Abstract
Background
Anatomy assessment using Computer Tomography (CT) and Magnetic Resonance (MRI) is performed in patients undergoing pulmonary vein isolation (PVI). The aim of this analysis was to investigate whether electroanatomical 3D map and CT/MRI image integration using the CartoMerge system improves efficacy, reduces procedure time or fluoroscopy usage.
Materials and methods
57 patients undergoing PVI were divided in two groups: “Merge” (n=45 pts) and “non-Merge” (n=14 pts) depending on usage of image integration. PVI isolation during procedure (acute PVI), procedure time, fluoroscopy time, number of radio frequency (RF) applications and AF recurrence during follow-up (Merge group: 12-24 months, non-Merge group: 9-18 months) were analyzed.
Results
Intra-proceduaral PVI was equal in both groups (93%). Long-term efficacy, defined as absence of AF recurrence, was insignificantly higher in the Merge group (69,8% vs 50%, p=0,11793). Procedure time was significantly longer in the Merge group – 239,1 (±55,5) min. vs 192,4 (±44,5). Fluoroscopy time was similar in both groups 29,9 (±12,23) vs 24,6 (±26,5) min, (p=0,579). Number of RF applications was significantly higher in the Merge group 48,5 (±25,2) vs 27,2 (±14,9).
Conclusions
CartoMerge did not improve the rate of acute PVI, long-term effectivity or fluoroscopy time. In the non-Merge group the procedure time was shorter and the number of applications was significantly smaller.
Citation
Drelich Ł, Królak T, Liżewska-Springer A, Kozłowski D, Kempa M, Raczak G. Usefulness of CartoMerge image integration module in catheter ablation of atrial fibrillation. Eur J Transl Clin Med. 2019;2(1):48-52Introduction
Atrial fibrillation (AF) is the most common arrhythmia, with age-related incidence, reaching 5-15% in among people >80 years old. The increased and irregular heart rate of AF usually causes palpitations, and in more severe cases fainting, syncope or heart failure. Chaotic and uncoordinated electrical activity in the atria during AF reduces their mechanical function. Blood stasis, especially in left atrium appendage, leads to formation of thrombus, a potential embolic material which may cause cerebral stroke.
The pharmacological treatment of AF concentrates on 3 main goals: to reduce the frequency of AF episodes, to control of ventricular rate during arrhythmia and prevent thromboembolism. Limited efficacy of available drugs ant their numerous side-effects determined development of an alternative, invasive method - transcathether cardiac tissue ablation.
In 1997 Haisseguerre demonstrated the relationship between ectopic foci in pulmonary veins and AF [1]. High frequency impulses generated in those areas are the arrhythmia inducing (“trigger”) and quite often sustaining factor (“driver”). Initially, ablation was targeted directly into those areas, but the coexistence of numerous foci and the risk of post-ablative vein stenosis led to the evolution of this procedure [1-5]. Nowadays, the most common method is pulmonary vein isolation (PVI) [6-9], with the objective of creating circular scars surrounding PVs (usually two scars, each surrounding a pair of left or right veins). Scars created during ablation are not able to conduct depolarization waves, thus in consequence the ectopic foci cannot influence LA tissue.
Pulmonary venous anatomy assessment is crucial for performing successful pulmonary vein isolation. Nowadays, electroanatomical systems are commonly used to create spatial maps of the LA and PVs. These tools provide more precise reflection of anatomical relations, increase precision of navigation and radio frequency (RF) application. Literature and clinical practice revealed high proportion of atypical pulmonary venous confluence variants. Imaging techniques like CT or nuclear magnetic resonance (NMR) allow determination of the number, size and the type of ostium of pulmonary vein [10-13]. Carto 3™ system (Biosense-Webster Inc.) used in our center is equipped in additional module – CartoMerge – allowing integration of 3D electroanatomical map with a 3D reconstruction of LA and PVs. The aim of this study was to investigate whether CartoMerge image integration module improves the PVI efficacy, reduces procedure time or fluoroscopy usage.
Materials and methods
The study population consisted of 57 patients undergoing PVI in the years 2012-2014. LA and PVs CT scan was routinely performed before the procedure. In the next step, the scans were imported to the Carto 3D system. Ablation strategy was the same in every patient. Ablation catheter and ring-shape Lasso diagnostic catheter were placed in LA via transseptal puncture, diagnostic electrodes were also placed in His bundle area (His) and in coronary sinus (CS) using transvenous access.
In the next step, electroanatomical 3D map were collected. In case of LA Point-by-Point or FAM technique was used – depending on operator’s preference. After completing the mapping, in the first group of patients 3D map was integrated with the CT scan. Whereas in the second group the 3D CT scan was displayed as a separate picture in the 3D Carto system window. Choice was based on operator’s preference.
After mapping, circumferential PV isolation was performed using an RF ablation catheter. Linear applications surrounding PVs in pairs were delivered – separately around pair of left and right veins. Ablation lines were located ~5-10 mm from the ostia of PVs in order to minimize the risk of post-ablation vein stenosis. Bidirectional conduction block in the ablation line was considered a criterium of electrical isolation. It was verified by pacing on one side of the line (LA and PVs) and signal analysis signals registered by the electrode on the opposite side. In some cases electrical cardioversion (ECV) under general anesthesia was necessary, resulting in prolonged procedure time.
Based on the procedure protocols and study registrations following data was analyzed: PV isolation during procedure (acute PVI), procedure time, fluoroscopy time, the number of RF applications. Moreover, the anatomy of PV ostia was analyzed using the 3D CT reconstructions and the radiologist’s report. 4 veins with separate ostia were defined as typical. Different number of veins or common ostium was defined as atypical. None of the patients presented anomalous pulmonary venous drainage (PV ostium in heart chamber other than LA).
Follow-up visits were scheduled at 3, 6, 9, 12, and 24 months after the procedure. Medical history focusing on AF was collected, standard ECG and 24h Holter ECG were performed. In case of suspicion of AF recurrence, additional visits and investigations were administered.
Patients were divided into “Merge” and “non-Merge” groups depending on the usage of image integration. Follow-up lasted 12-24 months in the Merge group and 9-18 months in the non-Merge group.
Results
The merge group consisted of 43 patients (F=15) with average age of 53 (SD=13). Whereas the non-Merge group consisted of 14 patients (F=2) with average age of 51 (SD=11), p=0,63. The difference in AF type distribution was statistically not significant: 72% of Merge group patients suffered from paroxysomal AF and 57,1% in the non-Merge group (p=0,373). Typical PV anatomy was found in 72% (n=31) of patients in the Merge group and 78,8% in non-Merge group (p=0,8976). Detailed distribution is presented in table 1. Proportion of patients having ECV during PVI was similar in both groups (37,2% vs 35,7%).
Table 1. Distribution of clinical findings among the two study groups

AF – atrial fibrillation, ECV – external cardioversion, PV – pulmonary vein, RMPV - Right Medial Pulmonary Vein
PVI success rate was equal in both groups (93%). The long-term efficacy, defined as absence of AF recurrence, was higher than in the Merge group (69,8% vs 50%), but this difference was statistically insignificant. Procedure time was significantly longer the in Merge group: 239,1 (±55,5) minutes vs 192,4 (±44,5). Fluoroscopy time was similar in both groups 29,9 (±12,23) vs 24,6 (±26,5) min, (p=0,579). The number of RF applications was significantly higher in the Merge group: 48,5 (±25,2) vs 27,2 (±14,9).
Table 2. Characteristics and outcomes of the PVI procedure among the two study groups
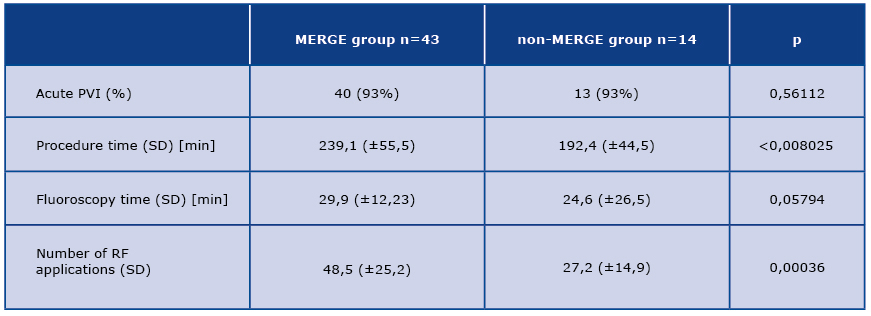
PV – pulmonary vein, RF – radio frequency, SD – standard deviation
Discussion
The CartoMerge module was designed to improve PVI efficacy, reduce number of complications, reduce fluoroscopy time and whole procedure time. First articles describing CT/NMR integration with Carto 3D map during PVI were published in 2005-2007 [14-17].
Bartagella et al. presented data from Carto Merge Italian Registry involving 12 centers [18]. Three different AF ablation strategies were analyzed: SOCA – segmental ostial pulmonary vein isolation (240 patients), CARTO – circumferential PV isolation using Carto 3D navigation (107 patients) and MERGE – CT/NMR scans integrated with 3D map using CartoMerge (226 patients). Total procedure time was significantly shorter in the MERGE group (210.3±63.4 vs. 231.7±70.7 min, p< 0.04), but longer than in the SOCA group (184.9± 58.4 min, P < 0.0001). Fluoroscopy time differences were insignificant (SOCA 56.5±22.5 vs. CARTO 55.0±25.3 vs. MERGE 54.3±25.5 min, p = 0.98). AF recurrence rate was lower in MERGE group (22.6%; P < 0.0001 compared to SOCA (44.6%) and CARTO (41.7%).
Kistler et al. analyzed the impact of CartoMerge on PVI efficacy in a randomized trial comparing navigation using Carto electroanatomical mapping (EAM group, 40 pts.) and CartoMerge (CT group, 40 pts.) incorporating 3D reconstructions from CT scans [19]. There was no significant difference in PVI efficacy during 6 month follow-up (EAM - 56% vs CT – 50%) and long-term follow- up, including patients after Re-Do procedure Do (77% vs 71%). Fluoroscopy and procedure time did not differ significantly. In contrast to the Italian Registry, LA CT scan was performed in both groups and available to the operator, but data was imported to Carto system in CARTO group. Those conclusions suggest, that the radiological PV anatomy assessment is crucial for PVI efficacy, not image integration itself.
In our study in both analyzed groups CT scan was performed and imported to Carto 3D system, so use of image integration was the only difference between both groups. In the non-Merge groups CT reconstruction was displayed as separate picture in Carto 3D system, so data processing was exactly the same in both groups. That allowed us to strictly evaluate the influence of image integration on PVI efficacy.
In our study the long-term efficacy in Merge group was higher, but this difference was statistically insignificant, probably due to the small size of the non-Merge group. Moreover, in this group the incidence of persistent AF was higher (statistically not significant), and that is well-known factor correlating with lower efficacy of PVI.
Conclusions
Compared to LA visualization without integrating the image with Carto 3D map, the Carto Merge module did not improve the rate of acute PVI, long-term effectivity or fluoroscopy time. In the non-Merge group procedure time was shorter and the number of RF applications was significantly smaller.
References
| 1. |
Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95(3):572–6.
|
| 2. |
Haissaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66.
|
| 3. |
Haissaguerre M, Shah DC, Jaïs P, Hocini M, Yamane T, Deisenhofer I, et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;102(20):2463–5.
|
| 4. |
Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102(21):2619–28.
|
| 5. |
Ouyang F, Bänsch D, Ernst S, Schaumann A, Hachiya H, Chen M, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110(15):2090–6.
|
| 6. |
Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–429.
|
| 7. |
Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47.
|
| 8. |
Mantovan R, Verlato R, Calzolari V, Baccillieri S, De Leo A, Turrini P, et al. Comparison between anatomical and integrated approaches to atrial fibrillation ablation: adjunctive role of electrical pulmonary vein disconnection. J Cardiovasc Electrophysiol. 2005;16(12):1293–7.
|
| 9. |
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S-A, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14(4):528–606.
|
| 10. |
Lin W-S, Prakash VS, Tai C-T, Hsieh M-H, Tsai C-F, Yu W-C, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins. Circulation. 2000;101(11):1274–81.
|
| 11. |
Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sánchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001;86(3):265–70.
|
| 12. |
Mansour M, Holmvang G, Sosnovik D, Migrino R, Abbara S, Ruskin J, et al. Assessment of pulmonary vein anatomic variability by magnetic resonance imaging:. implications for catheter ablation techniques for atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15(4):387–93.
|
| 13. |
Wood MA, Wittkamp M, Henry D, Martin R, Nixon J., Shepard RK, et al. A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am J Cardiol. 2004;93(1):49–53.
|
| 14. |
Mikaelian BJ, Malchano ZJ, Neuzil P, Weichet J, Doshi SK, Ruskin JN, et al. Integration of 3-Dimensional Cardiac Computed Tomography Images With Real-Time Electroanatomic Mapping to Guide Catheter Ablation of Atrial Fibrillation. Circulation. 2005;112(2):e35–e36.
|
| 15. |
Kistler PM, Earley MJ, Harris S, Abrams D, Ellis S, Sporton SC, et al. Validation of three-dimensional cardiac image integration: use of integrated CT image into electroanatomic mapping system to perform catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(4):341–8.
|
| 16. |
Dong J, Dickfeld T, Dalal D, Cheema A, Vasemreddy CR, Henrikson CA, et al. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(5):459–66.
|
| 17. |
Kistler PM, Schilling RJ, Rajappan K, Sporton SC. Image integration for atrial fibrillation ablation – pearls and pitfalls. Hear Rhythm. 2007;4(9):1216–21.
|
| 18. |
Bertaglia E, Bella PD, Tondo C, Proclemer A, Bottoni N, De Ponti R, et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMergeTM Italian Registry. Europace. 2009;11(8):1004–10.
|
| 19. |
Kistler PM, Rajappan K, Harris S, Earley MJ, Richmond L, Sporton SC, et al. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. Eur Heart J. 2008;29(24):3029–36.
|








