Abstract
Ivonescimab is an innovative bispecific antibody targeting both programmed cell death protein 1 (PD-1) and vascular endothelial growth factor (VEGF-A). Through its dual mechanism (immunomodulatory and anti-angiogenic) it can effectively enhance the body’s immune response to cancer and inhibit the formation of new blood vessels, thereby slowing tumor growth. Clinical trials have demonstrated high efficacy of ivonescimab in treating nonsmall cell lung cancer (NSCLC). When combined with chemotherapy, ivonescimab significantly prolongs progression-free survival compared to pembrolizumab-based therapy. A higher response rate was also observed, with an acceptable safety profile. Results from the HARMONi-A and HARMONi-2 studies suggest that ivonescimab may become the proposed therapeutic regimen for first-line treatment. Further studies are underway to evaluate the broader potential of ivonescimab, as it may represent a promising option pending approval. The globalization of ivonescimab research increases the likelihood of its registration as the first anti-PD-1/anti-VEGF-A bispecific antibody, thus potentially revolutionizing cancer therapy. This article provides a concise summary of the mechanism of action and key clinical evidence supporting the use of Ivonescimab in NSCLC.
Citation
Ficoń W, Dobosz M, Całyniuk B. Ivonescimab – the future of cancer therapy. Eur J Transl Clin Med. 2025;8(2):81-85Introduction
Ivonescimab (AK112 or SMT112) is the first humanized bispecific anti-programmed cell death protein 1 (anti-PD-1) and anti-vascular endothelial growth factor (anti-VEGF antibody) with a heterotetrameric structure composed of two IgG1 heavy chains and two kappa light chains, linked by disulfide bonds (-S-S-) [1-4]. The PD-1-targeting fragment is attached to the C-terminus of each anti-VEGF heavy chain (Figure 1). Modification of the PD-1 Fc region eliminated complement-dependent cytotoxicity. This drug’s dual activity enables simultaneous immune system activation (by enhancing recognition and destruction of cancer cells) and inhibition of angiogenesis, potentially slowing or halting tumor growth [5]. The approach based on simultaneous targeting of 2 molecular pathways offers the possibility of overcoming the resistance mechanisms typically observed in single-target therapies, providing a more integrated and effective treatment strategy for patients with non-small cell lung cancer (NSCLC) [6]. VEGF-A and PD-1 expression are strongly correlated in the tumor microenvironment. Blocking both mechanisms simultaneously results in synergistic target binding and enhanced antitumor activity with a better safety profile than separate anti-PD-1 and anti-VEGF therapies [7]. Originally developed by the Chinese biotechnology company Akeso Biopharma, ivonescimab was later licensed globally by Summit Therapeutics, paving the way for international development and commercialization [8]. In this paper we present the mechanism of action and key scientific evidence regarding the use of ivonescimab in NSCLC.
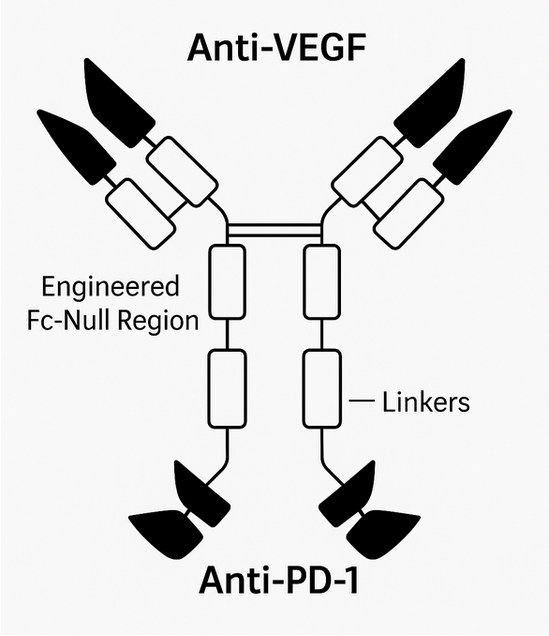
Figure 1. The structure of Ivonescimab
Based on figure published by Zhang et al. in their poster [9]
Material and methods
We searched scientific databases such as PubMed, Google Scholar and ScienceDirect to find articles on ivonescimab, its structure, mechanism of action and clinical trials. We also included information from its manufacturer's (Summit Therapeutics) website and the clinicaltrials.gov database. The inclusion criteria were as follows: full text review or original articles describing clinical trials or randomised controlled trials, published in peer-reviewed journals in 2024 and 2025.
Results
A total of 133 abstracts containing the keyword ‘ivonescimab’ were found as a result of the literature search. After selection, 42 abstracts were included, while 91 were excluded due to lack of open access to the full text, low quality or unclear methodology, and a different subject area than that covered in this review. The full texts of the remaining 42 articles were then searched and reviewed. Ultimately, 10 articles available in scientific databases and 5 articles published on the websites of the drug manufacturer and an international clinical trial database were included in this review.
Clinical trials
Phase 186I, II, and III clinical trials evaluating ivonescimab are currently underway. Phase I trials assess safety and determine the maximum tolerated dose, which is 20 mg/kg every 14 days. Ivonescimab is administered intravenously (IV) every 2 weeks in various concentrations, ranging from 0.3 mg/kg to 30 mg/kg, to patients who have advanced or metastatic solid tumors that are resistant to treatment, have relapsed after standard therapies, and for whom no effective standard therapy is available [1]. The maximum tolerated dose was determined based on drug tolerance and reported adverse events. This dose ensures high PD-1 receptor saturation and a significant reduction in free VEGF. Adverse effects such as skin rash, joint pain, and increased blood pressure (typical for ivonescimab’s mechanism of action) were reported by 27.5% of the trial participants [1]. In phase II trials the researchers evaluate the response to treatment (drug efficacy), selection of the optimal dose and the incidence of adverse effects in the wider population. In phase III trials, on the other hand, the aim is to compare the efficacy of the new drug with the current standard of therapy, while monitoring long-term treatment effects and complications. The results of these trials are key to the decision on marketing authorisation.
Phase II clinical trials
The first phase II trial evaluating ivonescimab was conducted among patients with advanced NSCLC in combination with chemotherapy [10]. All participants had stage III or IV NSCLC and were divided into 3 cohorts. The first included patients with advanced NSCLC who did not have epidermal growth factor receptor (EGFR). Participants in the second group had advanced NSCLC with EGFR-sensitive mutations, but previous EGFR-tyrosine kinase inhibitor (TKI) targeted therapy had failed. The final group consisted of patients with advanced-stage NSCLC who had not received a full course of platinum-based chemotherapy or anti-PD-1 therapy. Adverse effects were reported by all patients, mainly leukopenia (18.1%), neutropenia (16.9%) and thrombocytopenia (12%) [10]. The authors concluded that the combination of chemotherapy with concomitant administration of ivonescimab is promising due to a reduction in tumour volume irrespective of histological nature in the majority of subjects and relatively good tolerability [10].
Another phase II trial evaluated ivonescimab’s efficacy against squamous cell carcinoma (SCC) NSCLC and non-squamous cell carcinoma (non-SCC) NSCLC without EGFR/ALK mutations in combination with chemotherapy. The results of this study were evaluated based on the analyzed endpoints, which were safety and the objective response rate (ORR) in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. The high efficacy of the undertaken treatment was demonstrated irrespective of the NSCLC subtype, thus allowing a wide use of ivonescimab. In addition, adverse effects were observed in 10% of patients, most commonly nasal bleeding, proteinuria and rash, all of which are typical in immune therapy and chemotherapy [9].
Phase III clinical trials
The HARMONi-A phase III study analysed the efficacy and safety of ivonescimab combination therapy with chemotherapy in patients with EGFR-mutated advanced NSCLC who were previously treated with EGFR tyrosine kinase inhibitors (including third-generation EGFR-TKIs). That study involved 322 patients randomly allocated to 2 treatment groups: the first received ivonescimab in combination with chemotherapy (pemetrexed and carboplatin), while the second received placebo with the same chemotherapy regimen. After completion of the full 4 cycles of induction treatment, maintenance therapy was continued with ivonescimab or placebo in combination with pemetrexed. The median disease progression-free survival (PFS) time was 7.1 months in the ivonescimab group, a significant improvement compared to 4.8 months in the placebo group. The ORR was also higher in the ivonescimab group at 50.6% (95% confidence interval (CI), 42.6% - 58.6%), compared to 35.4% (95% CI, 28.0% - 43.3%) in the control group. The most common adverse effects reported in the HARMONi-A trial were related to chemotherapy [11].
The HARMONi-2 study was conducted in 55 hospitals across China. Patients over 18 years of age diagnosed with advanced or metastatic non-small cell lung cancer were divided into two equal groups. The first received 20 mg/kg ivonescimab (IV) every 3 weeks, while the second had 200 mg pembrolizumab administered (IV) every 3 weeks. Adverse effects resulting from ivonescimab did not lead to treatment discontinuation, as was the case in the 5 patients using pembrolizumab. Furthermore, the compared drugs had a similar safety profile (acceptable). The HARMONi-2 study showed that ivonescimab significantly prolongs PFS by approximately 11 months, compared to nearly 6 months in the pembrolizumab group. Ivonescimab also showed a higher response rate of 50% (95% CI 43-57) compared to 39% (95% CI 32-46) for pembrolizumab. Moreover, the therapeutic advantage was evident in different patient subgroups, regardless of PD-L1 expression level, histological type or the presence of liver and brain metastases. Based on the results of the HARMONi-2 study, it can be concluded that ivonescimab is a promising alternative to pembrolizumab for first-line treatment in patients with PD-L1-positive NSCLC, providing more favourable clinical outcomes [12].
Recruitment is currently underway for the HARMONi-3 trial, which aims to compare the use of ivonescimab with chemotherapy and pembrolizumab (also together with chemotherapy) as first-line treatment in patients with metastatic NSCLC of both SCC and non-SCC types [13]. Also, there is an open enrolment in the HARMONi-7 trial to compare the use of ivonescimab monotherapy with pembrolizumab as first-line treatment in patients with metastatic NSCLC with high PD-L1 expression (TPS ≥ 50%) [14].
Limitations and future directions
Despite the promising performance of ivonescimab in clinical trials, it is important to highlight a number of significant facts that limitat the interpretation of the available data. First of all, most of the published articles describe the results of phase I and II studies (focusing mainly on safety, tolerance and preliminary efficacy), resulting in a lack of data from large, international, randomised phase III studies. In addition, the number of participants in the published clinical trials is relatively small, limiting the possibility of generalising the results to a wider patient population. Due to the relatively recent development of ivonescimab, there is no information on the long-term effects of its use. Another significant limitation is the lack of registration and reimbursement of ivonescimab in Poland, which limits its availability outside of clinical trials. Ivonescimab was initially approved for marketing in China in May 2024, and its widespread use in that country began in April 2025. Ivonescimab received Fast Track designation from the US Food & Drug Administration (FDA) for the HARMONi clinical trial [15]. Currently, there are no PD-1-based bispecific antibodies approved by the FDA or the European Medicines Agency (EMA). An important aspect of future studies will also be the evaluation of long-term clinical outcomes such as overall survival, durability of response and quality of life of patients. Translational research should focus on identifying ivonescimab’s mechanisms of action, including the tumor microenvironment and the regulation of the immunomodulatory response, which will contribute to a better understanding of this drug’s clinical efficacy.
Conclusion
Approved for treatment in China, ivonescimab shows promising results in the treatment of advanced non-small cell lung cancer (NSCLC). Clinical trials are a key component in the development of effective cancer therapies. Ongoing trials are evaluating the potential for broader clinical use of ivonescimab, including its effectiveness in combination with other therapies and its prospective for use in other types of cancer. Extending the research to other countries will allow the evaluation of ivonescimab in patient groups other than Chinese and strengthen the credibility of the results obtained so far.
Conflict of interests
The authors declare no financial relationships with any commercial entity that could have influenced the outcomes or interpretations presented in this manuscript and report no conflicts of interest.
Funding
The authors did not receive any external funding for the preparation of this article. The work was carried out independently, without support from public, private or commercial institutions.
References
| 1. |
Frentzas S, Austria Mislang AR, Lemech C, Nagrial A, Underhill C, Wang W, et al. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J Immunother cancer [Internet]. 2024;12(4). Available from: http://www.ncbi.nlm.nih.gov/pubmed/38642937.
|
| 2. |
Zhou C, Chen J, Wu L, Wang L, Liu B, Yao J, et al. PL02.04 Phase 3 Study of Ivonescimab (AK112) vs. Pembrolizumab as First line Treatment for PD-L1-positive Advanced NSCLC: Primary Analysis of HARMONi-2. J Thorac Oncol [Internet]. 2024;19(10):S1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086424008864.
|
| 3. |
Chen Z, Wu L, Wang Q, Yu Y, Liu X, Ma R, et al. Brief Report: Ivonescimab Combined With Etoposide Plus Carboplatin as First Line Treatment for Extensive-Stage SCLC: Results of a Phase 1b Clinical Trial. J Thorac Oncol [Internet]. 2025;20(2):233–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086424024213.
|
| 4. |
Ivonescimab Overview [Internet]. Summit Therapeutics Inc. 2025 [cited 2025 Jul 18]. Available from: https://www.smmttx.com/ivonescimab-smt112/.
|
| 5. |
Wang L, Luo Y, Ren S, Zhang Z, Xiong A, Su C, et al. A Phase 1b Study of Ivonescimab, a Programmed Cell Death Protein-1 and Vascular Endothelial Growth Factor Bispecific Antibody, as First- or Second-Line Therapy for Advanced or Metastatic Immunotherapy Naive NSCLC. J Thorac Oncol [Internet]. 2024;19(3):465–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086423023122.
|
| 6. |
Al Matairi A, Hammadeh BM, Aldalati AY, Qtaishat FA, Nashwan AJ, Alzibdeh A. Efficacy and Safety of Ivonescimab in the Treatment of Advanced Non-small Cell Lung Cancer (NSCLC): A Systematic Review. Cureus [Internet]. 2025;17(1):e77381. Available from: http://www.ncbi.nlm.nih.gov/pubmed/39944427.
|
| 7. |
Coward J, Mislang ARA, Frentzas S, Lemech CR, Nagrial A, Jin X, et al. Safety and efficacy of AK112, an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors in a phase I dose escalation study. J Clin Oncol [Internet]. 2021;39(15_suppl):2515–2515. Available from: https://ascopubs.org/doi/10.1200/JCO.2021.39.15_suppl.2515.
|
| 8. |
Our Strategic Partnership with Akeso with an Aligned Mission [Internet]. Summit Therapeutics Inc. 2025 [cited 2025 Jul 18]. Available from: https://smmttx.com/ivonescimab-smt112/akeso-partnership/default.aspx.
|
| 9. |
Zhang L, Fang W, Zhao Y, Yang Y, Zhou N, Chen L, et al. Phase II results of ivonescimab (AK112/ SMT112), a novel PD-1/VEGF bispecific, in combination with chemotherapy for first line treatment of advanced or metastatic non-small cell lung cancer (NSCLC) without actionable genomic alterations (AGA) in EGFR/ALK. J Clin Oncol [Internet]. 2023;41(16_suppl):9087–9087. Available from: https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.9087.
|
| 10. |
Zhao Y, Chen G, Chen J, Zhuang L, Du Y, Yu Q, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinical Medicine [Internet]. 2023;62:102106. Available from: http://www.ncbi.nlm.nih.gov/pubmed/37593227.
|
| 11. |
Fang W, Zhao Y, Luo Y, Yang R, Huang Y, He Z, et al. Ivonescimab Plus Chemotherapy in Non-Small Cell Lung Cancer With EGFR Variant: A Randomized Clinical Trial. JAMA [Internet]. 2024;332(7):561–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/38820549.
|
| 12. |
Xiong A, Wang L, Chen J, Wu L, Liu B, Yao J, et al. Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China. Lancet (London, England) [Internet]. 2025;405(10481):839–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/40057343.
|
| 13. |
Clinical Study of Ivonescimab for First-line Treatment of Metastatic NSCLC Patients [Internet]. ClinicalTrials.gov 2025 [cited 2025 Jul 18]. Available from: https://clinicaltrials.gov/study/NCT05899608.
|
| 14. |
Clinical Study of Ivonescimab for First-line Treatment of Metastatic NSCLC Patients With High PD-L1 (HARMONi-7) [Internet]. ClinicalTrials.gov. 2025 [cited 2025 Jul 18]. Available from: https://clinicaltrials.gov/study/NCT06767514.
|
| 15. |
Ivonescimab Plus Chemotherapy Demonstrates Statistically Significant and Clinically Meaningful Improvement in Progression Free Survival in Patients with EGFR-Mutant Non-Small Cell Lung Cancer after EGFR TKI Therapy in Global Study [Internet]. Smmttx.com. 2025 [cited 2025 Sep 6]. Available from: https://smmttx.com/news/press-releases/news-details/2025/Ivonescimab-Plus-Chemotherapy-Demonstrates-Statistically-Significant-and-Clinically-Meaningful-Improvement-in-Progression-Free-Survival-in-Patients-with-EGFR-Mutant-Non-Small-Cell-Lung-Cancer-after-EGFR-TKI-Therapy-in-Global-Study/default.aspx.
|








