Does the age of patients with hereditary hemochromatosis at the moment of their first diagnosis have an additional effect on the standard echocardiographic parameters?
Abstract
Background
Hereditary haemochromatosis (HH) is an inherited disease in which gene mutation leads to excessive iron absorption and accumulation in different organs, including the heart, which causes damage. Whether the age of patients with HH at the moment of their first diagnosis has an additional effect on the standard echocardiographic parameters was the aim of the study.
Material and methods
We prospectively enrolled 20 HH patients, and 20 healthy age- and sex-matched volunteers. Analysis of standard echocardiographic parameters was performed and compared in subgroups of ≥50 and <50 years old (yo).
Results
Comparing HH patients with healthy volunteers in ≥50 yo subgroup, significant differences were found in parameters regarding diastolic function (IVS thickness, LVM index, Em, E/Em, PV S/D, LAA index and LAV index). In the <50 yo subgroup we did not find the abovementioned differences, however LVEF appeared to be lower in the HH patients.
Conclusions
Despite the lack of clinical symptoms of cardiovascular disease and the lack of deviations in the standard echocardiographic examination, there were a number of differences regarding LV diastolic function parameters in HH patients ≥50 yo, whereas differences regarding LV systolic function were more prominent in HH patients <50 yo when compared with healthy subjects.
Citation
Rozwadowska K, Daniłowicz-Szymanowicz L, Fijałkowski M, Sikorska K, Szymanowicz W, Lewicka E, Raczak G. Does the age of patients with hereditary hemochromatosis at the moment of their first diagnosis have an additional effect on the standard echocardiographic parameters?. Eur J Transl Clin Med. 2018;1(1):20-25Introduction
Hereditary haemochromatosis (HH) is one of the most common inherited metabolic diseases among Caucasians. In over 80% of cases, it is associated with homozygous mutations in the C282Y HFE gene and occasionally with mutations in other genes, whose products are involved in the regulation of iron turnover in the human body [1]. Dysfunction of molecules that control iron homeostasis leads to excessive iron absorption. As there is no regulatory mechanism for iron excretion from the human body, iron is deposited in many organs. Bioactive iron ions produce oxidative stress that destroys involved tissues. Cardiomyocytes, due to intense iron intake, are very susceptible to this oxidative stress-induced damage. The late symptom of the disease is congestive heart failure, which is responsible for approximately 1/3 of deaths from the natural course of hemochromatosis [1]. Genetic testing allows diagnosis at an early stage and the start of treatment early enough to inhibit the structural changes in organs, including the heart [1-2]. Literature describes a phenomenon of late heart damage in terms of both diastolic and systolic function in HH [2-4]. However, from a clinical point of view the group of patients with newly diagnosed HH, who have not presented any cardiac symptoms, seems to be very interesting, firstly – because of limited literature considering this group and secondly – because of possibility of early treatment introduction in the preclinical stage of the disease [5].
Aim
The aim of this study, is to assess whether the age of patients at the moment of HH diagnosis has an additional effect on the morphology and function of the heart analysed in standard echocardiography.
Materials and methods
Study group
We enrolled 20 patients who were diagnosed with HH <3 months ago but before the start of any HH-specific treatment. All patients had a clinical diagnosis of HH made based on the following criteria: clinical characteristics of the patients, abnormal iron turnover parameters and the presence of HFE gene mutation [6]. Additional criteria included: age ≥18 years, the lack of clinical symptoms of any cardiovascular diseases, and the lack of medical history of heart diseases, high blood pressure and diabetes. Twenty healthy age- and sex-matched volunteers constituted the control group.
Laboratory analysis
At the time of first visit, all HH patients had blood drawn and their levels of serum iron and ferritin level, transferrin saturation (TSAT), hemoglobin and glucose levels were measured.
Conventional Echocardiography
The patients were examined in the left lateral decubitus position using a GE VIVID E9 ultrasound system (GE Ultrasound, Horten, Norway) equipped with phased-array transducer (M5S). Standard echocardiographic parameters were obtained according to the principles described in the ASE/EACVI recommendations [7]. Data acquisitions were obtained from parasternal long- and short-axis views and the three standard apical views. For each view, three consecutive cardiac cycles were recorded during quiet respiration. Grayscale recordings were optimized for LV evaluation at a rate of 50- 80 frames/s and only patients with these parameters were included in the further analyses. All echocardiograms were stored digitally and further offline analysis was performed using an EchoPAC workstation (v201, GE Healthcare Horten, Norway).
Analysis of 2D and Doppler parameters
Left atrial diameters (LADs), LV end-diastolic diameters (LVEDD), LV end-systolic diameter (LVESD), interventricular septal (IVS) and posterior wall (PW) thickness were measured in the parasternal view. The relative wall thickness (RWT) was calculated as the sum of anteroseptal and posterior wall thickness divided by the LV end-diastolic dimension. LVMI was calculated according to the ASE/EACVI recommendations [7-8]. The LV volumes and LVEF were measured using the biplane Simpson’s rule. The mitral inflow velocity was obtained from the apical 4-chamber view by placing a pulsed-wave Doppler sample volume between mitral leaflet tips during diastole. The peak early (E) and late (A) transmitral flow velocities and deceleration time of E velocity (DT) were measured; the ratio of early-to-late peak velocities (E/A) was calculated. Tissue doppler imaging (TDI) was performed to measure mitral annulus excursion: pulsed wave sample volume was placed at the lateral and septal corner of the mitral valve annulus, early diastolic (Em) myocardial peak velocity was recorded and averaged from both positions, the E/Em ratio then was calculated. Systolic (S) and diastolic (D) waves and S/D ration were calculated using pulsed wave Doppler from pulmonary venous (PV) inflow. All parameters were then compared between the groups of ≥50 and <50 years of age. The study protocol was approved by the local Ethics Committee, and written informed consent was obtained from all participants.
Statistical analysis
Continuous data are presented as the median (25th– 75th percentile), while categorical data are expressed in proportions. We performed the Shapiro-Wilk test to check whether our data were normally distributed. The majority of the analysed parameters did not have a normal distribution, even after logarithmic data transformation, thus we selected appropriate statistical analysis methods based on non-parametric tests. The presentation of the continuous data was also caused by the lack of a normal distribution for most of the analysed parameters. Comparisons between groups were performed with the Mann–Whitney U test for independent continuous data and the Pearson’s chi-square test was applied for categorical data. P-values <0.05 were considered significant. The statistical analysis was conducted with the STATISTICA 9.0 (StatSoft, Tulsa OK, USA) package and R 2.25.2 environment.
Results
The HH patients’ genetic characteristics were as follows: 18 persons - C282Y/C282Y genotype, 1 persons - C282Y/H63D genotype, 1 person - C282Y/wt genotype. Table 1 shows the iron turnover biochemical results, as well as haemoglobin and glycaemia level at the start of the study.
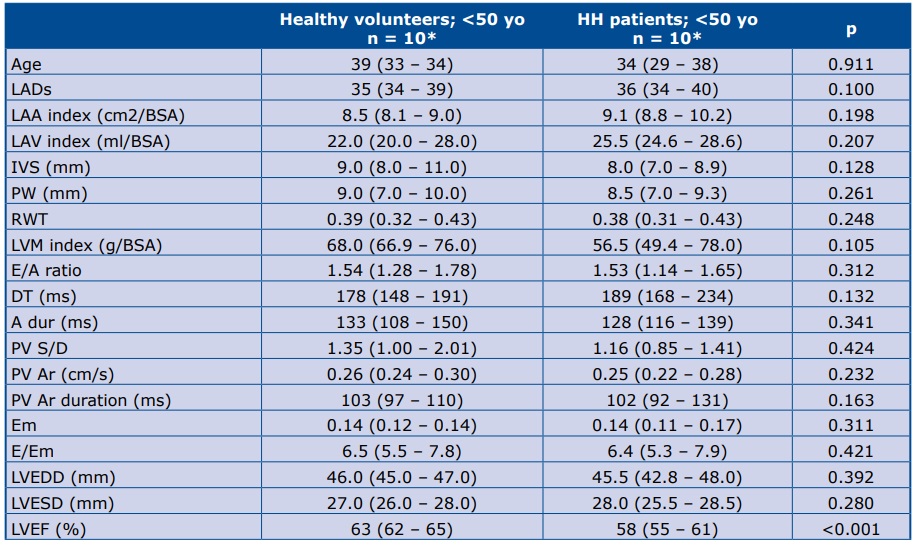
The echocardiographic parameters of all patients were in the normal range [7-8]. Comparing HH patients with healthy volunteers ≥50 years of age, significant differences were found in diastolic function parameters: IVS thickness, LVM index, Em, E/Em, PV S/D, LAA index and LAV index (Table 2).
Table 1. HH patients’ laboratory characteristics at the time of first contact
*Data are presented as the median (25th – 75th percentile); TSAT – transferrin saturation
Table 2. Comparison of echocardiographic parameters between HH patients and healthy volunteers ≥50 yo
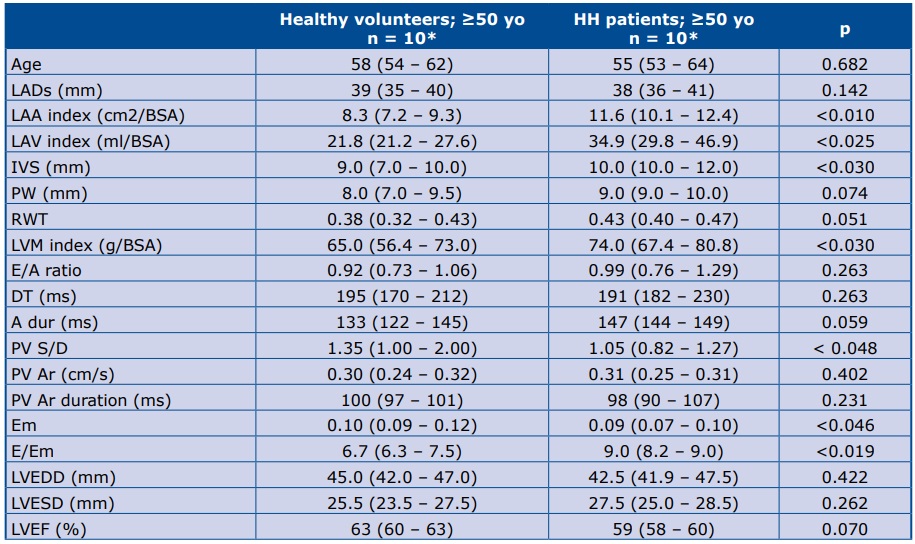
*Data are presented as the median (25th – 75th percentile) LADs – left atrial diameter; BSA – body surface area; LAA index – left atrium area/BSA; LAV index – left atrium volume/BSA; IVS – intraventricular septum (mm); PW – posterior wall; RWT – relative wall thickness; LVM index – left ventricle mass/BSA; E – early mitral velocity; A – late mitral velocity; E/A – E to A ratio; DT – deceleration time of E velocity; A dur – mitral A wave duration time; PV Ar – pulmonary vein A- wave velocity; PV Ar duration – pulmonary vein A-wave duration; Em – peak mitral annulus velocity; E/Em – early mitral inflow velocity to peak mitral annulus velocity ratio; LVEDD – left ventricle end diastolic diameter; LVESD – left ventricle end systolic diameter; LVEF – left ventricular ejection fraction
In the younger subgroups we did not find the above-mentioned differences, however LVEF appeared to be lower in the younger HH subgroup than in the healthy volunteers <50 years of age (Table 3).
Table 3. Comparison of echocardiographic parameters between HH patients and healthy volunteers <50 yo
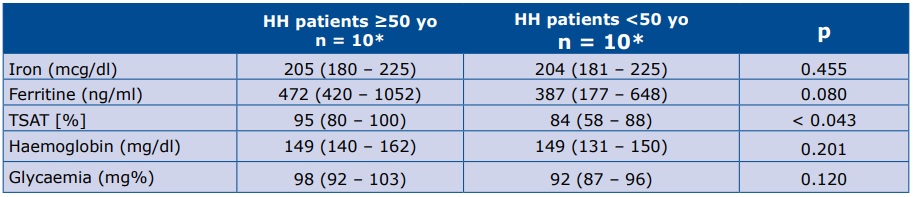
*Data are presented as the median (25th – 75th percentile) LADs – left atrial diameters; BSA – body surface area; LAA index – left atrium area/BSA; LAV index – left atrium volume/BSA; IVS – intraventricular septum (mm); PW – posterior wall; RWT – relative wall thickness; LVM index – left ventricle mass/BSA; E – early mitral velocity; A – late mitral velocity; E/A – E to A ratio; DT – deceleration time of E velocity; A dur – mitral A wave duration time; PV Ar – pulmonary vein A- wave velocity; PV Ar duration – pulmonary vein A-wave duration; Em – peak mitral annulus velocity; E/Em – early mitral inflow velocity to peak mitral annulus velocity ratio; LVEDD – left ventricle end diastolic diameter; LVESD – left ventricle end systolic diameter; LVEF – left ventricular ejection fraction
Discussion
Despite the lack of clinical signs of any heart disease, as well as the lack of any abnormalities in the standard echocardiographic examination, we found a number of differences between patients with newly diagnosed HH compared with healthy subjects. Previous studies focused mainly on patients with long-lasting and long-treated HH [2-4]. In our study we enrolled the patients with newly diagnosed HH, but unlike other authors we analysed echocardiographic parameters with special consideration of patient’s age [5]. We found that differences regarding LV diastolic function parameters were more apparent in HH patients ≥50 years of age, whereas differences in LV systolic function were more prominent in HH patients <50 years of age when compared with healthy subjects.
Age is an independent factor affecting cardiac structure and function [1, 9-16]. Referring to the Framingham and Baltimore studies, Dai et al. noted that LV wall thickness increased with age, resulting in more frequent presence of myocardial hypertrophy in older people, regardless of the prevalence of hypertension. In addition, advancing age increases the left ventricular filling pressure and, as a result, its diastolic dysfunction [9]. Moreover, enlargement of the left atrium is considered as a symptom of increased left ventricular filling pressure and is a sensitive indicator of the severity and duration of diastolic dysfunction [16].
In our study, we set an age limit of 50 years. There are publications where different cut-offs have been set, often closer to 60 years of age. For example, in a study dedicated to the echocardiographic assessment of left ventricular diastolic function, Nagueh et al. adopted age groups of 40-60 and >60 years of age [8]. Similarly, Dai et al. analysed the aging process in the heart [9]. However, in a study dedicated to diastolic dysfunction of the heart Kane et al. set the age groups at 45-64 and >65 years of age [14]. The division of age groups by every 10 years can be found in the literature, especially in large population studies [10-11, 15]. On the other hand, in a publication of the European Study Group on Diastolic Heart Failure, it was assumed that 50 years was the age limit for major changes in left ventricular diastolic function [17]. Loffredo et al. reported that 1% of people >50 years of age have heart failure of varying aetiology [18], which confirms the legitimacy of this age limit. An additional argument for us to select the age limit of 50, is the fact that in the current era of genetic testing HH is diagnosed in a population of relatively young people. When conducting separate analyses of the ≥50 and <50 years of age subgroups in our study, it was noted that the differences in diastolic parameters were present only in the older group of patients. Palka et al. [19] found significantly higher left atrium and left ventricle mass indexes in a similar age subgroup of HH patients, however, some patients included in their study were under long-term treatment by venesection.
Our results, suggesting worse left ventricle diastolic function in HH patients ≥50 years of age, may be explained by the influence of HH on that function, as well as the intensification of hypertrophy of the heart muscle, both of which are more advanced than they would be due to age alone. The results may even indirectly indicate a faster “heart aging” process in people with HH. Data from other studies support this hypothesis [20-21]. Using a mouse model of HH, Djemai et al. suggested a correlation between cardiomyopathy and cardiac iron deposition with aging in mice homozygous for the C282Y HFE gene [20]. Similarly, using another HH mouse model, Sukumaran et al. demonstrated that cardiac iron loading can accelerate the natural aging process of the heart, especially cardiac hypertrophy and fibrosis and potentially heart failure [21].
The above-mentioned differences in diastolic parameters were not described in the younger subgroups, but LVEF was significantly lower in HH patients. This could be explained by the greater influence of HH on systolic function in younger patients. However the small sample size in each of the age subgroups necessitates further verification of this hypothesis.
Study limitations
An important limitation of this study is its small sample size. However this is because we inclulded only patients with an early clinical diagnosis of HH but without any cardiovascular symptoms. Although our sample is relatively small, it is similar to that in previous studies on patients with early diagnosed HH. Due to the small size of the HH group, it was not possible to perform a more advanced statistical analysis.
Conclusion
The HH patients in our study group lacked clinical symptoms of cardiovascular disease and had normal findings in the standard echocardiographic examination, however when compared with healthy subjects they had a number of abnormalities in 2D and Doppler parameters. Specifically, the HH patients <50 years of age had abnormal LV systolic function, whereas LV diastolic dysfunction was more prominent in HH patients ≥50 years of age. Our findings require further corroboration on a larger patient sample and with the use of more advanced diagnostic techniques.
References
| 1. |
Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac Involvement in Hemochromatosis. Cardiol Rev. 2014;22(2):56–68.
|
| 2. |
Candell-Riera J, Lu L, Serés L, González JB, Batlle J, Permanyer-Miralda G, et al. Cardiac hemochromatosis: Beneficial effects of iron removal therapy. Am J Cardiol. 1983;52(7):824–9.
|
| 3. |
Davidsen ES, Hervig T, Omvik P, Gerdts E. Left ventricular long-axis function in treated haemochromatosis. Int J Cardiovasc Imaging. 2009;25(3):237–47.
|
| 4. |
Davidsen ES, Omvik P, Hervig T, Gerdts E. Left ventricular diastolic function in patients with treated haemochromatosis. Scand Cardiovasc J. 2009;43(1):32–8.
|
| 5. |
Shizukuda Y, Bolan CD, Tripodi DJ, Yau Y-Y, Smith KP, Sachdev V, et al. Left Ventricular Systolic Function During Stress Echocardiography Exercise in Subjects With Asymptomatic Hereditary Hemochromatosis. Am J Cardiol. 2006;98(5):694–8.
|
| 6. |
European Association for the Study of the Liver. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol. 2010;53(1):3–22.
|
| 7. |
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14.
|
| 8. |
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314.
|
| 9. |
Dai D-F, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac Aging: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid Redox Signal. 2012 Jun 15;16(12):1492–526.
|
| 10. |
Daimon M, Watanabe H, Abe Y, Hirata K, Hozumi T, Ishii K, et al. Gender Differences in Age-Related Changes in Left and Right Ventricular Geometries and Functions. Circ J. 2011;75(12):2840–6.
|
| 11. |
De Sutter J, De Backer J, Van de Veire N, Velghe A, De Buyzere M, Gillebert TC. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E′) and the ratio of transmitral early peak velocity to E′ (E/E′). Am J Cardiol. 2005;95(8):1020–3.
|
| 12. |
Hees PS, Fleg JL, Dong S-J, Shapiro EP. MRI and echocardiographic assessment of the diastolic dysfunction of normal aging: altered LV pressure decline or load? Am J Physiol Circ Physiol. 2004;286(2):H782–8.
|
| 13. |
Hung C-L, Gonçalves A, Shah AM, Cheng S, Kitzman D, Solomon SD. Age- and Sex-Related Influences on Left Ventricular Mechanics in Elderly Individuals Free of Prevalent Heart FailureCLINICAL PERSPECTIVE. Circ Cardiovasc Imaging. 2017;10(1):e004510.
|
| 14. |
Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, et al. Progression of Left Ventricular Diastolic Dysfunction and Risk of Heart Failure. JAMA. 2011;306(8):856–63.
|
| 15. |
Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, et al. Age- and Gender-Specific Changes in the Left Ventricular Relaxation: A Doppler Echocardiographic Study in Healthy Individuals. Circ Cardiovasc Imaging. 2009;2(1):41–6.
|
| 16. |
Tsang TS., Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90(12):1284–9.
|
| 17. |
Failure ESG on DH. How to diagnose diastolic heart failure. Eur Heart J. 1998;19(7):990–1003.
|
| 18. |
Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart Failure With Preserved Ejection Fraction: Molecular Pathways of the Aging Myocardium. Circ Res. 2014;115(1):97–107.
|
| 19. |
Palka P, Macdonald G, Lange A, Burstow DJ. The role of Doppler left ventricular filling indexes and Doppler tissue echocardiography in the assessment of cardiac involvement in hereditary hemochromatosis. J Am Soc Echocardiogr. 2002;15(9):884–90.
|
| 20. |
Djemai H, Thomasson R, Trzaskus Y, Mougenot N, Meziani A, Toussaint J-F, et al. A Mouse Model of Cardiomyopathy Induced by Mutations in the Hemochromatosis HFE Gene. Can J Cardiol. 2017;33(7):904–10.
|
| 21. |
Sukumaran A, Chang J, Han M, Mintri S, Khaw B-A, Kim J. Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci Rep. 2017;7(1):5756.
|









