Anthropometric measurements, nutritional status and body composition in children with cystic fibrosis – the prospective study
Abstract
Background
Cystic fibrosis(CF),despite much progress in therapy, remains the disease which affects nutrition. Nutrition is an important prognostic factor of the outcome of the disease. We want to evaluate physical development, nutrition and body composition in CF children.
Material and methods
75 children diagnosed with CF (9 months to 18 years old) were included into the study. 33 healthy children (9 months to 18 years old) constituted the control group. The study consisted of 2 stages. In the first the differences between groups were investigated. The second, took place a year later. At each time point the following measurements were performed: height, body mass, skin fold, arm circumference; BMI, FFM%, FM% and Frisancho index. FFM (fat free mass), FM (fat mass), muscle mass, TBW (total body water) were evaluated by mans of BIA(bioimpedance).
ResultsCF children were shorter than healthy children. Stunting affected 18,67% of CF patients at first examination and 21,6% a year later. Underweight was diagnosed in 28% of patients at the beginning and in 41.2% a year after. Underweight was the result of both little FM and scarce muscle mass.
Conclusions
Many children with cystic fibrosis suffers from short stature and underweight, which progresses within time. FFM decreases with the disease progress.
Citation
Kaźmierska K N, Lemanowicz-Kustra A, Jankowska A, Szlagatys-Sidorkiewicz A, Sapiejka E. Anthropometric measurements, nutritional status and body composition in children with cystic fibrosis – the prospective study. Eur J Transl Clin Med. 2020;3(1):34-42Introduction
Cystic fibrosis (CF) is caused by mutation of the chloride channel CFTR (Cystic fibrosis transmembrane conductance regulator) gene. This mutation is responsible for morphological changes which result in the accumulation of secretions in the external ducts and subsequent malfunction of exocrine organs of the digestive and respiratory system [1]. Patients with CF are prone to malnutrition due to malabsorption and increased energy expenditure
Treatment of CF is complex and should be interdisciplinary as it involves the pulmonary disease and its complications, pancreatic enzyme supplementation, nutritional treatment and physical therapy. Nutritional status is one of the prognostic factors of disease outcome [1]. It is well-known that during the course of cystic fibrosis unfavourable changes in body composition take place. One of them is depletion of muscle mass, which results in impairment of lung function, aggravated coughing and worse results in spirometry. That is why we aimed to evaluate prospectively assess anthropometric parameters and body composition in children suffering from cystic fibrosis.
Methods
Our study included 75 children with cystic fibrosis (33 girls and 42 boys) who were 9 months to 18 years of age (mean 8,7 years ± 5,3). Nine of them (4 girls, 5 boys) were < 2 years old, 14 patients (5 girls, 9 boys) were 2-5 years old, 35 (15 girls, 20 boys) were 6-14 years old and 17 patients (9 girls, 8 boys) were > 15years old. The 23 patients (30,6%) were diagnosed via neonatal screening. The CF diagnosis was confirmed via molecular examination of the CFTR gene. based Pancreatic exocrine insufficiency was diagnosed via stool elastase-1 activity in 65 patients (86,7%). None of the patients underwent nutritional intervention. All of the CF patients had oral nutrition in accordance with the applicable pediatric CF recommendations [2].
Lung function (FEV1 , FVC and FEF25-75) was assessed using a MES Lungtest 1000 spirometer (Kraków, Poland), calibrated according to the manufacturer's instructions. All of the study participants were in stable condition without respiratory exacerbation within the last 4 weeks and without signs of oedema or dehydration.
We conducted a two-step prospective study. Measurements were performed on the day of enrolment into the study and 12 months later. The number of patients in both stages of the study varied due to resignation from participation (several patients), absence on the follow-up visit and death (1 patient). At both time points, the patients were assessed using anthropometric measurements (body weight, height, BMI, arm circumference, triceps and subscapular skinfold) and bioimpedance analysis (BIA) of body composition (FFM, FM, Muscle Mass, TBW).
Height and length were measured with a Seca anthropometer (Hamburg, Germany) (> 18 months) and infantometer Seca (Hamburg, Germany) (< 18 months), with accuracy 0,5 cm. Body weight was assessed using a Radwag WPT 60/500W medical scale (Radom, Poland), with accuracy 100g (> 18 months) and 10g (< 18 months). Measurements were performed according to technique described by Martin and Seller [3]. The results were adjusted to body mass and height percentile charts designated for the Polish population [4]. Underweight was diagnosed when body mass was below 10th percentile and severe underweight was defined as below 3rd percentile. Height and length below 3rd percentile classified as short stature. Skinfold was measured with calliper with accuracy of 1 mm. The arm circumference was taken with a medical tape (accuracy of 0,1 cm). Nutritional status was estimated on the basis of BMI percentile charts published by the WHO for children and adolescents (birth-19 years of age) [5-6]. Underweight was diagnosed for BMI below the 15th percentile whereas severe underweight when it was below 3rd percentile. Fat mass was calculated via the Slaughter equation, using skinfold value [7]. Muscle mass was estimated by means of Frisancho index [8]
arm circumference without fat mass = [arm circumference - ( π x arm skinfold )]
Electric bioimpedance analysis (Maltron BIO SCAN 920-2, Rayleigh, U.K.) was used to assess fat free mas (FFM), total body water (TBW), intracellular water (ICW), extra cellular water (ECW), muscle mass (MM) and bone mass (BM). The measurement was accurate to 0,1 kg for muscle mass, fat mass and fat free mass and 1% of their percentage content. All BIA measurements were obtained using tetra polar system, i.e. four self-adhesive electrodes. The data were obtained during a pediatric examination, with patients wearing only underwear. To minimize testing errors, the patients were in a supine position for 5-10 minutes before the test, with limb loose at an angle of 30°-45° in the long axis to the torso [9].
The reference group consisted of 33 healthy children (14 girls and 19 boys) age 9 months-18 years (mean age 116 ± 65 months, median 137 months). The children in this group had no chronic or acute diseases and were hospitalized due foreign body in the digestive tract or planned corrective orthopaedic plastic surgery. The study protocol was approved by the local Bioethics Committee.
Results
There were significant differences in anthropometric measurements between children with CF and healthy ones. In accordance with the Polish percentile charts, underweight was diagnosed in 28% of the study group and severe underweight (< 3pc) affected 17.33% of these children [4] [see Graph1]. Short stature (< 3pc) was diagnosed in 18.7% of the patients [see Graph 2].
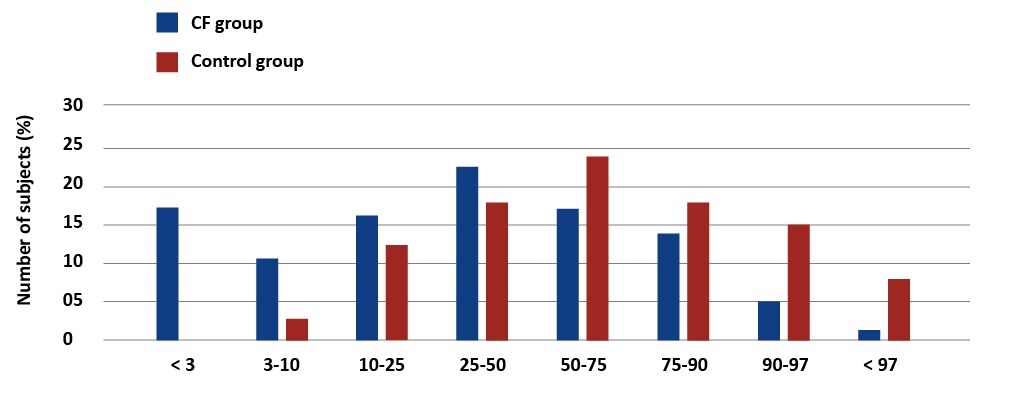
Graph 1. Percentage distribution of body weight values in children with CF and the reference group. Palczewska, Niedźwiedzka (IMiDz) 1999
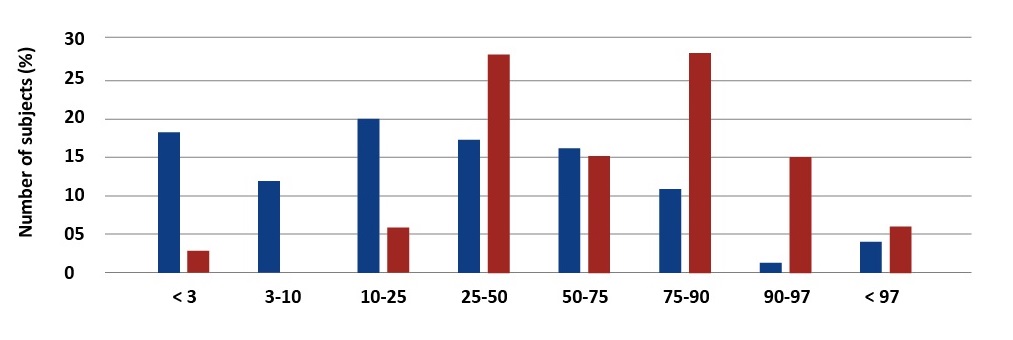
Graph 2. Percentage distribution of body weight values in children with CF and the reference group. Palczewska, Niedźwiedzka (IMiDz) 1999
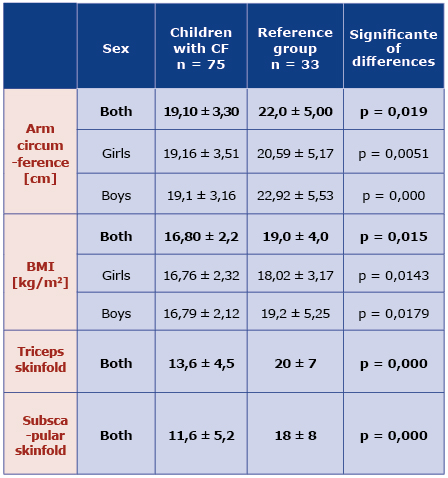
Children with CF had significantly smaller arm circumference than healthy children, for both girls and boys (p = 0.019) [Table 1]. Triceps skinfold in the CF group was in the range between 4.0 mm and 25 mm (mean 13,6 mm ± 4,5) and the mean value was significantly lower than in the reference group (p = 0.000). The sub scapular skinfold in the study group was 12.55-29 cm (mean 11.6 ± 5.2 cm) and was also smaller than in the control group [Table 1].
Table 1. Mean BMI and anthropometric features in the CF and reference groups
Based on the WHO standards, 20% (15) of patients with CF had BMI < 15th percentile and 8% (8 patients) were severely underweight (< 3 percentile) [Graph 3].
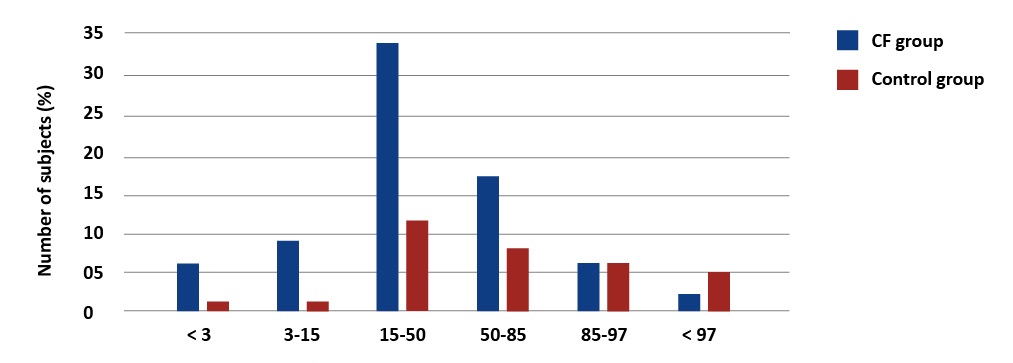
Graph 3. Percentage distribution of body weight values in children with CF and the reference group. WHO BMI Percentile Charts
The results analysis showed that mean content of fat tissue in children with CF was lower than in healthy ones. Fat free mass was estimated indirectly (FFM = 100% - FM). Mean percentage of fat mass (FM%) was higher in the study group compared to the control group (p = 0.000). Fat free mass was also evaluated on the basis of arm circumference by means of Frisancho equation [8]. There was difference between the two groups (p = 0.0222). Neither the mean FFM% nor mean FM% in the study group was different from the control group. Girls with CF had higher mean fat content than boys with CF (p = 0.006). There was tendency to lower TBW in children with CF in comparison with healthy children (p = 0.008) together with lower ICW (p = 0.049) [Table 2].
Table 2. Comparison of body composition parameters obtained using electrical bioimpedance in the CF and reference groups.
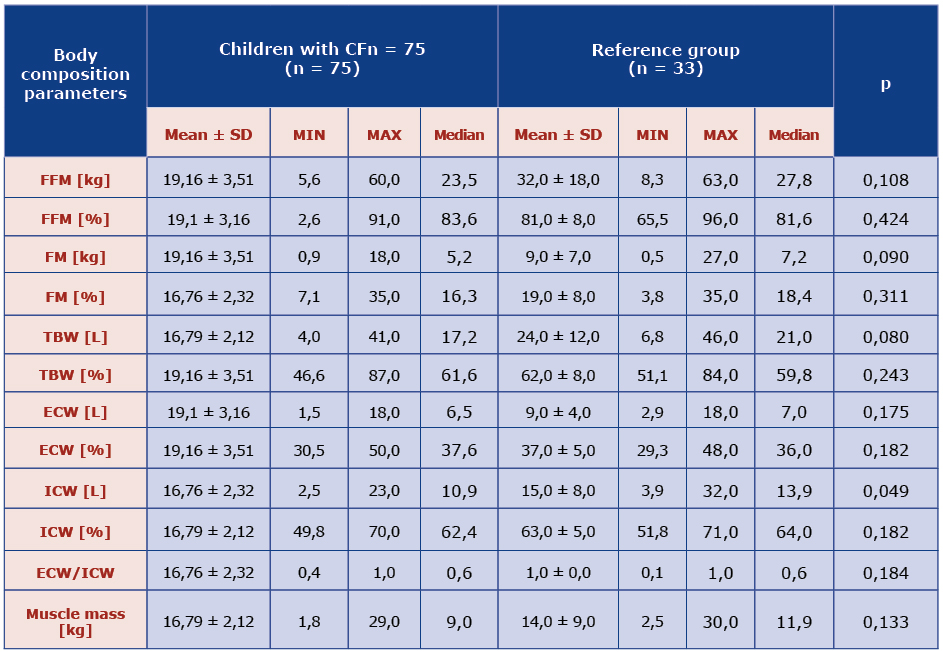
There was a positive relationship between anthropometric and bioimpedance values of FM% (p = 0,12; R = 0,288) and FFM% (p = 0,59; R = 0,219), whereas the Frisancho index was significantly correlated to muscle mass (compatibility 82%) (p = 0,00; R 0,817).
After a year of follow-up, the mean body mass in the study group increased to 34.8 ± 13.7 kg. Underweight was diagnosed in 41.2% children and 10 children (19.2%) were severely underweight (< 3rd percentile) [Table 3]. Thus, there were more underweight children than 12 months earlier, an increase by 11% (3rd-10th percentile). At the same time, the number of children with body mass > 50 percentile decreased.
There was significant difference in height as well. After a year, the mean height was 140.5 ± 24.5cm and there were 3% more stunted children (height < 3rd percentile) than 12 months earlier [Table 3]. However, there were more children whose height ranged between 25th and 50th percentile.
Table 3. Comparison of mean body components and somatic features in CF children obtained on the day of the initial examination and after one year.
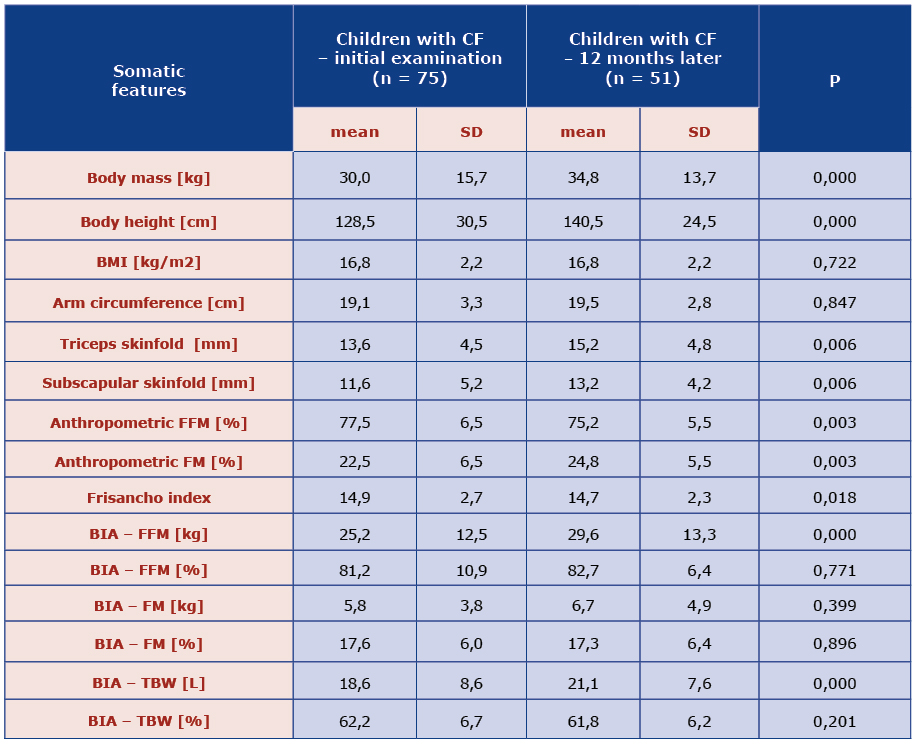
The mean subscapular and triceps skinfolds were thicker after a year: 15.2 ± 4.8 mm and 13.2 ± 4.2 mm respectively [Table 3]. According to the WHO standards, underweight affected 33.3% children suffering from CF, 13.7% were severely underweight. Anthropometric measurements revealed that children form study group had significantly more fat mass content (p = 0.003) and less fat free mass after a year of follow-up. Similarly, in the BIA there was also more FM (kg) and less FFM (kg). The differences in the Frisancho index revealed statistically significant reduction of muscle mass after a year (p = 0,018) [Table 3].
It was observed that children with a lower body mass percentile have worse spirometry results (FEV1 , FVC, FEF25-75) [Table 4]. In addition, no relationship was observed between the remaining somatic features and lung function.
Table 4. Comparison of lung function with somatic features and body composition parameters obtained by the method of anthropometry and electrical bioimpedance in children with CF.
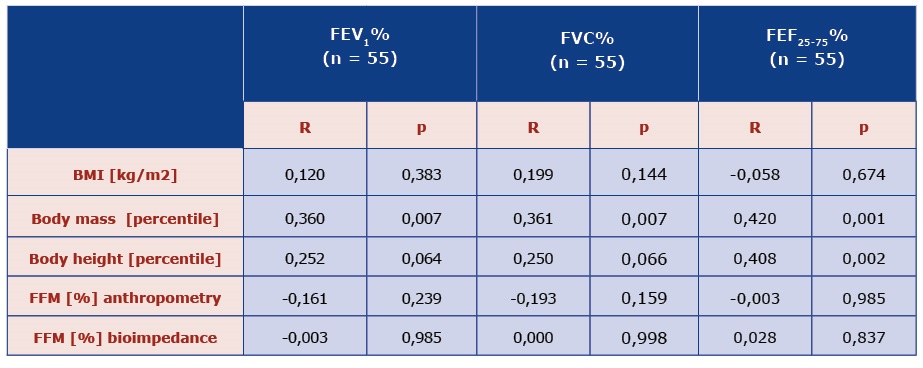
BMI – body mass index, FEV1 – forced expiratory volume in 1 second, FFM - fat free mass, FEF25-75 – forced expiratory flow at 25-75% of vital capacity, FVC – Forced vital capacity
Discussion
Cystic fibrosis is one of the most prevalent autosomal recessive genetic disorders in the Caucasian population [10]. The screening program performed in Poland during 2006-2010 helped to diagnose CF in 221 newborns (out of 1 212 487) and the incidence was estimated 1/4394 in Polish population [11]. Despite significant improvement in diagnosis and treatment of CF, several issues concerning both the disease and its complications need to be solved. For example, there is no gold standard for the assessment of nutritional status in children with CF, despite the fact that it is an important prognostic factor, particularly regarding the condition of the respiratory system. Walkowiak et al. emphasized the necessity of holistic evaluation of physical development [12]. On the other hand Sharma et al. proved that malnutrition was an important prognostic factor for the lifespan [13].
There are only few studies on nutrition in Polish patients with CF. Tutak-Słupska et al. found that 40% of children with CF in Bydgoszcz were undernourished (< 10th percentile) and 24% of them were severely undernourished (< 3rd percentile) [14]. Moreover, 28% were stunned and 16% had height < 3rd percentile. A prospective study performed at the Institute of Mother and Child in Warsaw demonstrated that children suffering from CF had lower BMI, lower arm circumference, lower Frisancho index and scarce fat tissue compared to healthy children [8,15]. The same study group was examined 4 years later and boys had less dynamic physical development (growth and body mass gain) whereas girls slower body mass gain in comparison with reference group [15]. A study from a different part of Poland revealed that stunting and even more frequent wasting are still observed despite nutritional treatment [16]. In our study we found that despite of the increase in mean values of body mass and height, the percentage of children who were underweight (from 28% to 40.6%) and severely undernourished (from 13% to 21,6%) has increased. Short stature affected 21.6% (18.6% preliminary). Our results show that anthropometric measurements (particularly body mass) worsen over time and one may conclude that so does malnourishment.
We know that body composition measurement methods and techniques should not be used interchangeably [17]. Similarly, King et al. compared 76 adults with CF in Australia using anthropometry, BIA and dual energy x-ray absorptiometry (DXA) [18]. Their results indicated a correlation between the findings obtained from various methods, but the there was a significant difference between the values. They observed false results due to CF-specific conditions (e.g. hypernatremia interfering with BIA), as well as improper patient preparation. These authors postulated that BIA should not be the reference method for assessing body composition in adults with CF. Our results also show that there are significant differences in the FM percentage and FFM measurements obtained via anthropometry and BIA and they coincided in only 29% and 22% of the patients respectively. However, when assessing the amount of muscle mass the results were strongly correlated.
There are many cross-sectional studies about the body content of children with CF, however few of them are prospective. Such, it is difficult to compare the results and make conclusions adjustable for whole population of children with CF. These studies used various methods for body content measurement, the groups were different in terms of age and number and ethnicity.
Charatsi et al. found that patients with preserved BMI and weight may have FFM depletion and may be at risk of severe pulmonary disease [19]. Moreover, using DXA Sheikh et al. found lower FFM in patients with CF [20]. Doulgareki et al. showed that children with CF and meconium ileus have lower bone mineral density and lower fat mass in DXA compared to those with CF alone [21]. FFM was lower among children with CF and there was a positive correlation between respiratory efficiency and FFM in a study by Alvares [22]. In another study of 18 participants 12-39 years of age, FFM evaluated by BIA correlated with respiratory function better than BMI [23].
In our study, we observed that mean percentage of FM in children with CF was smaller but their muscle muss content was similar to healthy children. Data from the follow-up examination revealed a significant reduction of FFM% and mean value of Frisancho index as an indicator of % muscle mass in body. This reduction is an alarming phenomenon in children with CF because FFM reflects their clinical condition. It is well-known that muscle mass is the biggest protein and energy source during malnutrition [24]. Since the best indicator of energy expenditure in both health and illness is precisely muscle mass. In 1982 Miller et al. noticed that during chronic inflammatory disease and excessive breathing effort, catabolic processes in muscles increase [25]. Similarly, Umałowska et al. demonstrated that chronic Pseudomonas aeruginosa infection affects nutritional condition and correlates with reduction of both TBM and FFM [26]. In chronic lung disease progressive muscle mass loss due to the increase in resting energy expenditure on one hand, and scarce dietary protein supplementation on the other, is well known. Emphysema results in diminished effectiveness of the respiratory muscles, but malnutrition plays its part too [27].
There are many articles about the relation between FFM and diminished strength of respiratory muscles in patients with CF, which results in impaired function and worse spirometry results as well as diminished coughing reflex [28-31]. However, chronic lung disease as well as numerous infections promote catabolism and as the FFM is reduced the chronic lung disease is aggravated [32]. Meta-analysis by Calella et al. demonstrated that in patients with cystic fibrosis fat free mass and bone mineral density are lower than in healthy children, which leads to lower muscle strength [33].
The treatment of choice in CF which could influence protein metabolism and increase fat free mass is a protein-rich diet, anabolic steroids and physiotherapy [34]. Aerobic exercise increases the life expectancy of children with CF and physical activity increases muscle strength, improves quality of life [35-38] and alleviates airways clearance. Regular FFM assessment is very important because it allows fast introduction of proper therapy if FFM% starts to diminish.
Conclusions
We demonstrated that despite the significant progress in the diagnosis and treatment, underweight and short stature still affect many of patients with CF and progress with time. Deteriorating changes such as fat free mass loss take place in the course of CF. Diagnostic tests of nutritional abnormalities include anthropometry and determination of different body components via bioelectrical impedance. They should be done in early age as that enables faster implementation of nutritional treatment. These measures should be accompanied by physical activity especially in patients with FFM loss.
References
| 1. |
Pencharz PB, Durie PR. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clin Nutr [Internet]. 2000 Dec;19(6):387–94. Available from: https://doi.org/10.1054/clnu.1999.0079.
|
| 2. |
Walkowiak J, Pogorzelski A, Sands D. Zasady rozpoznawania i leczenia mukowiscydozy. [The principles of diagnosing and treatment of cystic fibrosis]. Zalecenia Polskiego Towarzystwa Mukowiscydozy. 2009.
|
| 3. |
Martin R, Saller K. Lehrbuch der Anthropologie, in systematischer Darstellung. Stuttgart: Fischer; 1957.
|
| 4. |
Palczewska I, Niedzwiedzka Z. Wskazniki rozwoju somatycznego dzieci I mlodziezy Warszawskiej. Med Wieku Rozw / Dev Period Med. 2001;5(2 Suppl 1):18–118.
|
| 5. |
Design of the WHO multicentre growth refrence study. Methodology [Internet]. 2015. Available from: https://www.who.int/childgrowth/standards/Chap_2.pdf?ua=.
|
| 6. |
International Pediatric Association Endorsement The New WHO Growth Standards for Infants and Young Children [Internet]. 2006. Available from: https://www.who.int/childgrowth/Endorsement_IPA.pdf.
|
| 7. |
Slaughter MH, Lohman TG, Boileau R, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol [Internet]. 1988;60(5):709–23. Available from: https://www.jstor.org/stable/41464064?seq=1.
|
| 8. |
Frisancho AR. Triceps skin fold and upper arm muscle size norms for assessment of nutritional status. Am J Clin Nutr [Internet]. 1974 Oct 1;27(10):1052–8. Available from: https://doi.org/10.1093/ajcn/27.10.1052.
|
| 9. |
Lewitt A, Mądro E, Krupienicz A. Podstawy teoretyczne i zastosowania analizy impedancji bioelektrycznej (BIA). Endokrynol Otyłość i Zaburzenia Przemiany Mater. 2007;2(4):79–84.
|
| 10. |
McKay KO. Cystic fibrosis: Benefits and clinical outcome. J Inherit Metab Dis [Internet]. 2007 Aug 1;30(4):544–55. Available from: https://doi.org/10.1007/s10545-007-0620-0.
|
| 11. |
Sobczyńska-Tomaszewska A, Ołtarzewski M, Czerska K, Wertheim-Tysarowska K, Sands D, Walkowiak J, et al. Newborn screening for cystic fibrosis: Polish 4 years’ experience with CFTR sequencing strategy. Eur J Hum Genet [Internet]. 2013;21(4):391–6. Available from: https://www.nature.com/articles/ejhg2012180.
|
| 12. |
Walkowiak J, Krawczyński M, Gawęcki J. Nieuchronne niedożywienie [Internet]. Serwis Mukowiscydoza. [cited 2020 Apr 20]. Available from: https://www.imed.pl/index.php?PAGE=telegram&TEL CUR ID=281&return=archives.
|
| 13. |
Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJS, et al. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax [Internet]. 2001 Oct 1;56(10):746 LP – 750. Available from: http://thorax.bmj.com/content/56/10/746.abstract.
|
| 14. |
Tutak-Słupska M, Stępień-Jaszowska B, Staszak-Kowalska R, Krawczyk-Karlińska J, Zając M. Ocena stanu odżywienia i składu ciała pacjentów z mukowiscydozą. Pediatr Pol [Internet]. 2012;87(2):146–53. Available from: https://doi.org/10.1016/S0031-3939(12)70609-1.
|
| 15. |
Walkowiak J. Stan odżywienia i rozwój fizyczny dzieci chorych na mukowiscydozę w świetle podstawowych wskaźników wagowych i wzrostowych. Przegląd Pediatryczny. 1998;28(3):208–12.
|
| 16. |
Szczepanik M, Krawczyński M, Cichy W, Walkowiak J. Rozwój fizyczny dzieci z mukowiscydozą z województwa wielkopolskiego. Ped Prakt. 2000;8:397–410.
|
| 17. |
Beaumesnil M, Chaillou E, Wagner A-C, Rouquette A, Audran M, Giniès J-L. Composition corporelle des patients mucoviscidosiques – comparaison de 3 techniques de mesure : anthropométrie, absorptiométrie biphotonique et impédancemétrie. Arch Pédiatrie [Internet]. 2011 Apr;18(4):370–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0929693X11000273.
|
| 18. |
King S, Wilson J, Kotsimbos T, Bailey M, Nyulasi I. Body composition assessment in adults with cystic fibrosis: comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition [Internet]. 2005 Nov;21(11–12):1087–94. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0899900705002182.
|
| 19. |
Charatsi AM, Dusser P, Freund R, Maruani G, Rossin H, Boulier A, et al. Bioelectrical impedance in young patients with cystic fibrosis: Validation of a specific equation and clinical relevance. J Cyst Fibros [Internet]. 2016;15(6):825–33. Available from: http://www.sciencedirect.com/science/article/pii/S1569199316300534.
|
| 20. |
Sheikh S, Zemel BS, Stallings VA, Rubenstein RC, Kelly A. Body Composition and Pulmonary Function in Cystic Fibrosis [Internet]. Vol. 2, Frontiers in Pediatrics . 2014. p. 33. Available from: https://www.frontiersin.org/article/10.3389/fped.2014.00033.
|
| 21. |
Doulgeraki A, Petrocheilou A, Petrocheilou G, Chrousos G, Doudounakis S-E, Kaditis AG. Body composition and lung function in children with cystic fibrosis and meconium ileus. Eur J Pediatr [Internet]. 2017;176(6):737–43. Available from: https://doi.org/10.1007/s00431-017-2906-z.
|
| 22. |
Alvarez JA, Ziegler TR, Millson EC, Stecenko AA. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition [Internet]. 2016;32(4):447–52. Available from: http://www.sciencedirect.com/science/article/pii/S0899900715004189.
|
| 23. |
Papalexopoulou N, Dassios TG, Lunt A, Bartlett F, Perrin F, Bossley CJ, et al. Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir Med [Internet]. 2018 Sep;142:60–5. Available from: http://www.sciencedirect.com/science/article/pii/S0954611118302531.
|
| 24. |
Cahill Jr GF. Starvation in man. N Engl J Med [Internet]. 1970;282(12):668–75. Available from: https://www.nejm.org/doi/pdf/10.1056/NEJM197003192821209.
|
| 25. |
Miller M, Ward L, Thomas BJ, Cooksley WG, Shepherd RW. Altered body composition and muscle protein degradation in nutritionally growth-retarded children with cystic fibrosis. Am J Clin Nutr [Internet]. 1982 Sep 1;36(3):492–9. Available from: https://doi.org/10.1093/ajcn/36.3.492.
|
| 26. |
Umławska W, Krzyżanowska M, Zielińska A, Sands D. Effect of selected factors associated with the clinical corse of disease on nutritional status in children with cystic fibrosis. Adv Clin Exp Med [Internet]. 2014;23(5):775–83. Available from: http://www.advances.umed.wroc.pl/en/article/2014/23/5/775/.
|
| 27. |
Rothkopf MM, Stanislaus G, Haverstick L, Kvetan V, Askanazi J. Invited Review: Nutritional Support in Respiratory Failure. Nutr Clin Pract. 1989;4(5):166–72.
|
| 28. |
Enright S, Chatham K, Ionescu AA, Unnithan VB, Shale DJ. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J Cyst Fibros [Internet]. 2007;6(6):384–90. Available from: https://doi.org/10.1016/j.jcf.2007.02.006.
|
| 29. |
Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr [Internet]. 1998 Jul 1;68(1):35–41. Available from: https://doi.org/10.1093/ajcn/68.1.35.
|
| 30. |
Mohamed EI, Maiolo C, Iacopino L, Pepe M, Daniele N, Lorenzo A. The Impact of Body-Weight Components on Forced Spirometry in Healthy Italians. Lung [Internet]. 2002 May 27;180(3):149–59. Available from: http://link.springer.com/10.1007/s004080000089.
|
| 31. |
Ionescu AA, Chatham K, Davies CA, Nixon LS, Enright S, Shale DJ. Inspiratory Muscle Function and Body Composition in Cystic Fibrosis. Am J Respir Crit Care Med [Internet]. 1998 Oct;158(4):1271–6. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm.158.4.9710079.
|
| 32. |
Ionescu AA, Nixon LS, Luzio S, Lewis-Jenkins V, Evans WD, Stone MD, et al. Pulmonary Function, Body Composition, and Protein Catabolism in Adults with Cystic Fibrosis. Am J Respir Crit Care Med [Internet]. 2002 Feb 15;165(4):495–500. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm.165.4.2104065.
|
| 33. |
Calella P, Valerio G, Brodlie M, Donini LM, Siervo M. Cystic fibrosis, body composition, and health outcomes: a systematic review. Nutrition [Internet]. 2018 Nov;55–56:131–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0899900718301825.
|
| 34. |
Shepherd R, Cooksley WGE, Cooke WDD. Improved growth and clinical, nutritional, and respiratory changes in response to nutritional therapy in cystic fibrosis. J Pediatr [Internet]. 1980 Sep;97(3):351–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022347680801806.
|
| 35. |
Shei R-J, Mackintosh KA, Peabody Lever JE, McNarry MA, Krick S. Exercise Physiology Across the Lifespan in Cystic Fibrosis. Front Physiol [Internet]. 2019 Nov 5;10:1382. Available from: https://www.frontiersin.org/article/10.3389/fphys.2019.01382/full.
|
| 36. |
Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol [Internet]. 2002 Mar 1;33(3):194–200. Available from: https://doi.org/10.1002/ppul.10015.
|
| 37. |
Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter CR, Kelsey S, et al. Strength vs aerobic training in children with cystic fibrosis. Chest [Internet]. 2004 Oct;126(4):1204–14. Available from: https://doi.org/10.1378/chest.126.4.1204.
|
| 38. |
Klijn PHC, Oudshoorn A, van der Ent CK, van der Net J, Kimpen JL, Helders PJM. Effects of anaerobic training in children with cystic fibrosis: a randomized controlled study. Chest [Internet]. 2004;125(4):1299–305. Available from: http://www.sciencedirect.com/science/article/pii/S0012369215320894.
|













