stud. 2016;32(1):1-3.
|
| 6. |
Głuszek S, Kozieł D. Prevalence and progression of acute pancreatitis in the świętokrzyskie voivodeship population. Polish J Surg. 2012;84(12):618-25.
|
| 7. |
Xiao AY, Tan MLY, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45-55.
|
| 8. |
Andersson B, Appelgren B, Sjödin V, Ansari D, Nilsson J, Persson U, et al. Acute pancreatitis – costs for healthcare and loss of production. Scand J Gastroenterol. 2013;48(12):1459-65.
|
| 9. |
Bogdan J, Elsaftawy A, Kaczmarzyk J, Jabłecki J. Epidemiological characteristic of acute pancreatitis in Trzebnica district. Polish J Surg. 2012;84(2):70-5.
|
| 10. |
Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-15.
|
| 11. |
De Waele J. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):1-15.
|
| 12. |
Mayor S. A fifth of acute pancreatitis cases are not diagnosed promptly, inquiry warns. BMJ. 2016;354:i3746.
|
| 13. |
Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38(5):539-48.
|
| 14. |
Yadav D. Recent advances in the epidemiology of alcoholic pancreatitis. Curr Gastroenterol Rep. 2011;13(2):157-65.
|
| 15. |
Pérez-Mateo M. How we predict the etiology of acute pancreatitis. JOP. 2006;7(3):257-61.
|
| 16. |
Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252-61.
|
| 17. |
Farthing M, Roberts SE, Samuel DG, Williams JG, Thorne K, Morrison-Rees S, et al. Survey of digestive health across Europe: final report. Part 1: the burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United Eur Gastroenterol J. 2014;2(6):539-43.
|
| 18. |
Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13(47):6296-313.
|
| 19. |
Appelros S, Borgström A. Incidence, aetiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. Br J Surg. 1999;86(4):465-70.
|
| 20. |
Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54(3):426-36.
|
| 21. |
Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20(38):1387-29.
|
| 22. |
Hollemans RA, van Brunschot S, Bakker OJ, Bollen TL, Timmer R, Besselink MGH, et al. Minimally invasive intervention for infected necrosis in acute pancreatitis. Expert Rev Med Devices. 2014;11(6):637-48.
|
| 23. |
Mauri G, Mattiuz C, Sconfienza LM, Pedicini V, Poretti D, Melchiorre F, et al. Role of interventional radiology in the management of complications after pancreatic surgery: a pictorial review. Insights Imaging. 2015;6(2):231-9.
|
| 24. |
Connor S, Raraty MGT, Howes N, Evans J, Ghaneh P, Sutton R, et al. Surgery in the treatment of acute pancreatitis minimal access pancreatic necrosectomy. Scand J Surg. 2005;94(2):135-42.
|
| 25. |
Fernández-Cruz L, Lozano-Salazar RR, Olvera C, Higueras O, López-Boado MA, Astudillo E, et al. Acute necrotizing pancreatitis: therapeutic alternatives. Cir Esp. 2006;80(2):64-71.
|
| 26. |
Segal D, Mortele KJ, Banks PA, Silverman SG. Acute necrotizing pancreatitis: role of CT-guided percutaneous catheter drainage. Abdom Imaging. 2007;32(3):351-61.
|
| 27. |
Seewald S, Ang TL, Teng KCYK, Soehendra N. EUS‐guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21:S61-5.
|
| 28. |
Bhatia M. Acute pancreatitis as a model of SIRS. Front Biosci. 2009;14(1):2042-50.
|
| 29. |
Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42(6):886–91.
|
| 30. |
Munhoz-Filho CH, Batigalia F, Funez HLX. Clinical and therapeutic correlations in patients with slight acute pancreatitis. ABCD Arq Bras Cir Dig (São Paulo). 2015;28(1):24-7.
|
| 31. |
Andrén Sandberg A, Borgström A. Early prediction of severity in acute pancreatitis. Is this possible? JOP. 2002;3(5):116-25.
|
| 32. |
Agarwal N, Pitchumoni CS, Sivaprasad A V. Evaluating tests for acute pancreatitis. Am J Gastroenterol. 1990;85(4):356-66.
|
| 33. |
Kim YS, Lee BS, Kim SH, Seong JK, Jeong HY, Lee HY. Is there correlation between pancreatic enzyme and radiological severity in acute pancreatitis? World J Gastroenterol. 2008;14(15):2401-5.
|
| 34. |
Lee W-S, Huang J-F, Chuang W-L. Outcome assessment in acute pancreatitis patients. Kaohsiung J Med Sci. 2013;29(9):469-77.
|
| 35. |
Sarathi Patra P, Das K, Bhattacharyya A, Ray S, Hembram J, Sanyal S, et al. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg. 2014;101(13):1721-8.
|
| 36. |
Manrai M, Kochhar R, Gupta V, Yadav TD, Dhaka N, Kalra N, et al. Outcome of acute pancreatic and peripancreatic collections occurring in patients with acute pancreatitis. Ann Surg. 2018;267(2):357-63.
|
| 37. |
Van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ali UA, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141(4):1254-63.
|
| 38. |
Smoczyński M, Marek I, Dubowik M, Rompa G, Kobiela J, Studniarek M, et al. Endoscopic drainage/debridement of walled-off pancreatic necrosis – single center experience of 112 cases. Pancreatology. 2014;14(2):137-42.
|
| 39. |
Jagielski M, Smoczyński M, Adrych K. Endoscopic treatment of walled-off pancreatic necrosis complicated with pancreaticocolonic fistula. Surg Endosc. 2018;32(3):1572-80.
|
| 40. |
Jagielski M, Smoczyński M, Jabłońska A, Adrych K. The Development of Endoscopic Techniques for Treatment of Walled-Off Pancreatic Necrosis: A Single-Center Experience. Gastroenterol Res Pract. 2018;2018:1-9.
|
| 41. |
Jagielski M, Smoczyński M, Studniarek M, Adrych K. Spontaneous regression of asymptomatic walled-off pancreatic necrosis. Arch Med Sci. 2018;14(1).
|
| 42. |
Jagielski M, Smoczyński M, Jabłońska A, Marek I, Dubowik M, Adrych K. The role of endoscopic ultrasonography in endoscopic debridement of walled-off pancreatic necrosis–a single center experience. Pancreatology. 2015;15(5):503-7.
|
| 43. |
Da Costa DW, Boerma D, Van Santvoort HC, Horvath KD, Werner J, Carter CR, et al. Staged multidisciplinary step‐up management for necrotizing pancreatitis. Br J Surg. 2014;101(1):e65–79.
|