Abstract
Diabetes mellitus (DM) is one of the biggest health problems of the 21st century. The complications of DM are macrovascular (ischemic diseases of the heart and brain) and microvascular (retinopathy, nephropathy, neuropathy). The aim of this review was to describe the role of micro ribonucleic acids (miRNAs) as markers for the development of distant complications of diabetes. A search was conducted in the PubMed, Scopus, Google Scholar and EMBASE databases for articles published in 2014-2024. Fifty-three original articles, systematic reviews and meta-analyses were included in the review. MiRNAs are involved in metabolic pathways responsible for the onset and development of DM and their altered expression may serve as a non-invasive biomarker for the development of distant DM complications. Among the most significant is miR-21, responsible for normal angiogenesis, whose expression was higher in patients with macroangiopathy and retino-, nephro- and neuropathy. Certain miRNAs may have potential as potential therapeutic targets, e.g. miR-203 (ischemic heart disease), miR-181c (retinopathy) and miR-184-5p (neuropathy). MiRNAs are potential biomarkers for the development of distant complications of diabetes and may serve as therapeutic targets for their reduction.
Citation
Bigosiński K, Granat M, Salamon K, Góra J, Caliński M, Bednarz H, Dubaj M, Porada D. The milestone – role of miRNAs as predictors of diabetes complications: a literature review. Eur J Transl Clin Med. 2025;8(2):58-70Introduction
Diabetes mellitus (DM) is a growing global health and economic challenge, with 828 million cases reported in 2022 and projections reaching 1.31 billion by 2050, mainly in low- and middle-income countries [1-3]. Furthermore, in 2021 approximately 11.8% of global deaths in the working-age population (< 60 years) were estimated to be caused by DM or its complications [1-3].
Chronic complications of DM lead to poor outcomes (increased morbidity, mortality, and healthcare costs) and are traditionally classified as microvascular (retinopathy, nephropathy, neuropathy) and macrovascular (coronary artery disease [CAD], stroke, peripheral artery disease [PAD]) [4-5]. However, Yu et al. suggested a revision of this classification, in accordance with the affected tissues: vascular, parenchymal and hybrid (both vascular and parenchymal) [5].
Microribonucleic acids (miRNAs) are small non-coding RNAs (20-25 nucleotides) that regulate gene expression post-transcriptionally [6]. Circulating miRNAs are stable in body fluids due to protection in exosomes or protein complexes, show potential as non-invasive diagnostic and prognostic biomarkers. In DM, miRNAs modulate key pathogenic mechanisms, including beta-cell responses to metabolic, genetic, and inflammatory stimulation [6-7]. Specific miRNAs, such as miR-34 and miR-146, are of particular interest [7]. Moreover, miRNAs offer the potential for the early detection of a particular type of DM and its specific complications [8-9]. Given the urgent need for effective biomarkers of DM, this paper we present a comprehensive review of the available data on the involvement of miRNAs in predicting its complications [10].
Material and methods
A literature search was conducted independently by 3 authors using the PubMed, Scopus, Google Scholar, and EMBASE databases. The results were then compared. The following keywords were used: ‘diabetes,’ ‘complications,’ ‘miRNAs,’ ‘miRNAs,’ ‘markers,’ ‘retinopathy,’ ‘neuropathy,’ ‘nephropathy,’ and the ‘AND’ operator. The search was limited to articles published in the years 2014-2024. We excluded non-full-text records (abstract-only), editorials, letters to the editor and non-systematic reviews. The articles were searched sequentially in the given databases, and the results were combined by the same 3 authors. The inclusion criteria were as follows: article published in 2014-2024, article format original/research article (systematic review and meta-analysis), studies corresponding to the role of miRNAs in DM, articles describing distant complications of DM, describing patients >18 years of age, or studies in animal models. The exclusion criteria were: articles published before 2014, inappropriate format (editorial, letter to the Editor, abstract, poster, case report), articles describing acute complications of DM, and articles on DM in children. The discrepancies between the searchers were minor. After analysis, articles that did not meet the inclusion criteria were rejected, and a combined list of articles for the final review was developed. Initially, 476 studies were identified. After excluding duplicate articles and screening the titles and abstracts, 70 articles remained. After a substantive analysis, 53 articles were included in the review.
Results and discussion
Macroangiopathy
Among the highest risks of death and characteristic complications of DM are vascular disorders. Morrison et al. demonstrated that patients with vascular complications of DM have elevated levels of HDL-related miRNAs. MiR-181c-5p may underlie proangiogenic processes, with 14-fold higher activity in subjects with PAD (1454 ± 1346%) than in the healthy group (100 ± 121%) and patients without PAD (82 ± 77%). This could also be applied to miRNA-181c5p in plasma, however the difference was not statistically significant. In contrast, HDL-bound miRNA-27b-3p showed 10-fold higher activity in patients with PAD (260 ± 232%) than in those without lesions (27 ± 23%) [11].
The adverse effect of DM on the prognosis of patients with heart failure (HF) and CAD has been known for a long time. The correlation between miR-1 and miR-21 expression and these conditions was studied by Al-Hayali et al. in a group of 35 patients with insulin-dependent DM. The results showed significantly 0.22-fold lower serum levels of miR-1 in patients with heart failure than in a group with type 2 DM alone, as well as in a group with CAD (p < 0.001). Moreover, miR-21 expression was higher in patients with heart failure (1.7 times) and CAD (1.37 times) than in patients without these conditions. Additionally, they demonstrated positive correlations between miR-21 and NT-proBNP and galactin-3. When miR-21 levels were > 1.695 and NT-proBNP > 4747 pg/ mol, the probability of DM and heart failure co-occurring was 95.2%. In contrast, in cases where miR-21 levels were < 1.695 and NT-proBNP levels were < 4747 pg/mol, the probability of DM alone was 66.7%. In cases where the miR-21 value was > 1.695 and the galactin-3 value was > 9.25 ng/mol, the probability was as high as 74.4% [12].
Other researchers observed decreased miR-130a and miR130b levels in patients with DM and CAD compared to those with DM alone and in the control group [13]. In their studies of mice, Dai et al. reported an unknown role for miR-21 in alleviating the symptoms and progression of diabetic cardiomyopathy by affecting gelsolin. A notable decrease in miR-21 expression was observed in the hearts with diastolic dysfunction. The supply of exogenous miR-21 effectively protected the heart from the early onset of impaired diastolic function in the reduced emission of reactive oxygen species, increased bioavailability of nitric oxide, and mitigation of cardiomyocyte hypertrophy in diabetic mice [14].
Lopes et al. tested miRNA values from the left ventricle of diabetic rodents to elucidate their role in diabetic cardiomyopathy. They noted decreased expression of rno-miR-877, rno-miR-320 and rno-miR-214 in the study group, together with increased expression of rno-miR-17, rno-miR-187, rnomiR-34a, rno-miR-322, rno-miR-188, rno-miR-532 and rnomiR-21. These results demonstrate the potential usefulness of the aforementioned miRNAs in the early detection of diabetic heart complications [15].
Chen et al. measured miR-30c levels in the myocardium of rodents with diabetic cardiomyopathy and healthy controls. A notable reduction in miR-30c was observed in the myocardium of subjects with cardiomyopathy compared to controls. Moreover, miR-30c overexpression reduced diabetic myocardial dysfunction. In addition, the data showed that overexpression of miR-30c silences the autophagy-initiating protein BECN1, thereby protecting myocardial function in diabetic rodents. However, the reduction of miR-30c expression increased the expression of BECN1 protein and through it, autophagy and progression of pathology within the myocardium [16]. In addition, Chen et al. observed a relationship between decreased levels of miR-133 in cardiac muscle and increased levels of fibrosis biomarkers in a mouse model of diabetic cardiomyopathy. Overexpression of miR-133 can even reverse DM-induced cardiac remodeling by attenuating these biomarkers [17].
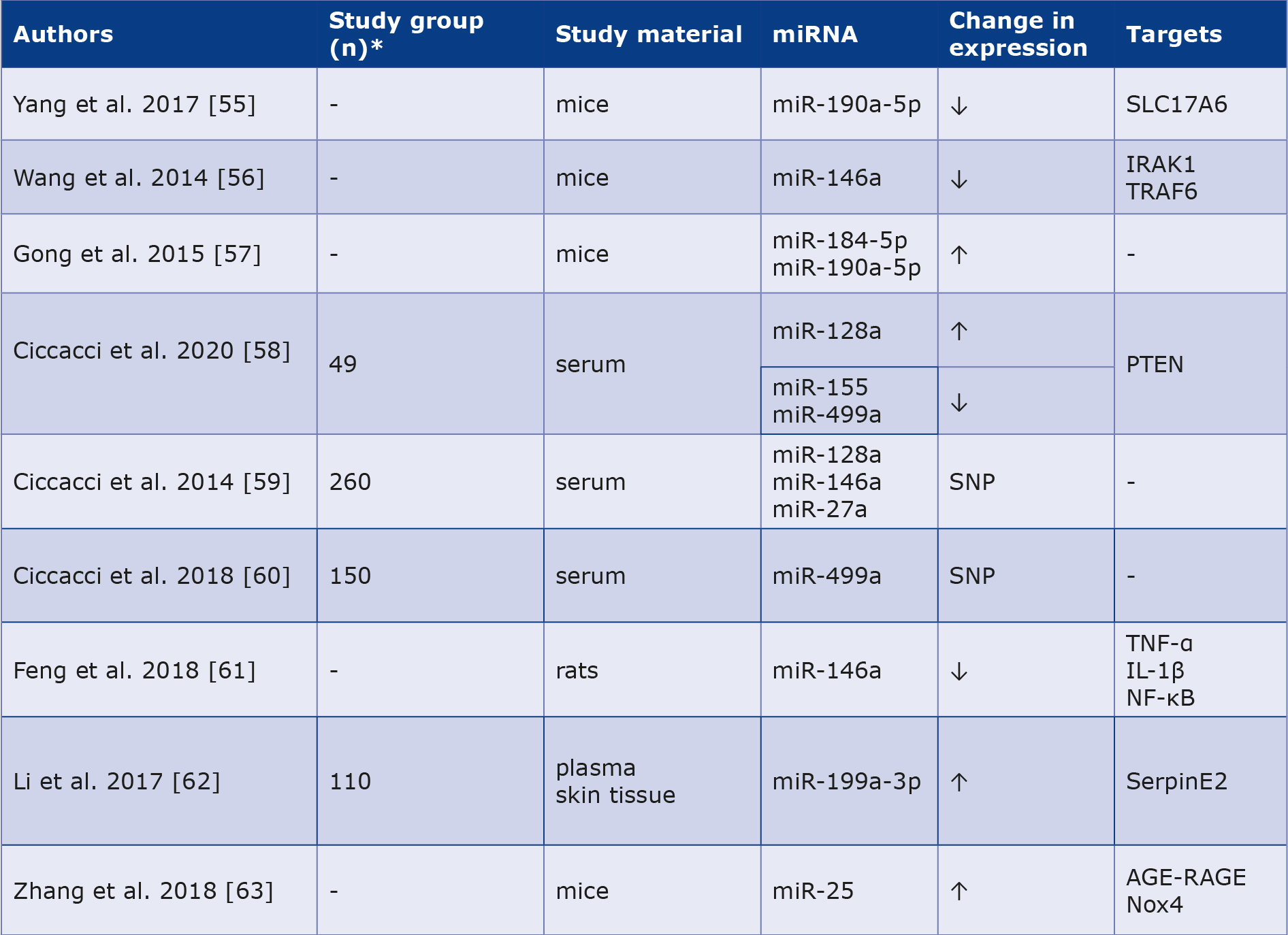
Liu et al. were the first to eport the role of miR-222 in the protective effect of physical exercise on the cardiovascular system. Mice exposed to swimming showed higher expression of miR-222 in cardiomyocytes, which stimulated cell stimulation and growth. In contrast, miR-222 inhibition led to apoptosis of cardiomyocytes, indicating its potential therapeutic relevance [18]. Other researchers have found that increased levels of circulating miR-1 and miR-133 are correlated with a higher probability of CAD in patients with type 2 DM [19]. Similar conclusions were published in a study of miR-126, the expression of which was significantly reduced in the type 2 DM and CAD groups compared to the reference group [20]. Jansen et al. noted that DM alters vascular endothelial miRNA expression in circulating endothelial microvesicles. MiR-126 and miR-26a expression levels were lower in the DM group than in the controls. The group with reduced expression of the molecules mentioned above was more likely to have concomitant CAD [21]. Xubin et al. noted that increased expression of miR-203 could act as a cardio-protective factor in diabetic cardiomyopathy by inhibiting the PI3K/Akt path [22]. Deng et al. investigated that the level of circulating miR-24 was significantly reduced in the peripheral blood of patients with type 2 DM and CAD compared to controls [23]. The summary is presented in Table 1.
Table 1. Role of miRNAs as potential biomarkers in diabetic macroangiopathy [11-23]
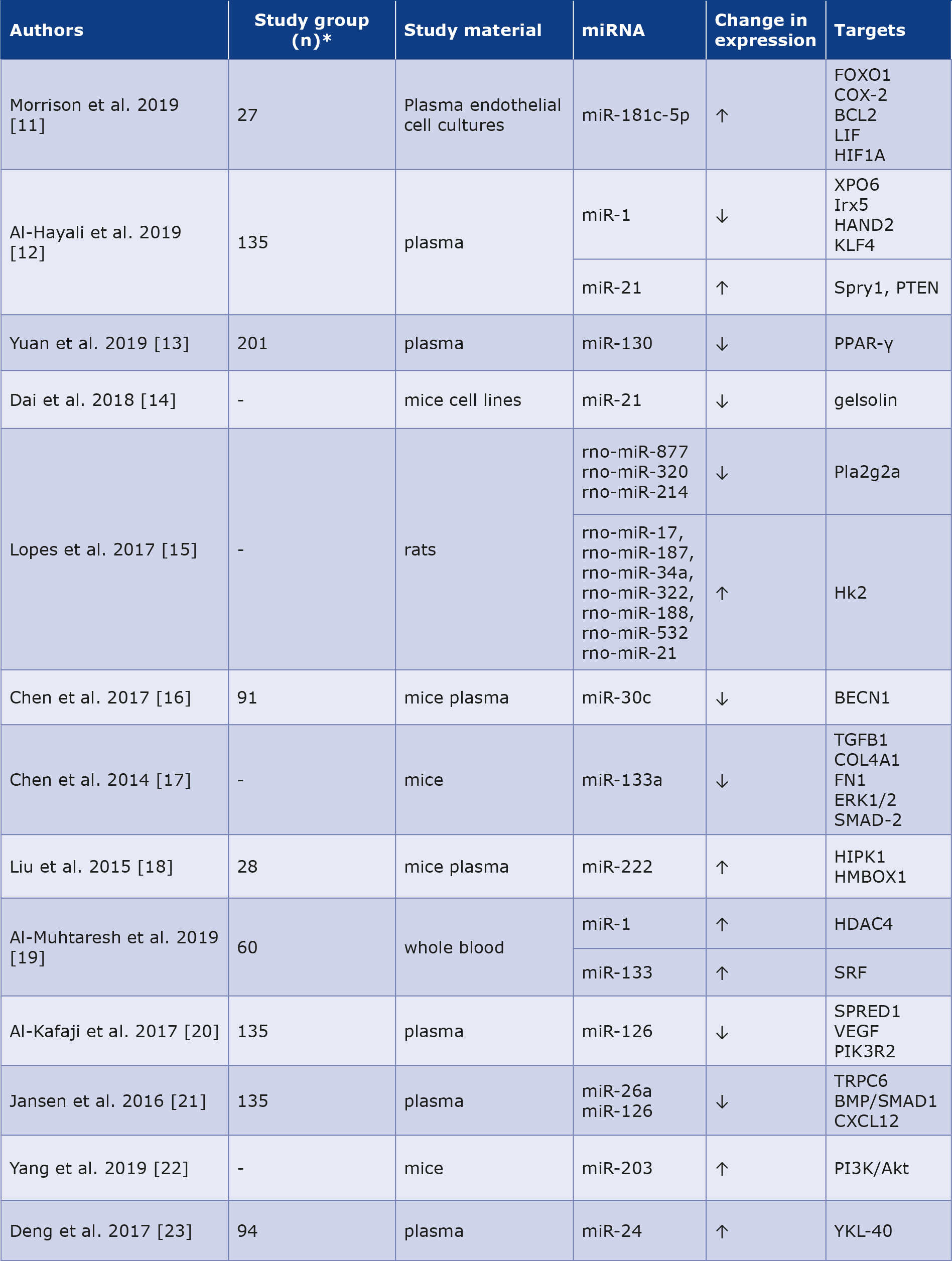
* Not all articles mentioned the exact number of people and animals participating in the descbed study.
Retinopathy
Hyperglycemia causes vascular wall dysfunction and that is why diabetic retinopathy (DR) is one of the most common complications of DM and the most common cause of vision loss. One study showed that miR-21 expression was increased in patients with non-proliferative DR (n = 73) compared to controls (n = 115) and increased in patients with proliferative DR (n = 51) than in those with non-proliferative DR. Increased miR-21 levels were associated with the development of DR and can be used as an indicator of its severity [24]. Moreover, Qing et al. proved that the merger of miR-21, miR-181c and miR-1179 is useful in distinguishing the proliferative DR from the non-proliferative form. They noted that miR-21 was strongly correlated with angiogenesis in hyperglycemia. Simultaneously, miR-181c expression was higher in endothelial cells in a DM-like environment, indicating that it is related to vascular proliferation at high glucose concentrations [25]. McAuley et al. noted that the A allele of the miR-126 polymorphism is associated with sight-threatening DR compared to those without DR or early-stage disease. It acts as a vascular endothelial growth factor (VEGF) and boosts the probability of progression of the DR, thus it could become a potential therapeutic target [26]. Other scholars have identified miR-1281 as a sensitive biomarker for the early detection of DR. It had the most elevated expression in patients with non-proliferative DR compared to healthy controls, and its expression was increased in retinal cell cultures in high-glucose environments [27]. The authors observed an overexpression of miR-423-5p in DR with enhanced hyperglycemia-induced apoptosis in retinal pigment epithelial cells [28]. Moreover, Santovito et al. found that DR was correlated with higher circulating miR-25-3p and miR-320b and lower miR-495-3p levels compared to the subjects without DR and healthy controls [29]. Blum et al. showed a reduced expression of miR-423 in a group with DR compared to controls [30]. Moreover, García de la Torre et al. observed that the level of miR-126 did not differ between the groups with and without DR, whereas the level of miRNA-221 was increased [31]. Gomaa et al. noticed that miR-200b expression was approximately 5 times higher in vitreous body samples collected from patients with proliferative DR [32]. In another study, miR-15a, miR-320a, miR-320b, miR-93, miR-29a, and miR-423-5p were significantly elevated in patients with proliferative DR [33]. Li et al. investigated the role of miR-200b in the evolution of DR. They found that its increased expression could reduce the expression of VEGFA,mitigating DR progression [34]. According to Liang et al., miR-28-3p, miR-151a-5p, and miR148a-3p were correlated with the progression of DR and can serve as non-invasive diagnostic markers [35]. Liu et al. discovered that miR-211 may also serve as a new marker with high sensitivity and specificty for DR by affecting Sirtuin 1 [36]. Murray et al. showed overexpression of miR-200b in Akita mice retinas (experimental benchmark of insulin-dependent DM). This miRNA reduces expression of Oxr1. These results suggest that downregulating miRNA-200b expression while enabling Oxr1 expression may have a protective role against DR progression [37]. Rezk et al. found that the group with DR had a lower miR-126 level compared to the group without it [38]. Zampetaki et al., in a group of patients with type 1 DM (n = 300), identified high expression of miR-320a and miR-27b as potential biomarkers of DR progression [39]. Moreover, Zou et al. identified increased miR-93 as a new promising diagnostic biomarker of DR progression [40]. Pastukh et al. (n = 40) showed that miR-122 expression increased in subjects with severe DR compared to healthy controls. However, when the illness progressed to proliferative DR, miR-122 expression decreased [41]. Moreover, Huihui et al. identified elevated expression of both miR-3197 and miR2116-5p as potential diagnostic biomarkers for DR [42]. The above data are summarized in Table 2. The role of miRNAs as diagnostic and prognostic markers in DR is shown in Figure 1.
Table 2. Role of miRNAs as potential biomarkers in diabetic retinopathy [24-42]
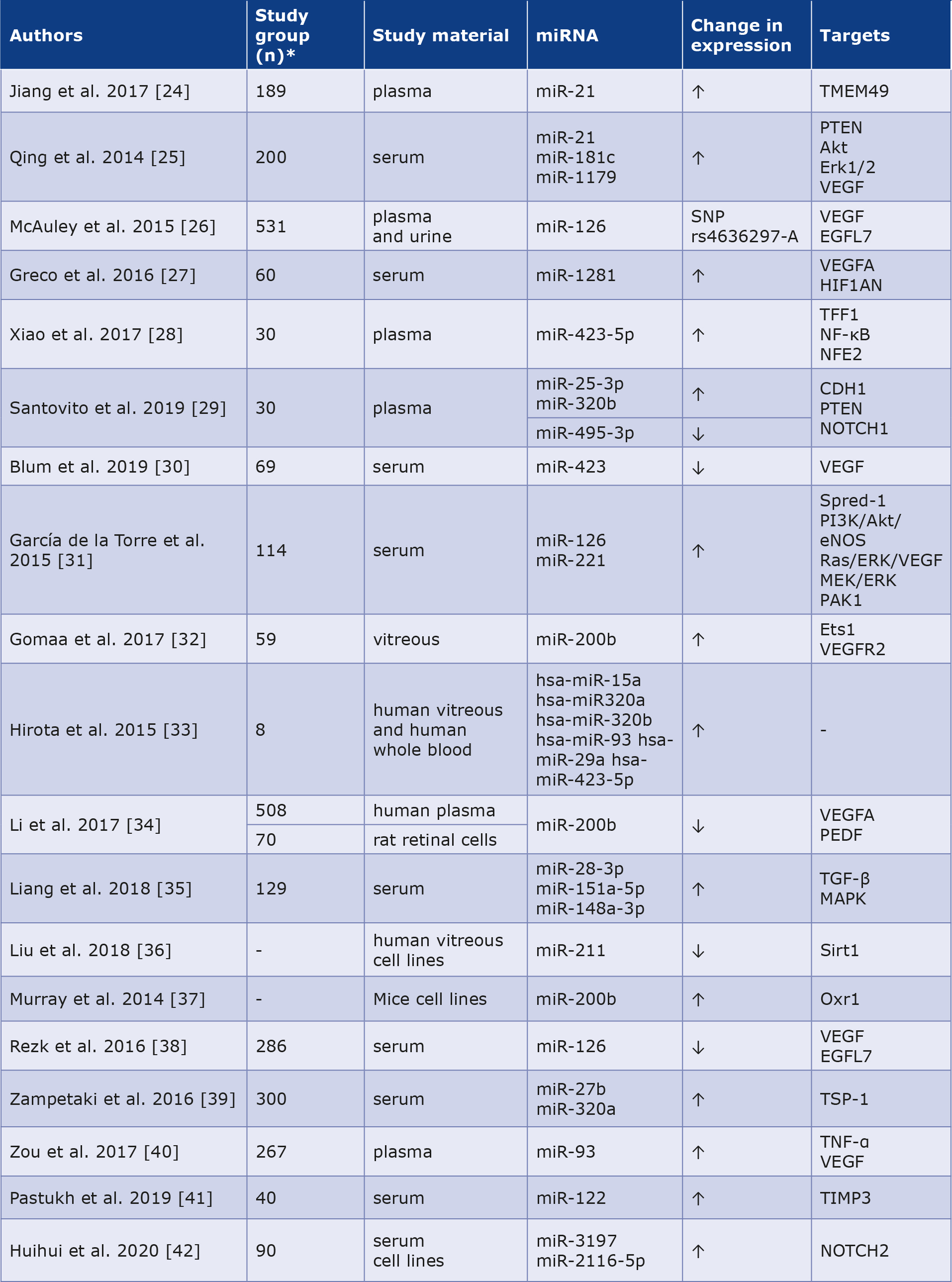
* Not all articles mentioned the exact number of people and animals participating in the descbed study.
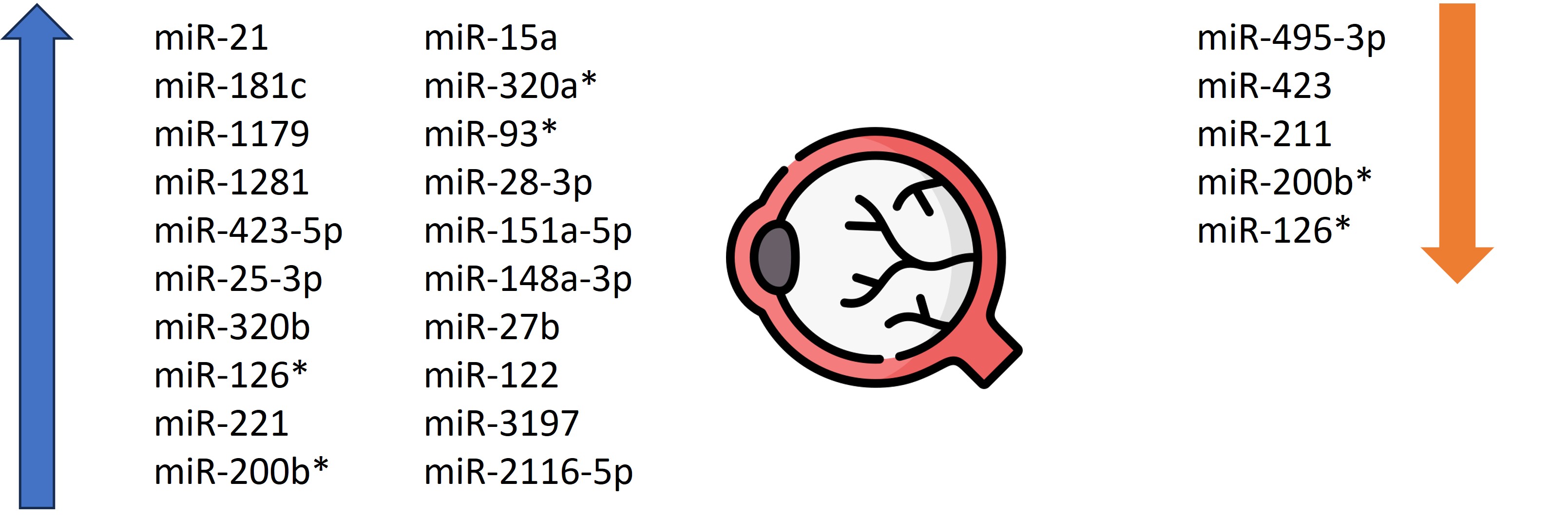
Figure 1. The role of miRNAs as diagnostic and prognostic markers in diabetic retinopathy.
* molecules appearing in 2 or more articles
Nephropathy
Diabetic nephropathy (DN) manifests in approximately one-third of diabetic patients and is associated with multiple complications and premature mortality. The disease progresses as the number of podocytes is reduced, the mesangial matrix expands, the glomerular basement membrane thickens, and the glomeruli undergo sclerosis. Initially, DN is often asymptomatic and the patient can be unaware of its progression. Zang et al. reported significantly higher urinary miR-21-5p (2.13-fold) and lower miR-30b-5p (0.82-fold) in DN patients versus subjects without kidney injury, supporting their use in early detection [43]. Beltrami et al. examined the profile of 754 miRNAs and noted increases in miR-126 (2.8-fold), miR-155 (1.8-fold), and miR-29b (4.6-fold) levels in urine from a group with confirmed DN (n = 20) compared to controls (n = 20). Concurrently, comparing patients without DN and the control group was statistically significant for miR-126 (3.1-fold increase) and miR-155 (1.6-fold increase), with a trend toward increased miR-29b (4.1-fold increase). Histopathological analysis revealed that the detection of miRNA-126 and miR-29b was significantly higher in podocyte cells and endothelial cells from glomeruli, while miR-155 was higher in proximal tubule epithelial cells, which could also be used as a valuable biomarker of DN progression [44]. Moreover, An et al. proved that increased urinary miRNA-196a expression is correlated with the level of kidney injury and may constitute a valuable biomarker of progressive fibrotic lesions in DN. Urinary miR-196a levels correlated positively with proteinuria (rho = 0.385), duration of DM (rho = 0.255) and systolic blood pressure (rho = 0.267). Additionally, miR-196a was associated with glomerular sclerosis and renal interstitial fibrosis in patients with DN [45]. Zhao et al. discovered that miR-142-3p is weakly expressed in renal tubular epithelial cells stimulated by hyperglycemia. Increasing its expression can attenuate apoptosis and oxidative stress in chronic hyperglycemia [46]. Moreover, miR-31 expression was reduced in the group with type 2 DM and coexisting DN was reduced relative to that in the group without complications. Interestingly, this difference was more prominent in the group with macroalbuminuria than in that with microalbuminuria [47]. Assmann et al. showed increased expression of miR-21-3p and miR378-3p in a group with DN. In contrast, miR-16-5p and miR29a-3p showed decreased expression compared with a group of patients with type 1 DM and moderate DN [48]. Furthermore, Delić et al. concluded that urinarymiR-320c levels were elevated in DN patients compared to diabetic patients without complications and healthy controls [49]. Huang et al. found that increased level of miR-155 and miR-146a in subjects with DM and animal models leads to inflammation-induced glomerular endothelial damage, inducing the progression of DN [50]. Other researchers have observed that miR-155 deficiency attenuates DN during chronic exposure to hyperglycemia [51]. Liu et al. noted that miR-25 is downregulated in patients and animals with DN and the administration of miR25 to mice inhibited the progression of renal damage [52]. Zanchi et al. concluded in their DN rat model with tubulointerstitial fibrosis, there was an 18-fold higher expression of miR184 than in controls [53]. Another molecule with decreased expression in advanced DN was miR-98, and its overexpression inhibits the disease progression [54]. The data on DN are presented in Table 3. The role of miRNAs as diagnostic and prognostic markers in DN is shown in Figure 2.
Table 3. Role of miRNAs as potential biomarkers in diabetic nephropathy [43-54]
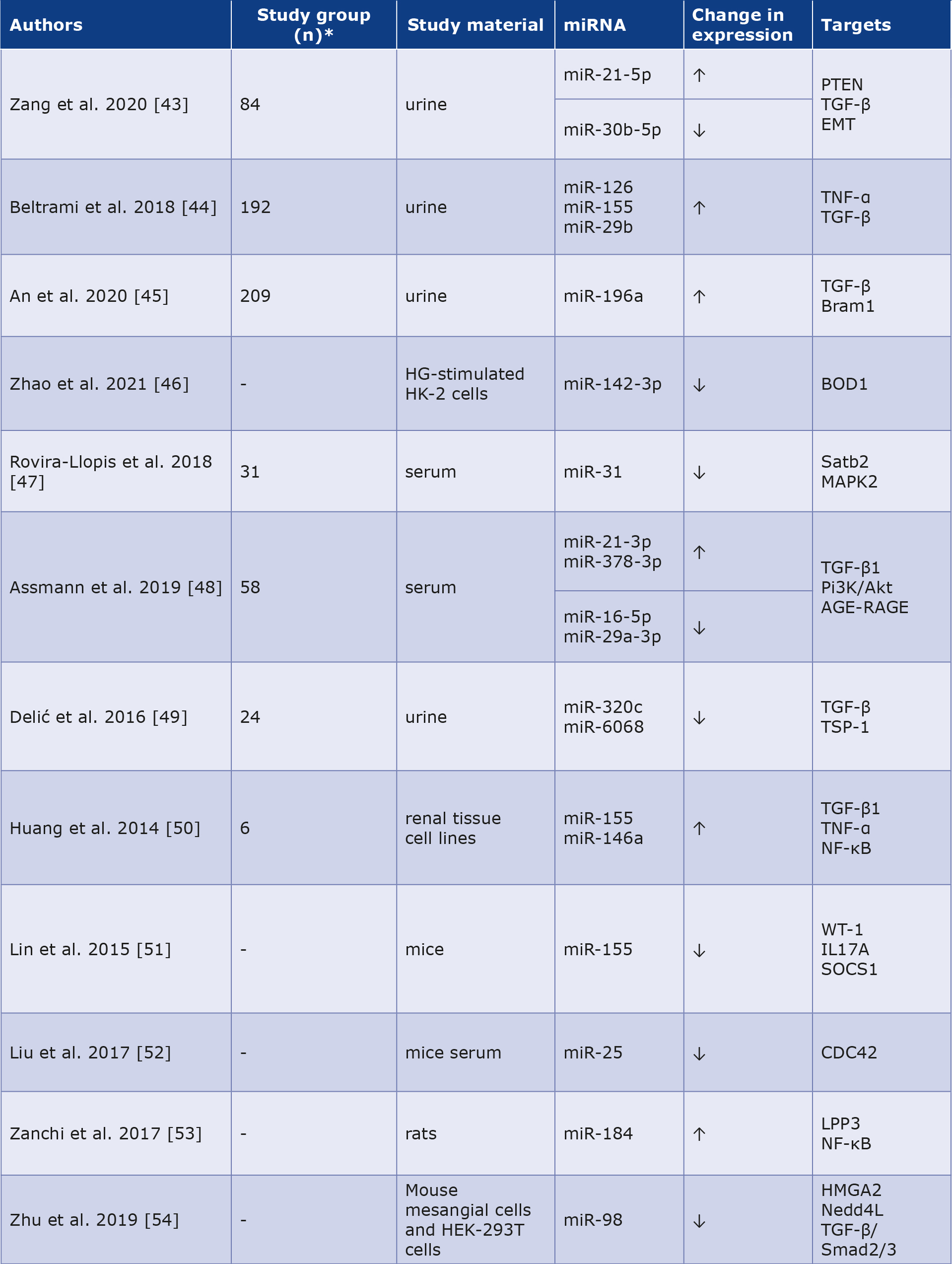
* Not all articles mentioned the exact number of people and animals participating in the descbed study.
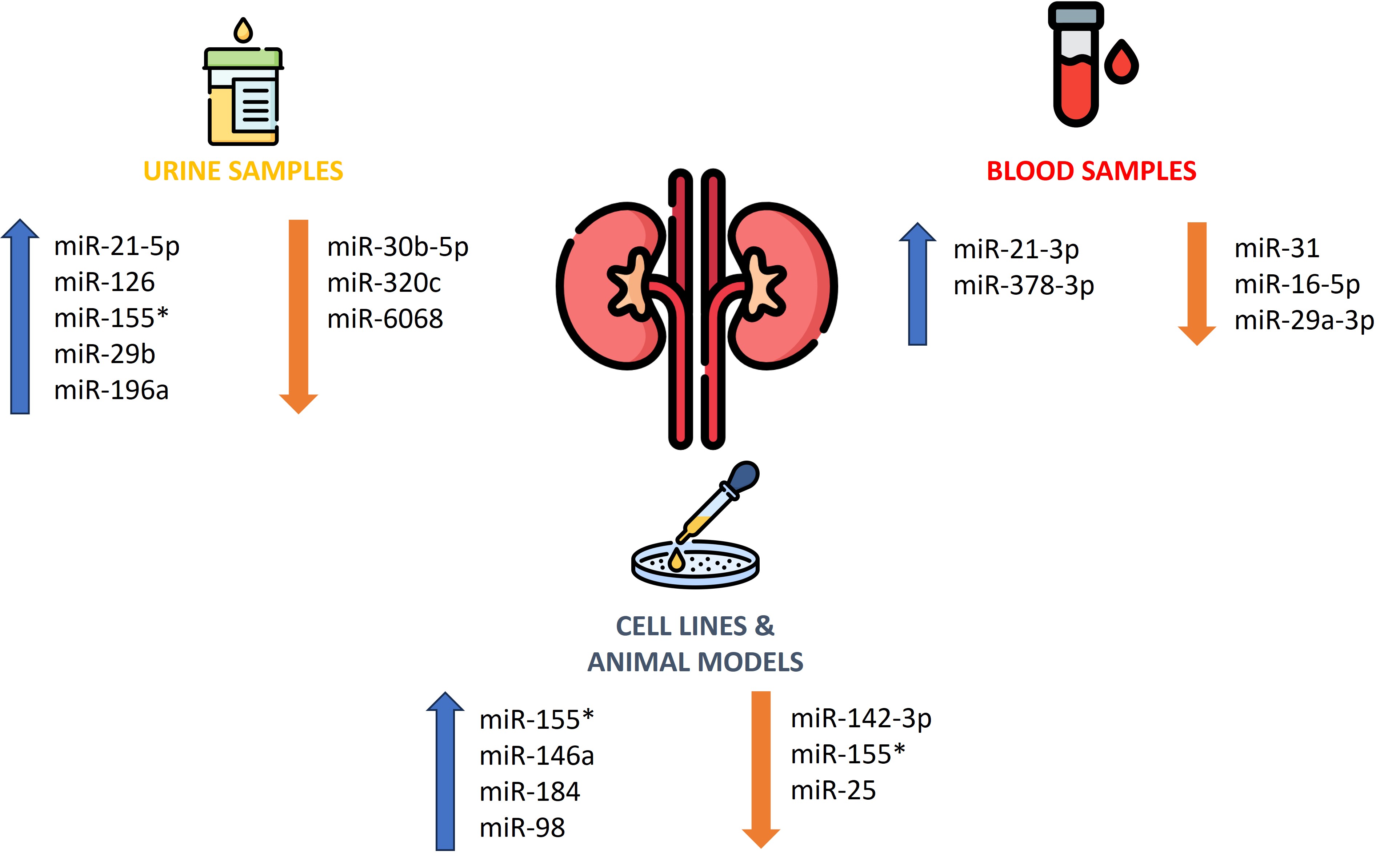
Figure 2. The role of miRNAs as diagnostic and prognostic markers in diabetic nepropathy.
* molecules appearing in 2 or more articles
Neuropathy
Diabetic neuropathy is a significant complication of DM and it occurs due to chronic exposure to elevated blood glucose levels. Recent studies and observations have shown that miRNAs are crucial for the onset of neuropathic pain. The contribution of miR-190a-5p to the overall pathomechanism is therefore significant. In an experiment on mice with induced DM and neuropathy, significantly reduced miR-190a-5p expression was identified after sampling the dorsal horns of the lumbar spine. Regarding the positive effects of expression-enhancing therapy and even the regression of some neuropathic lesions, this offers great hope for the evolution of a novel treatment strategy for this disease [55]. Moreover, a study by Wang et al. provided reliable evidence for the role of miR-146a in the initiation of apoptosis of dorsal root ganglion neurons in an environment of chronic hyperglycemia and they found that DM reduced miR-146a expression in the mice model [56]. Another study identified miR-184-5p and miR-190a-5p as valuable therapeutic targets for patients with diabetic neuropathy [57]. Ciccacci et al. demonstrated increased levels of miR-128a in patients with diabetic polyneuropathy, whereas miR-155 and miR-499a levels were decreased in the patient group [58]. The same authors in another study noted that the T allele SNP in miR-128a was associated with a higher risk, while the C allele SNP in miR-146a had a lower risk of developing diabetic polyneuropathy. Moreover, the SNP in miR-27a was correlated with the probability of initial cardiovascular autonomic neuropathy, while the SNP in miR-146a proved to have a risk-reducing role [59]. The same group demonstrated that miR-499a may be involved in the development of diabetic neuropathy, specifically showing a higher risk of developing severe cardiovascular autonomic neuropathy [60]. Furthermore, Feng et al. noted decreased miR-146a expression in rats with diabetic neuropathy compared to those without it [61]. Li et al. proved that expression of miR-199a3p was higher in the diabetic neuropathy group compared to patients without this complication [62]. Other researchers have pointed out that miR-25 plays a protective role against diabetic neuropathy, while the use of drugs that inhibit miR-25 may contribute to the development of this complication [63]. The data are presented in Table 4. The role of miRNAs as diagnostic and prognostic markers in diabetic neuropathy is shown in Figure 3.
Table 4. Role of miRNAs as potential biomarkers in diabetic neuropathy [55-63]
* Not all articles mentioned the exact number of people and animals participating in the descbed study.
SNP – single nucleotide polymorphism
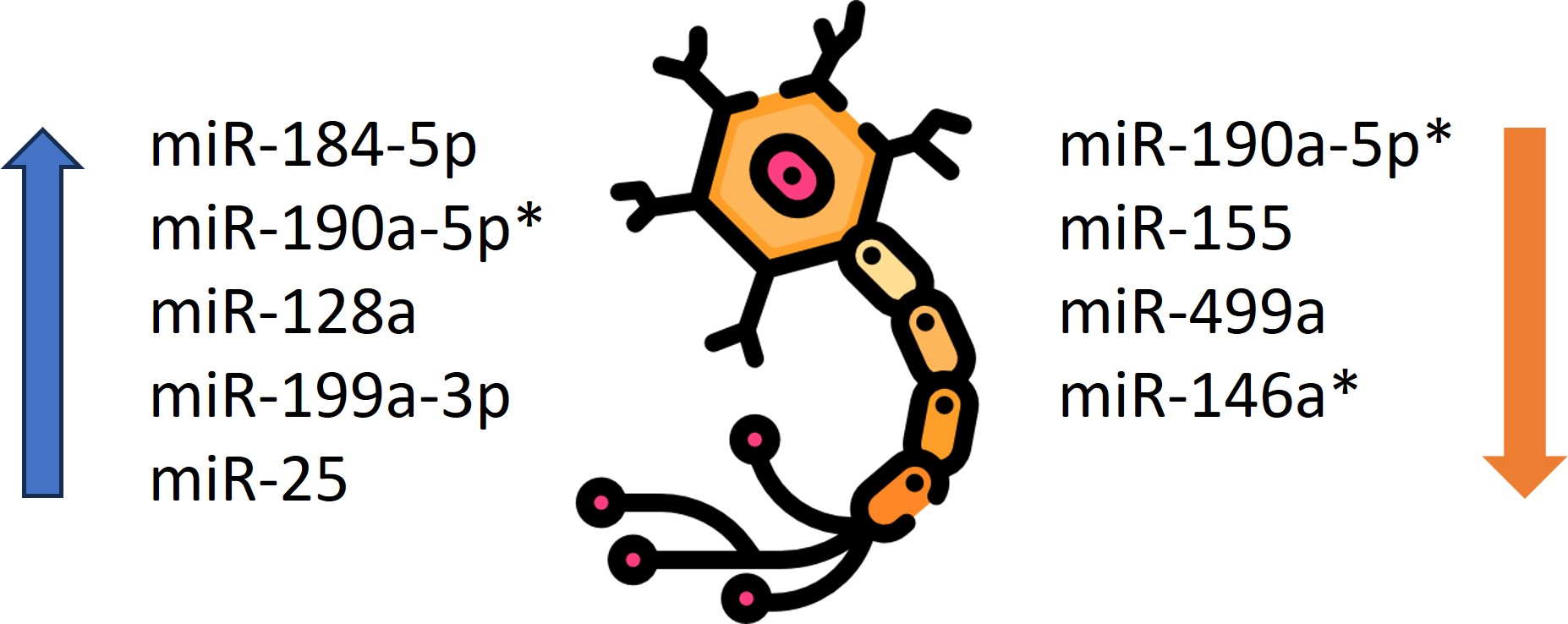
Figure 3. The role of miRNAs as diagnostic and prognostic markers in diabetic neuropathy.
* molecules appearing in 2 or more articles
Limitations of the study
We used a narrative rather than a systematic review method. No quality or risk of bias assessment was conducted. Due to the relatively small number of studies in human groups, data from both human groups and animal models were included in our review.
Conclusions
MiRNAs play an essential function in metabolic pathways and the pathogenesis of DM. MiRNAs are potential biomarkers for the evolution of distant consequences of DM and may serve as their therapeutic targets. Randomized trials on large groups of human populations are still lacking. More research is needed to understand the role and potential future diagnostic use of miRNAs.
Conflict of interest
None.
Funding
None.
References
| 1. |
Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet [Internet]. 2023;402(10397):203–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673623013016.
|
| 2. |
The Lancet. Diabetes: a defining disease of the 21st century. Lancet [Internet]. 2023;401(10394):2087. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673623012965.
|
| 3. |
Zhou B, Rayner AW, Gregg EW, Sheffer KE, Carrillo-Larco RM, Bennett JE, et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet [Internet]. 2024;404(10467):2077–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673624023171.
|
| 4. |
Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes 2017. J Diabetes Res [Internet]. 2018;2018:1–4. Available from: https://www.hindawi.com/journals/jdr/2018/3086167/.
|
| 5. |
Yu MG, Gordin D, Fu J, Park K, Li Q, King GL. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr Rev [Internet]. 2024;45(2):227–52. Available from: https://academic.oup.com/edrv/article/45/2/227/7252742.
|
| 6. |
Ho PTB, Clark IM, Le LTT. MicroRNA-Based Diagnosis and Therapy. Int J Mol Sci [Internet]. 2022;23(13):7167. Available from: https://www.mdpi.com/1422-0067/23/13/7167.
|
| 7. |
Li X, Dai A, Tran R, Wang J. Text mining-based identification of promising miRNA biomarkers for diabetes mellitus. Front Endocrinol (Lausanne) [Internet]. 2023;14. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2023.1195145/full.
|
| 8. |
Kraczkowska W, Stachowiak L, Pławski A, Jagodziński PP. Circulating miRNA as potential biomarkers for diabetes mellitus type 2: should we focus on searching for sex differences? J Appl Genet [Internet]. 2022;63(2):293–303. Available from: https://doi.org/10.1007/s13353-021-00678-5.
|
| 9. |
Dinesen S, El-Faitarouni A, Frisk NLS, Sørensen AE, Dalgaard LT. Circulating microRNA as Biomarkers for Gestational Diabetes Mellitus—A Systematic Review and Meta-Analysis. Int J Mol Sci [Internet]. 2023;24(7):6186. Available from: https://www.mdpi.com/1422-0067/24/7/6186.
|
| 10. |
He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med [Internet]. 2021;11(6). Available from: https://onlinelibrary.wiley.com/doi/10.1002/ctm2.468.
|
| 11. |
Morrison KR, Solly EL, Shemesh T, Psaltis PJ, Nicholls SJ, Brown A, et al. Elevated HDL-bound miR-181c-5p level is associated with diabetic vascular complications in Australian Aboriginal people. Diabetologia [Internet]. 2021;64(6):1402–11. Available from: https://link.springer.com/10.1007/s00125-021-05414-6.
|
| 12. |
Al-Hayali MA, Sozer V, Durmus S, Erdenen F, Altunoglu E, Gelisgen R, et al. Clinical Value of Circulating Microribonucleic Acids miR-1 and miR-21 in Evaluating the Diagnosis of Acute Heart Failure in Asymptomatic Type 2 Diabetic Patients. Biomolecules [Internet]. 2019;9(5):193. Available from: https://www.mdpi.com/2218-273X/9/5/193.
|
| 13. |
Yuan Y, Peng W, Liu Y, Xu Z. Circulating miR‐130 and its target PPAR‐γ may be potential biomarkers in patients of coronary artery disease with type 2 diabetes mellitus. Mol Genet Genomic Med [Internet]. 2019;7(9). Available from: https://onlinelibrary.wiley.com/doi/10.1002/mgg3.909.
|
| 14. |
Dai B, Li H, Fan J, Zhao Y, Yin Z, Nie X, et al. MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc Diabetol [Internet]. 2018;17(1):123. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-0767-z.
|
| 15. |
Luchessi AD. mRNA-miRNA integrative analysis of diabetes-induced cardiomyopathy in rats. Front Biosci [Internet]. 2017;9(2):483. Available from: https://imrpress.com/journal/FBS/9/2/10.2741/S483.
|
| 16. |
Chen C, Yang S, Li H, Yin Z, Fan J, Zhao Y, et al. Mir30c Is Involved in Diabetic Cardiomyopathy through Regulation of Cardiac Autophagy via BECN1. Mol Ther - Nucleic Acids [Internet]. 2017;7:127–39. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2162253117301452.
|
| 17. |
Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, Chakrabarti S. Cardiac miR‐133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med [Internet]. 2014;18(3):415–21. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jcmm.12218.
|
| 18. |
Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. miR-222 Is Necessary for Exercise-Induced Cardiac Growth and Protects against Pathological Cardiac Remodeling. Cell Metab [Internet]. 2015;21(4):584–95. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1550413115000662.
|
| 19. |
Al-Muhtaresh HA, Salem AH, Al-Kafaji G. Upregulation of Circulating Cardiomyocyte-Enriched miR-1 and miR-133 Associate with the Risk of Coronary Artery Disease in Type 2 Diabetes Patients and Serve as Potential Biomarkers. J Cardiovasc Transl Res [Internet]. 2019;12(4):347–57. Available from: http://link.springer.com/10.1007/s12265-018-9857-2.
|
| 20. |
Al-Kafaji G, Al-Mahroos G, Abdulla Al-Muhtaresh H, Sabry MA, Abdul Razzak R, Salem AH. Circulating endothelium-enriched microRNA-126 as a potential biomarker for coronary artery disease in type 2 diabetes mellitus patients. Biomarkers [Internet]. 2017;22(3–4):268–78. Available from: https://www.spandidos-publications.com/10.3892/etm.2016.3395.
|
| 21. |
Jansen F, Wang H, Przybilla D, Franklin BS, Dolf A, Pfeifer P, et al. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol [Internet]. 2016;15(1):49. Available from: http://cardiab.biomedcentral.com/articles/10.1186/s12933-016-0367-8.
|
| 22. |
Yang X, Li X, Lin Q, Xu Q. Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene [Internet]. 2019;715:143995. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378111919306547.
|
| 23. |
Deng X, Liu Y, Luo M, Wu J, Ma R, Wan Q, et al. Circulating miRNA-24 and its target YKL-40 as potential biomarkers in patients with coronary heart disease and type 2 diabetes mellitus. Oncotarget [Internet]. 2017;8(38):63038–46. Available from: https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.18593.
|
| 24. |
Jiang Q, Lyu X-M, Yuan Y, Wang L. Plasma miR-21 expression: an indicator for the severity of Type 2 diabetes with diabetic retinopathy. Biosci Rep [Internet]. 2017;37(2). Available from: https://portlandpress.com/bioscirep/article/37/2/BSR20160589/83197/Plasma-miR-21-expression-an-indicator-for-the.
|
| 25. |
Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, et al. Serum MiRNA Biomarkers serve as a Fingerprint for Proliferative Diabetic Retinopathy. Cell Physiol Biochem [Internet]. 2014;34(5):1733–40. Available from: https://karger.com/article/doi/10.1159/000366374.
|
| 26. |
McAuley AK, Dirani M, Wang JJ, Connell PP, Lamoureux EL, Hewitt AW. A genetic variant regulating miR-126 is associated with sight threatening diabetic retinopathy. Diabetes Vasc Dis Res [Internet]. 2015;12(2):133–8. Available from: https://journals.sagepub.com/doi/10.1177/1479164114560160.
|
| 27. |
Greco M, Chiefari E, Accattato F, Corigliano DM, Arcidiacono B, Mirabelli M, et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients With Diabetic Retinopathy. Front Endocrinol (Lausanne) [Internet]. 2020;11. Available from: https://www.frontiersin.org/article/10.3389/fendo.2020.00528/full.
|
| 28. |
Xiao Q, Zhao Y, Xu J, Li W-J, Chen Y, Sun H-J. NFE2/miR-423-5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol cell Biol [Internet]. 2019;20(1):39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31455213.
|
| 29. |
Santovito D, Toto L, De Nardis V, Marcantonio P, D’Aloisio R, Mastropasqua A, et al. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Sci Rep [Internet]. 2021;11(1):4136. Available from: https://www.nature.com/articles/s41598-021-83047-w.
|
| 30. |
Blum A, Meerson A, Rohana H, Jabaly H, Nahul N, Celesh D, et al. MicroRNA-423 may regulate diabetic vasculopathy. Clin Exp Med [Internet]. 2019;19(4):469–77. Available from: http://link.springer.com/10.1007/s10238-019-00573-8.
|
| 31. |
García de la Torre N, Fernández-Durango R, Gómez R, Fuentes M, Roldán-Pallarés M, Donate J, et al. Expression of Angiogenic MicroRNAs in Endothelial Progenitor Cells From Type 1 Diabetic Patients With and Without Diabetic Retinopathy. Investig Opthalmology Vis Sci [Internet]. 2015;56(6):4090. Available from: http://iovs.arvojournals.org/article.aspx?doi=10.1167/iovs.15-16498.
|
| 32. |
Gomaa AR, Elsayed ET, Moftah RF. MicroRNA-200b Expression in the Vitreous Humor of Patients with Proliferative Diabetic Retinopathy. Ophthalmic Res [Internet]. 2017;58(3):168–75. Available from: https://karger.com/article/doi/10.1159/000475671.
|
| 33. |
Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol [Internet]. 2015;253(3):335–42. Available from: http://link.springer.com/10.1007/s00417-014-2692-5.
|
| 34. |
Li E-H, Huang Q-Z, Li G-C, Xiang Z-Y, Zhang X. Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci Rep [Internet]. 2017;37(2). Available from: https://portlandpress.com/bioscirep/article/37/2/BSR20160572/83287/Effects-of-miRNA-200b-on-the-development-of.
|
| 35. |
Liang Z, Gao KP, Wang YX, Liu ZC, Tian L, Yang XZ, et al. RNA sequencing identified specific circulating miRNA biomarkers for early detection of diabetes retinopathy. Am J Physiol Metab [Internet]. 2018;315(3):E374–85. Available from: https://www.physiology.org/doi/10.1152/ajpendo.00021.2018.
|
| 36. |
Liu H-N, Cao N-J, Li X, Qian W, Chen X-L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem Biophys Res Commun [Internet]. 2018;505(4):1236–43. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X18321983.
|
| 37. |
Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma J. MicroRNA-200b Downregulates Oxidation Resistance 1 ( Oxr1 ) Expression in the Retina of Type 1 Diabetes Model. Investig Opthalmology Vis Sci [Internet]. 2013;54(3):1689. Available from: http://iovs.arvojournals.org/article.aspx?doi=10.1167/iovs.12-10921.
|
| 38. |
Rezk NA, Sabbah NA, Saad MSS. Role of MicroRNA 126 in screening, diagnosis, and prognosis of diabetic patients in Egypt. IUBMB Life [Internet]. 2016;68(6):452–8. Available from: https://iubmb.onlinelibrary.wiley.com/doi/10.1002/iub.1502.
|
| 39. |
Zampetaki A, Willeit P, Burr S, Yin X, Langley SR, Kiechl S, et al. Angiogenic microRNAs Linked to Incidence and Progression of Diabetic Retinopathy in Type 1 Diabetes. Diabetes [Internet]. 2016;65(1):216–27. Available from: https://diabetesjournals.org/diabetes/article/65/1/216/34936/Angiogenic-microRNAs-Linked-to-Incidence-and.
|
| 40. |
Zou H-L, Wang Y, Gang Q, Zhang Y, Sun Y. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol [Internet]. 2017;255(6):1159–66. Available from: http://link.springer.com/10.1007/s00417-017-3638-5.
|
| 41. |
Pastukh N, Meerson A, Kalish D, Jabaly H, Blum A. Serum miR-122 levels correlate with diabetic retinopathy. Clin Exp Med [Internet]. 2019;19(2):255–60. Available from: http://link.springer.com/10.1007/s10238-019-00546-x.
|
| 42. |
Ji H, Yi Q, Chen L, Wong L, Liu Y, Xu G, et al. Circulating miR-3197 and miR-2116-5p as novel biomarkers for diabetic retinopathy. Clin Chim Acta [Internet]. 2020;501:147–53. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009898119321011.
|
| 43. |
Zang J, Maxwell AP, Simpson DA, McKay GJ. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci Rep [Internet]. 2019;9(1):10900. Available from: https://www.nature.com/articles/s41598-019-47504-x.
|
| 44. |
Beltrami C, Simpson K, Jesky M, Wonnacott A, Carrington C, Holmans P, et al. Association of Elevated Urinary miR-126, miR-155, and miR-29b with Diabetic Kidney Disease. Am J Pathol [Internet]. 2018;188(9):1982–92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944017311446.
|
| 45. |
An Y, Zhang C, Xu F, Li W, Zeng C, Xie L, et al. Increased urinary miR-196a level predicts the progression of renal injury in patients with diabetic nephropathy. Nephrol Dial Transplant [Internet]. 2020;35(6):1009–16. Available from: https://academic.oup.com/ndt/article/35/6/1009/5231923.
|
| 46. |
Zhao N, Luo Q, Lin R, Li Q, Ma P. MiR-142-3p ameliorates high glucose-induced renal tubular epithelial cell injury by targeting BOD1. Clin Exp Nephrol [Internet]. 2021;25(11):1182–92. Available from: https://link.springer.com/10.1007/s10157-021-02102-y.
|
| 47. |
Rovira-Llopis S, Escribano-Lopez I, Diaz-Morales N, Iannantuoni F, Lopez-Domenech S, Andújar I, et al. Downregulation of miR-31 in Diabetic Nephropathy and its Relationship with Inflammation. Cell Physiol Biochem [Internet]. 2018;50(3):1005–14. Available from: https://karger.com/article/doi/10.1159/000494485.
|
| 48. |
Assmann TS, Recamonde-Mendoza M, Costa AR, Puñales M, Tschiedel B, Canani LH, et al. Circulating miRNAs in diabetic kidney disease: case–control study and in silico analyses. Acta Diabetol [Internet]. 2019;56(1):55–65. Available from: http://link.springer.com/10.1007/s00592-018-1216-x.
|
| 49. |
Delić D, Eisele C, Schmid R, Baum P, Wiech F, Gerl M, et al. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. Jeyaseelan K, editor. PLoS One [Internet]. 2016;11(3):e0150154. Available from: https://dx.plos.org/10.1371/journal.pone.0150154.
|
| 50. |
Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: implications for glomerular endothelial injury. BMC Nephrol [Internet]. 2014;15(1):142. Available from: https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-142.
|
| 51. |
Lin X, You Y, Wang J, Qin Y, Huang P, Yang F. MicroRNA-155 Deficiency Promotes Nephrin Acetylation and Attenuates Renal Damage in Hyperglycemia-Induced Nephropathy. Inflammation [Internet]. 2015;38(2):546–54. Available from: http://link.springer.com/10.1007/s10753-014-9961-7.
|
| 52. |
Liu Y, Li H, Liu J, Han P, Li X, Bai H, et al. Variations in MicroRNA-25 Expression Influence the Severity of Diabetic Kidney Disease. J Am Soc Nephrol [Internet]. 2017;28(12):3627–38. Available from: https://journals.lww.com/00001751-201712000-00022.
|
| 53. |
Zanchi C, Macconi D, Trionfini P, Tomasoni S, Rottoli D, Locatelli M, et al. MicroRNA-184 is a downstream effector of albuminuria driving renal fibrosis in rats with diabetic nephropathy. Diabetologia [Internet]. 2017;60(6):1114–25. Available from: http://link.springer.com/10.1007/s00125-017-4248-9.
|
| 54. |
Zhu Y, Xu J, Liang W, Li J, Feng L, Zheng P, et al. miR-98-5p Alleviated Epithelial-to-Mesenchymal Transition and Renal Fibrosis via Targeting Hmga2 in Diabetic Nephropathy. Int J Endocrinol [Internet]. 2019 Jan 1;2019(1):4946181. Available from: https://doi.org/10.1155/2019/4946181.
|
| 55. |
Yang D, Yang Q, Wei X, Liu Y, Ma D, Li J, et al. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J Pain Res [Internet]. 2017;Volume 10:2395–403. Available from: https://www.dovepress.com/the-role-of-mir-190a-5p-contributes-to-diabetic-neuropathic-pain-via-t-peer-reviewed-article-JPR.
|
| 56. |
Wang L, Chopp M, Szalad A, Zhang Y, Wang X, Zhang RL, et al. The role of miR-146a in dorsal root ganglia neurons of experimental diabetic peripheral neuropathy. Neuroscience [Internet]. 2014;259:155–63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S030645221301004X.
|
| 57. |
Gong Q, Lu Z, Huang Q, Ruan L, Chen J, Liang Y, et al. Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem Biophys Res Commun [Internet]. 2015;456(2):615–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X1402169X.
|
| 58. |
Ciccacci C, Latini A, Colantuono A, Politi C, D’Amato C, Greco C, et al. Expression Study of Candidate miRNAs And Evaluation of their Potential use as Biomarkers of Diabetic Neuropathy. Epigenomics [Internet]. 2020;12(7):575–85. Available from: https://www.tandfonline.com/doi/full/10.2217/epi-2019-0242.
|
| 59. |
Ciccacci C, Morganti R, Di Fusco D, D’Amato C, Cacciotti L, Greco C, et al. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol [Internet]. 2014;51(4):663–71. Available from: http://link.springer.com/10.1007/s00592-014-0582-2.
|
| 60. |
Ciccacci C, Latini A, Greco C, Politi C, D’Amato C, Lauro D, et al. Association between a MIR499A polymorphism and diabetic neuropathy in type 2 diabetes. J Diabetes Complications [Internet]. 2018;32(1):11–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1056872717303215.
|
| 61. |
Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther [Internet]. 2018;Volume 12:171–7. Available from: https://www.dovepress.com/involvement-of-microrna-146a-in-diabetic-peripheral-neuropathy-through-peer-reviewed-article-DDDT.
|
| 62. |
Li Y-B, Wu Q, Liu J, Fan Y-Z, Yu K-F, Cai Y. miR-199a-3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol Med Rep [Internet]. 2017;16(3):2417–24. Available from: https://www.spandidos-publications.com/10.3892/mmr.2017.6874.
|
| 63. |
Zhang Y, Song C, Liu J, Bi Y, Li H. Inhibition of miR-25 aggravates diabetic peripheral neuropathy. Neuroreport [Internet]. 2018;29(11):945–53. Available from: https://journals.lww.com/00001756-201808010-00011.
|














