Challenges in orthodontic treatment of patients after childhood cancer disease – a literature review
Abstract
Nowadays, cancer in children is increasingly common. Thanks to effective treatment, survival rates are continually rising. However, the applied chemotherapy, radiotherapy, or combined treatment leaves a number of side effects. There are disturbances in growth, including within the bones of the craniofacial complex, as well as developmental anomalies in dentition. Among these, the most frequently observed are defects in the structure of tooth roots, tooth agenesis and microdontia. These disorders cause aesthetic and occlusal problems, therefore there is a need to modify the orthodontic treatment plan for patients after cancer therapy. The higher risk of caries in these patients (due to xerostomia and enamel hypoplasia) complicates or even makes it impossible to achieve the intended results of orthodontic treatment. We analysed the available literature in Scopus, PubMed and Google Scholar databases from the years 2010-2022 to understand the challenges orthodontists face when treating patients who experienced cancer in childhood.
Citation
Maciejewska J, Racka-Pilszak B. Challenges in orthodontic treatment of patients after childhood cancer disease – a literature review. Eur J Transl Clin Med. 2025;8(2):43-57Introduction
Nowadays, an increasing number of children are diagnosed with cancer, most commonly leukaemia, central nervous system (CNS) tumours and lymphomas [1-3]. Treatment includes radiotherapy, chemotherapy, surgical methods, bone marrow transplantation or their combinations [3-8]. Thanks to advances in medicine, the survival rate of patients with childhood cancers increased to about 80% [3-4, 6, 9], hence patients who underwent such treatment in childhood are increasingly met in orthodontic clinics [1, 5, 7, 10-11]. It is estimated that currently 1/900 young adults have successfully undergone oncological treatment in childhood [1, 3]. Oncological therapies cause a series of adverse effects and orthodontists must be aware of the impact of the therapeutic procedures applied on the craniofacial complex and the oral cavity tissues, including the bite and dentition. Orthodontic treatment of these patients presents real challenges not only for the patient and the orthodontist, but also the patient’s family [11-12]. Neill et al. demonstrated that 85% of orthodontists did not acquire knowledge on treating children post-cancer treatment during their specialty training and such cases are usually handled by older, more experienced orthodontists [6]. Therefore, regardless of work experience it is crucial for every orthodontist to continually update their knowledge to competently treat such patients [12]. The purpose of this review is to discuss the challenges faced by orthodontists working with patients who have undergone oncological treatment in childhood, with particular emphasis on the limitations of possible orthodontic procedures.
Material and methods
Electronic databases PubMed, Scopus and Google Scholar were searched using the keywords “cancer”, “carcinoma”, “orthodontic treatment”, yielding 17 (Scopus), 182 (PubMed) and 15900 (Google Scholar) results (see Figure 1). The search results were limited to English and Polish language only, which resulted in 15 (Scopus), 179 (PubMed) and 11700 (Google Scholar) items. The results were further narrowed down to publication years 2010-2022, which reduced the number of records to 13 (Scopus), 162 (PubMed) and 11400 (Google Scholar). Articles on adult patients, epidemiology and duplicates were excluded, and a total of 26 articles were selected. Their content was analysed and finally 21 articles that matched the topic and contained valuable information were included in the review (see Table 1).
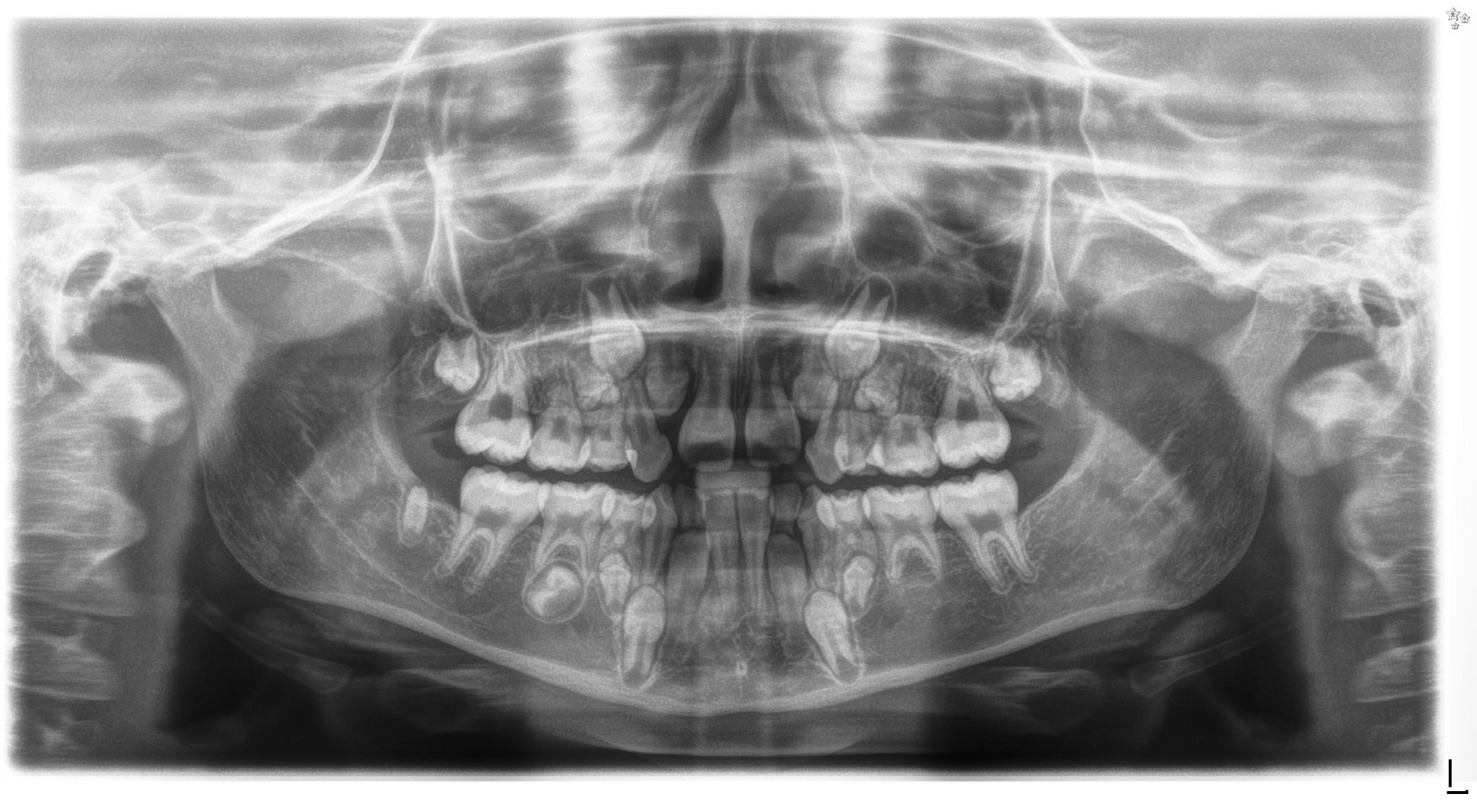
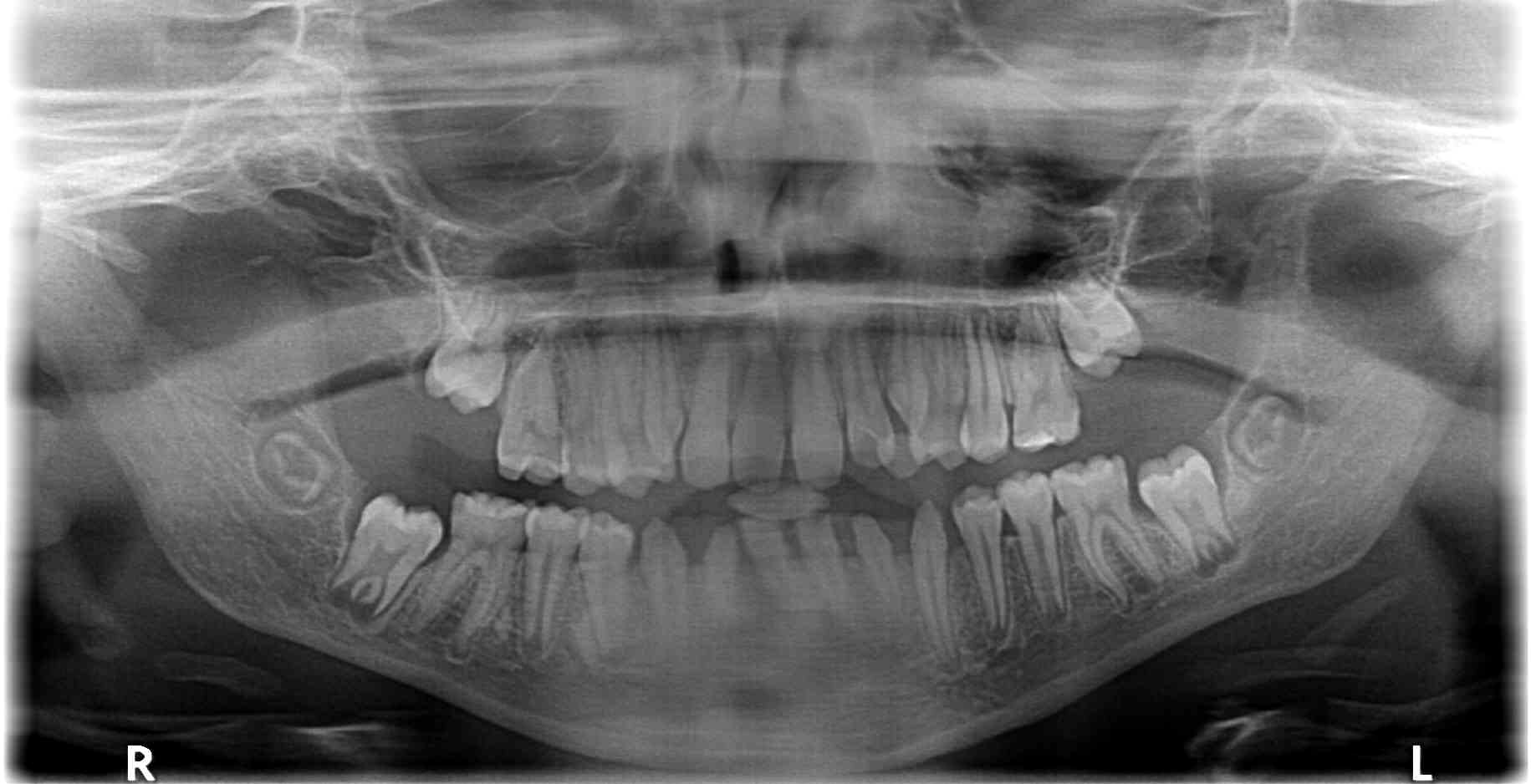
Figure 1. Panoramic radiograph of a 9-year-old girl treated for acute lymphoblastic leukaemia from the age of 15 months with subsequent 2 years of radiotherapy, chemotherapy, immunotherapy and antibiotic therapy, along with allogenic hematopoietic stem cell transplantation and two administrations of mesenchymal stem cells. Visible root shortening of all permanent first molar teeth, V-shaped roots of teeth 16 and 26, narrow roots of teeth 22, 36 and 46, absence of tooth buds 15, 25, 35 and 37, residual bud of tooth 47, microdontic buds of teeth 17, 14, 24, 27, 34 and 44 with disturbed mineralization (irregular crown outlines).
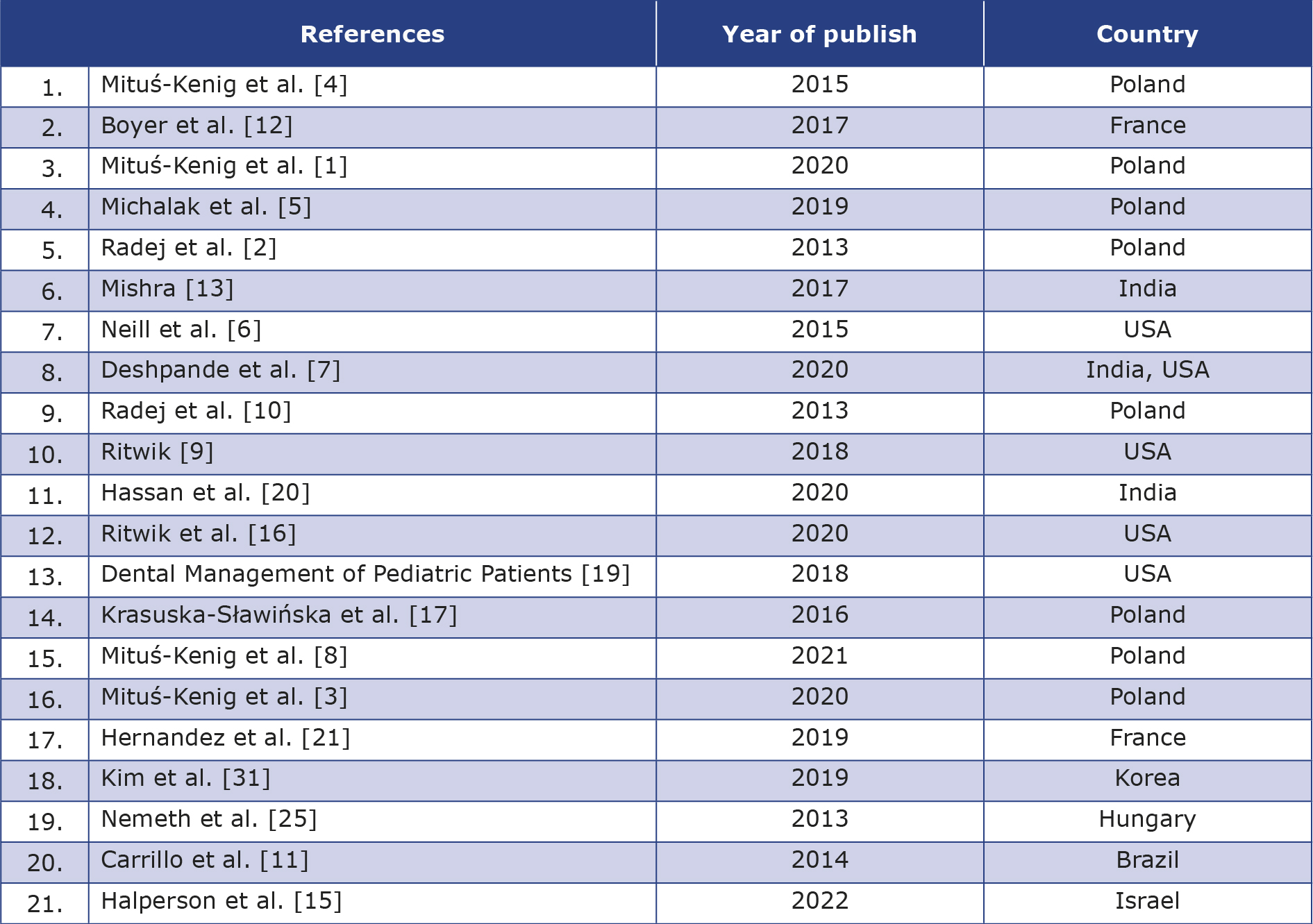
Table 1. List of included articles
Results and discussion
The reviewed articles did not include any statistical data, therefore analysis of factors such as sample size variations, potential biases or effect sizes was not possible. For this reason we were able to conduct a narrative review only. Articles included in this study were rated according to the Scale for the Assessment of Narrative Review Articles (SANRA) (Table 2) [13].
Table 2. Articles included in this study rated according to the Scale for the Assessment of Narrative Review Articles (SANRA);
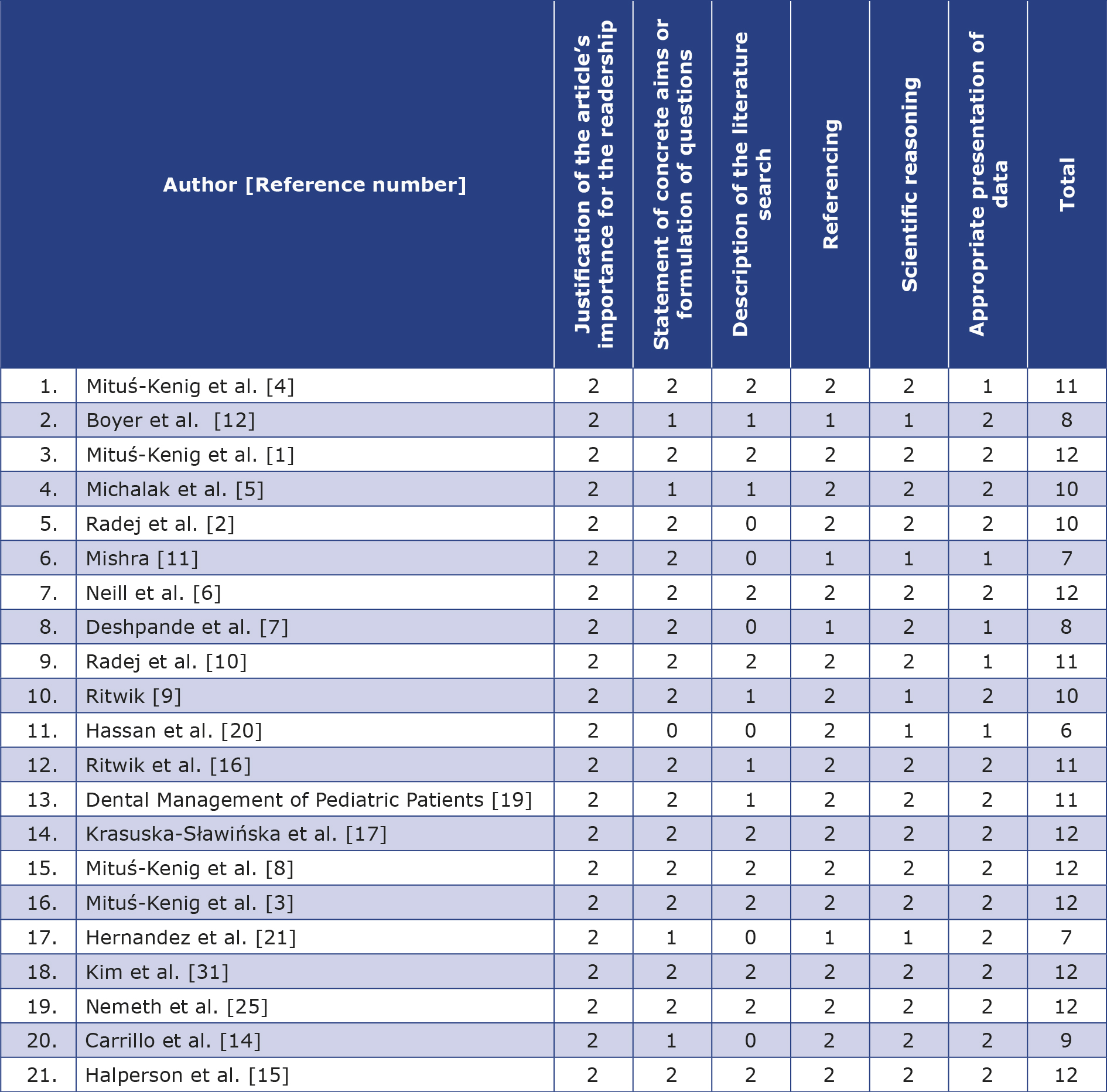
0 – low standard; 2 – high standard
Adverse effects
The adverse effects of oncological therapies conducted in childhood are caused by the cancer itself, the applied treatment (including immunosuppressive therapy), the supportive care or their combinations [5-6]. The severity and extent of adverse effects depend on the patient’s age (and thus their stage of development), psychological state, tumour-related factors (location, stage and extent at the time of detection), treatment (type, intensity and duration), as well as genetically conditioned sensitivity [4, 10, 14]. Systemic complications can be immediate or distant and influence the overall growth and development of children, including their hormonal, cardiovascular, respiratory, nervous, skeletal and reproductive systems [4, 15]. The development of the cranium, cervical vertebrae and the entire oral cavity (including teeth and jaws) is also altered [6, 9].
Overall, the younger the patient then the risk of adverse effects within the craniofacial bones is greater, particularly in children treated for cancer before the age of 5 [1, 8, 10]. This increases the risk of altered odontogenesis, which is also affected by exposure to higher doses of chemotherapeutic agents and radiation [1, 11]. Greater susceptibility to adverse effects were found in females [10] and during puberty [5, 10]. Childhood cancers usually respond well to chemotherapy due to the rapid growth of tumour tissue, however these drugs are not selective and also destroy healthy cells [12, 16]. Additionally, multi-drug chemotherapy ± radiotherapy complicate the assessment of the individual agent’s influence on the dental pulp and stages of odontogenesis [8, 14-15, 17]. Radiotherapy to the (CNS) results in a reduction in growth hormone and TSH secretion, leading to pituitary and thyroid function disorders [5, 10]. Roman et al. observed that chemotherapy was the only oncologic treatment method that disrupted children’s growth and caused growth hormone deficiency both during and after treatment [18]. Concurrent malnutrition during treatment and additional steroid therapy also impede a child’s growth [7]. All of this leads to changes in the onset of puberty and growth delay in the patient. Reduced growth may also be due to early puberty and shortening the duration of the growth spurt [10].
The most significant consequence of radiotherapy is hypovascularization and cytotoxic effects on growth plate chondrocytes [11-12]. Reduced blood supply to the bones leads to osteoradionecrosis, which is rare in childhood [5]. Chemotherapy interferes with bone development, leading to decreased mineral density, which may persist throughout life [2, 10-11]. In addition, it damages the bone remodelling system with a predominance of osteoclast action contributes to increased bone resorption and pathological fractures [11-12]. Radiotherapy to the head and neck area (e.g. during treatment of CNS tumours) during childhood results in various deformities of the craniofacial region (e.g. reduced cranial base), bone and soft tissue hypoplasia (including maxillary hypoplasia) and facial asymmetry [10-11]. Significant reduction in the height of alveolar bone in the anterior and lateral segments has been observed after combined chemo-radiotherapy in children, as well as shortening of all linear measurements in cephalometric analysis [10]. Individuals who have undergone full-body irradiation before bone marrow transplantation are particularly susceptible to growth delay in the temporomandibular joints leading to disorders, e.g. the condylar processes assuming a pathological anterior position [10]. Trismus and temporomandibular joint pain may also occur, affecting nutrition and oral hygiene [7].
Cancer treatment damages epithelial cells, leading to the thinning of the mucous membrane oral of the oral cavity which becomes very sensitive to, even the slightest, leading to easy irritation, injury and inflammation, resulting in painful ulcers and erosions [8, 12]. Cancer therapy reduces the regenerative abilities of the mucous membrane shows and its adverse effects are exacerbated by the presence of dental caries, dental plaque and other irritating factors (e.g. dental fillings or orthodontic brackets) [12]. Infections within the oral cavity are more likely to occur [5, 12]. Salivary glands produce in lesser quantity and poorer quality saliva (increased density and viscosity), resulting in xerostomia which may persist after completion of therapy and impede chewing and speaking [7-9, 12, 16, 19]. All these factors affect the pH of saliva, dental plaque formation, the composition of the mucosal microflora and the willingness and quality of maintaining oral hygiene [8, 12]. The quantity of cariogenic bacteria (particularly Streptococcus mutans) increases along with susceptibility to periodontal diseases and opportunistic fungal, bacterial and viral infections [5, 7, 9, 16, 20]. The progression of these infections may be atypical due to accompanying neutropenia [9, 16, 20]. Acute oral complications typically arise 5-7 days after the start of chemotherapy, corresponding to changes in the blood count parameters [9].
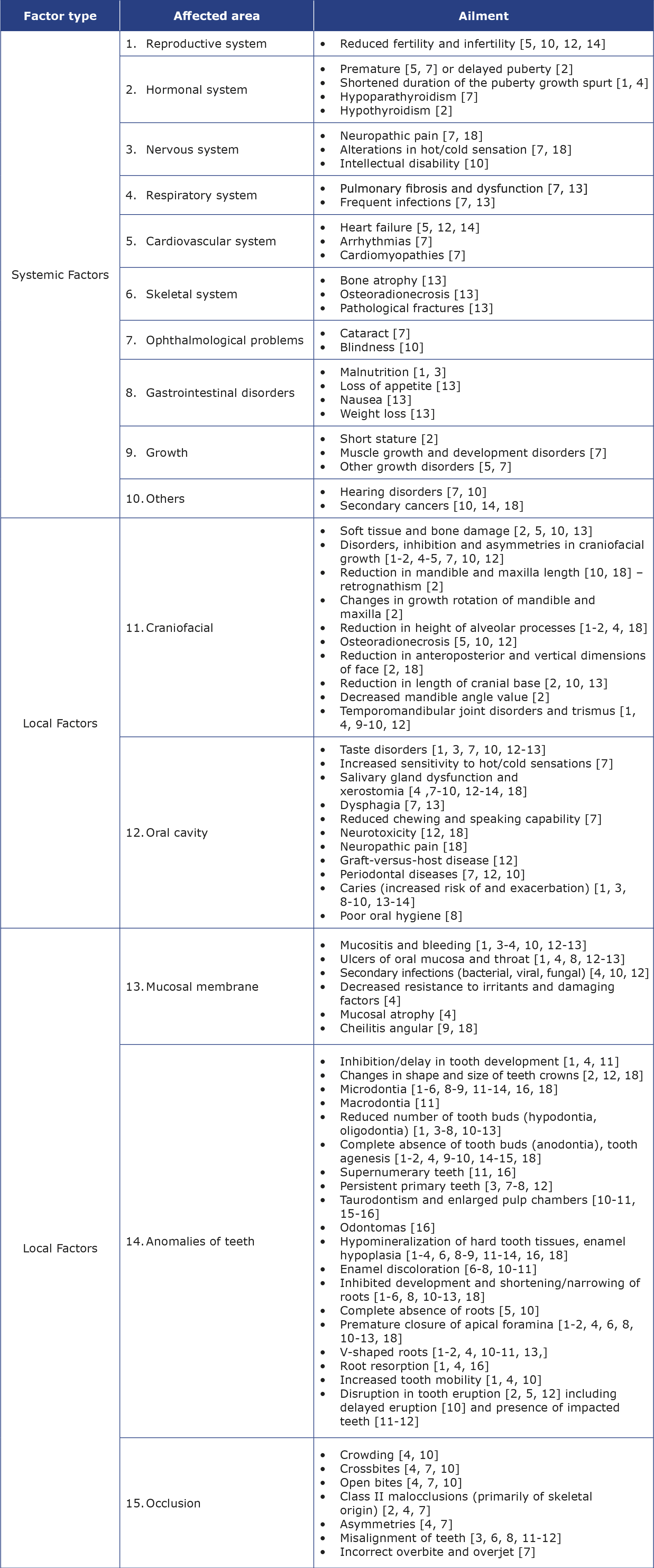
Long-term adverse effects of cancer treatment also include abnormalities in dental development, manifested by changes in both crowns and roots [5]. Table 3 contains a summary of adverse effects of cancer treatment. Their degree can vary from mild to severe and there is a strong correlation between the dosage and type of cancer treatment, its duration, the patient’s age, dental development stage and the frequency and severity of developmental tooth abnormalities [5, 9, 15-17, 19, 21]. The risk of disruptions during odontogenesis increases in children < 5 years of age and with increasing doses of radiation or chemotherapeutic agents [2, 6, 8]. The use of additional medications (e.g. antibiotics, immunosuppressants) also plays a role [21].
Table 3. Summary of the adverse effects of cancer treatment
Combined radiochemotherapy or radiation targeted at the head and neck area appears to increase the risk of dental anomalies [15]. Developmental tooth defects result from the direct action of chemotherapeutics on odontoblasts, which also delays the development of Hertwig’s epithelial root sheath, as well as indirectly through the influence of early chemotherapy complications such as vomiting and mucosal inflammation [17]. Amelogenesis and dentinogenesis may be disrupted during radiotherapy when the radiation beam is directed at the oral cavity or its immediate surroundings, but it has little impact on tooth formation when targeting distant body parts [5]. Radiotherapy affects cells during their mitotic division, disrupting enamel and dentin formation, whereas at very high doses it also damages non-proliferating cells [11].
The first signs of dental developmental disorders can be expected after 1-2 years of cancer treatment and they are visible on X-ray images [15] (Figure 2). Cancer treatment leads to changes in the shape, size of crowns and roots, degree of mineralization, enamel and dentin structure with frequent dental aplasia, therefore [1, 4, 6, 11, 15] hypodontia, microdontia, enamel hypoplasia and developmental root defects are typically observed [1, 4, 6, 11, 15]. Staining, discoloration and grooves on tooth crowns are often present [7, 10]. The tooth eruption process is also affected (often due to root development issues), leading to occlusal disturbances [2, 15]. Delays in primary tooth shedding and in permanent tooth replacement are frequent outcomes [3, 6-8].
Figure 2. Panoramic radiograph of a 12-year-old girl treated for a malignant eye tumour with chemotherapy from 5 weeks of age to 6 months of age. Visible V-shaped roots of teeth 16 and 26, reduced conical crowns of teeth 32 and 42 and root shortening of the lower incisors
All the above-mentioned adverse effects predispose to worsened aesthetics, function, dental misalignments and contribute to the development of malocclusions, which are mostly of skeletal aetiology [3, 6, 8-9, 15-16]. Crowding is observed, resulting from a lack of space in the dental arch as a consequence of maxillary hypoplasia [4, 10]. Malocclusions include crossbites, open bites, class II malocclusions and asymmetries [4, 7]. It’s important to note that hypodontia also leads to malocclusion by inhibiting facial skeletal growth [7]. Presence of microdontic teeth and reduced numbers of tooth buds result in unwanted interdental spaces and tooth displacements, leading to changes in tooth alignment and malocclusion development [1, 4].
In general, the systemic adverse effects of oncologic treatment occur in approximately 50% of patients [1-2, 4]. Dahloff and Huggare found that 93% of patients experienced at least 1 adverse effect, with an average of 3.7 adverse effects per person [22]. On the other hand, Geenen et al. stated that it affects nearly 75% of individuals [23]. Ritwik et al. emphasized that up to 60% of children treated for cancer suffer from infertility, heart failure and secondary tumours in the future [16]. Patients described by Radej et al. experienced stunted growth and thyroid dysfunction as complications of their oncologic treatment [2]. As adverse effects are most common after radiotherapy [10], the current standards recommend minimizing its use in favour of chemotherapy and surgery [10, 12].
Inflammation of oral mucosa (mucositis) after radiotherapy, chemotherapy and bone marrow transplantation affects up to 80%, 40% and 75% of paediatric patients, respectively [9]. It is noteworthy that mucositis is perceived by patients as the most painful complication of oncological treatment [16, 20].
Radiotherapy of the craniofacial structure leads to bone growth changes. It is noteworthy that the mandible was reported 4 times more sensitive to radiation compared to the maxilla [10]. There is a particular risk for the development of craniofacial disorders, (e.g. underdeveloped mandible), particularly with simultaneous chemo-radiotherapy. Sonis et al. noted greater retrognathism of the mandible in children irradiated under the age of 5 [24]. Radiotherapy in this age group often results in up to 14.72 times more frequent microdontia and root growth inhibition than in patients treated without it, although this risk decreases between the ages of 5-9 and above 10 [6]. Halperson et al. reported that tooth malformations were more common in patients treated at the age of 6 and younger (56%) compared to those treated between the ages of 6-12 (44%) [15]. The most significant growth occurs under the age of 5 and the adverse effects of chemo and radiotherapy are most apparent during adolescence [11]. Cephalometric measurements have shown a decrease exceeding 5% in the distance between sella-pogonion, sella-nasion and articulare-pogonion points. The most significant impairment in growth concerns the maxillary alveolar bone – its height decreased even by 50% [10].
Caries
Nemeth et al. address the issue of dental caries after cancer treatment. Researchers found that its prevalence is as high as 81.6%, which is 4.6% higher compared to healthy individuals [25]. Notably, post-radiation caries is extensive, has a rapid onset and aggressive progression [15]. The intensity of caries development can also be influenced by changes in oral microflora and a sweeter, claggy diet to compensate for feeding difficulties during cancer treatment [15, 25]. Treatment with chemotherapy only often results in fewer teeth affected by caries, missing teeth and fillings compared to individuals subjected to any amount of radiotherapy [15].
Oncological treatment and odontogenesis
Proc et al. demonstrated that dental abnormalities occur more frequently in cancer survivors (62%) compared to healthy individuals (13%) [26]. Halperson et al. reported that tooth anomalies after exclusive chemotherapy occurred in 43% of individuals and in 60% after radiotherapy in the head and neck region [15]. Teeth present in the irradiated field receive 45% of the applied dose [5]. According to Dahloff et al., a 10 Gy dose is the radiation dose that induces cell changes in developing permanent teeth [27]. Higher doses lead to the death of ameloblasts and odontoblasts, inhibiting further tooth tissue development and partially formed teeth remain in the bone due to root agenesis [5]. Nishimura et al. and Cubukcu et al. did not find any correlation between conventional chemotherapy duration and odontogenic disturbances [28-29]. Although no clear difference in the risk of dental abnormalities based on the child’s gender was observed, microdontia was more common in females, while caries was more prevalent in males [15]. Neill et al. reported that individuals treated with chemotherapy had 2.93 times fewer dental complications than those treated with combined therapy [6]. In contrast, treatment with radiotherapy or a combination of therapeutic methods yielded 5.07 times higher risk of root growth inhibition or microdontia. Researchers found that 72% of paediatric patients experienced ≥ 1 complication related to the stomatognathic system, while only 28% had no such complications. The most common complications were misalignment of teeth, root growth inhibition and changes in their development [6]. Halperson et al. stated that dental anomalies occurred in 46% of individuals after cancer therapy [15]. Vincristine is mainly responsible for these complications, although Halperson et al. did not find any specific chemotherapeutic agent to be more associated with dental abnormalities than others [15, 17].
According to Krasuska-Sławinska et al., 46.7% of patients after cancer treatment presented more than one developmental dental defect, most commonly shortening of roots (affecting 60% of patients), mainly in the permanent first molars (21.6%), followed by incisors and premolars (15% each), less frequently second molars (10%) [17]. The occurrence of these defects was not influenced by early chemotherapy complications, but by the age at the beginning of therapy and the administered doses. Tooth agenesis affected 26.67% of patients, while microdontia affected 21.67% [17]. Similarly, Cubukcu et al. noted a more frequent presence of developmental root deformities in 86.4% of patients previously treated for cancer [29]. Michalak et al. observed root problems, e.g. absence of roots, arrest of root development and abnormal shape of the newly formed permanent tooth roots (narrow and V-shaped) [5]. Radej et al. also highlighted the presence of shortened roots, their V-shape and inhibition of further development [2].
Enamel
Krasuska-Sławinska et al. noticed a positive correlation between enamel defects in permanent teeth, the age at the beginning of chemotherapy and its duration [17]. Enamel defects occur significantly more often in children after cancer treatment, including enamel hypoplasia and areas of opacities, affecting 76.7% of children. Vomiting post-chemotherapy was linked to enamel opacities, whereas mucositis was associated with enamel hypoplasia [17].
Microdontia
Krasuska-Sławinska et al. observed tooth size changes in up to 8 teeth of each paediatric patients after cancer treatment [17]. The number of affected teeth increased with the chemotherapy dose and duration, as well as the occurrence of vomiting and mucositis during treatment [17]. Microdontia of premolars and permanent second molars was most common. Other authors have highlighted that microdontia affects 19% of incisors and 45% of permanent second molars [1, 4]. Microdontia and tooth agenesis following chemotherapy before the age of 4 affected 66.7% of individuals, reaching 100% in high-dose cases [17]. Halperson et al. observed that when children began cancer treatment at age 6 or younger, they often experienced microdontia (33%), while those starting treatment later (between 6 and 12 years) had more hypocalcification or enamel hypoplasia (23%) [15]. Radej et al. also noted microdontia of second premolars and second molars in their patients after cancer treatment [2].
Hypodontia
According to Halperson et al. hypodontia is the most serious developmental disorder of dentition after cancer treatment, impacting dental arch symmetry, function and aesthetics [15]. Krasuska-Sławinska et al. indicated that the number of missing teeth increases with the chemotherapy dose and treatment duration, mainly affecting premolars (75% of children), second molars (25%) and lower incisors (12.5%) [17]. Michalak et al. found missing tooth buds of upper and lower second premolars and lower second molars in their studies as an adverse effect of cancer treatment [5]. Radej et al. observed mainly the absence of lower second molar buds in their patients after cancer treatment [2]. A cross-sectional study involving children after radiotherapy in Lyon (France), showed that 83% of them microdontic teeth and premature closure of root apical foramen, whereas facial asymmetries and delayed growth affected 74% [30].
Mental well-being
Hernandez et al. point out that odontogenic disorders also have psychological aspects. Dental abnormalities and malocclusion in children after cancer treatment represent vare stigmas that remind them of traumatic experiences and can impair the quality of life in adulthood [21]. All authors agree that complications after childhood cancer treatment significantly impact the potential for orthodontic treatment later in life. Yet, such patients require orthodontic treatment, which can help boost their self-confidence and enhance their overall self-esteem. Mituś-Kenig et al. demonstrated a positive impact of orthodontic treatment on the quality of life in patients with a cancer history, where the treatment duration had no significant effect [3].
Orthodontic treatment
First of all, a comprehensive orthodontic diagnosis is necessary, recognizing all tooth abnormalities alongside a full health evaluation and a review of the patient’s medical history [2, 6-7, 31]. Finding out the cancer diagnosis date and the end date of oncological therapy, along with obtaining written consent from the oncologist, is imperative to commence orthodontic treatment [7, 10]. Whenever possible, prior test results should be utilized to minimize additional radiation exposure to the patient [4]. The orthodontist must comprehend the underlying disease and assess the risk of complications resulting from cancer treatment [2, 11] Risk factors for orthodontic treatment complications in patients with a cancer history are presented in Table 4 [2, 5, 10].
Table 4. Summary of the risk factors for orthodontic treatment complications in patients with a cancer history [2, 5, 10]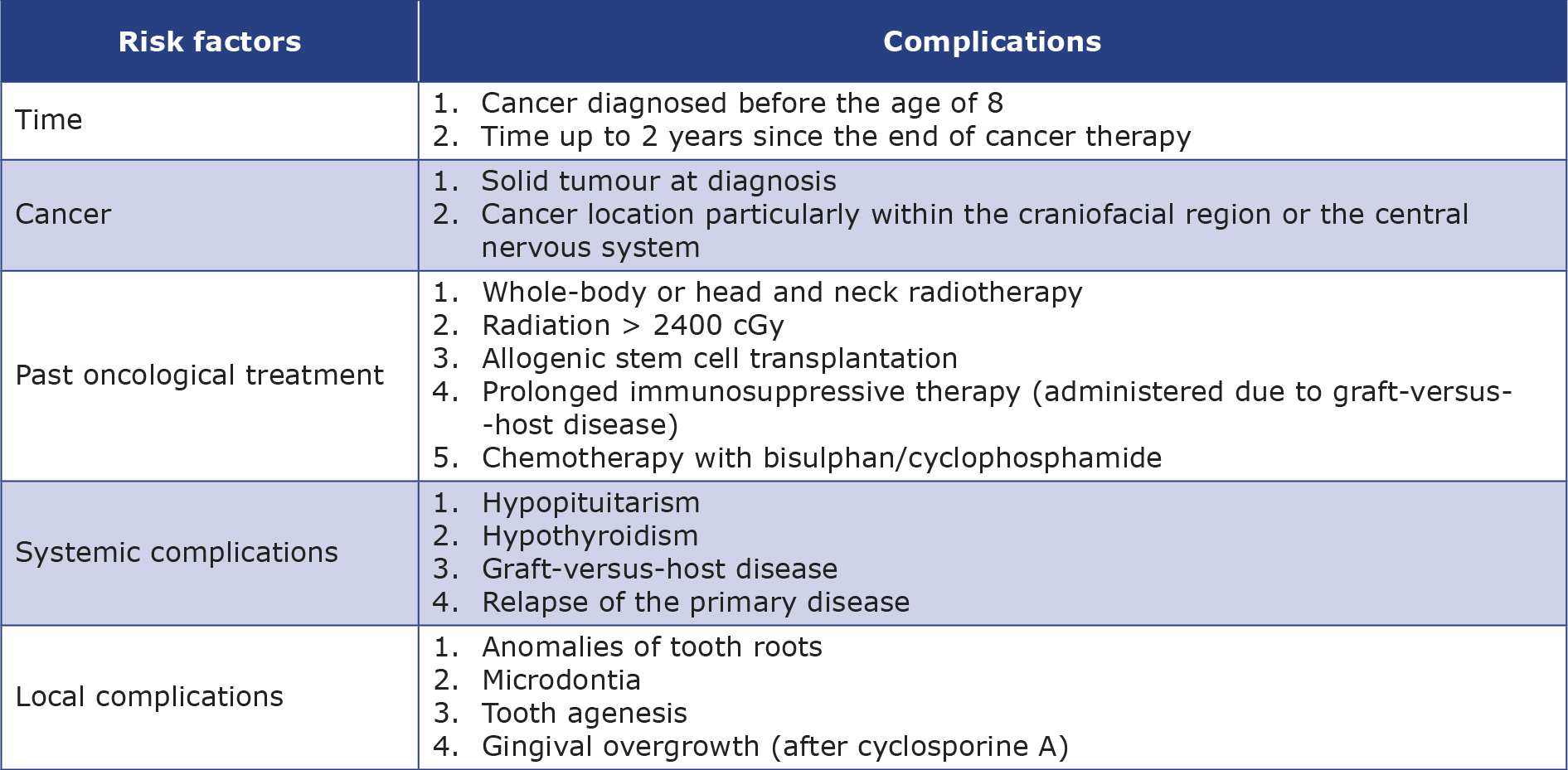
When formulating an individual orthodontic treatment plan, it is necessary to consider the complications arising from radiotherapy and chemotherapy, the patient’s overall health and medical prognosis [2, 4-5, 7, 10-11]. The outcome of the treatment of malocclusion is influenced by the degree of craniofacial growth disorders and the level of dental development following oncological treatment [2]. The orthodontist must anticipate various difficulties during the treatment and assess the options and accept possible compromises in the treatment results [4-5, 11]. Sometimes, it is necessary to develop an alternative treatment plan in case of failure due to shortened growth spurt and slowed growth of the child [2]. If any doubts arise, the orthodontist should contact the oncologist [2, 10, 12]. The reduction in the range of jaw opening, which accompanies temporomandibular joint dysfunctions after chemotherapy, presents an additional difficulty while making dental impressions and attaching the orthodontic appliance [5].
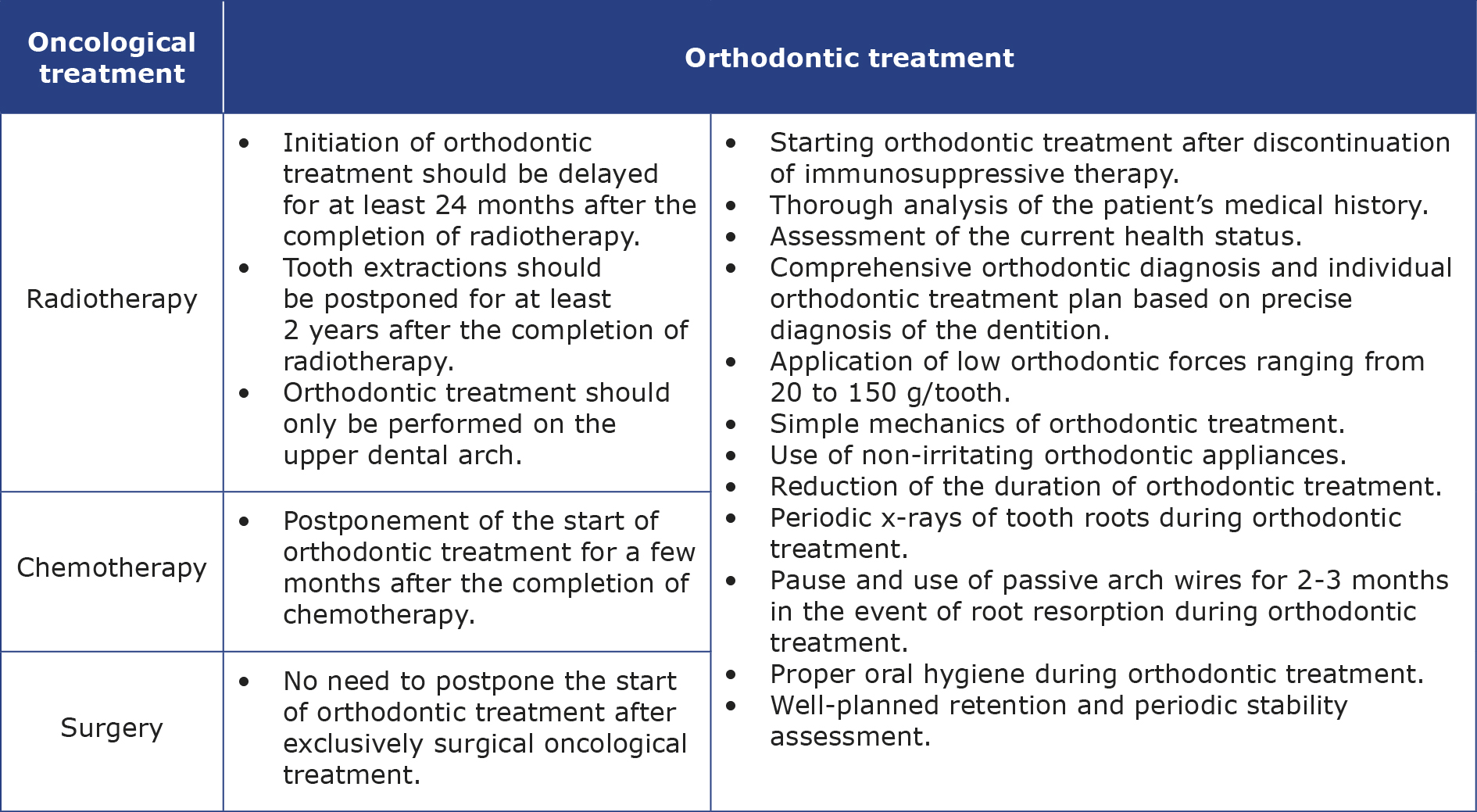
Orthodontic treatment must be not only deferred until the completion of the full cycle of anti-cancer therapy, but it is also recommended to postpone it by at least 24 months after the end of oncological treatment if no symptoms of cancer are present during this time [1-2, 4-5, 10-11]. This is related to bone metabolism disturbances caused by oncological treatment and the risk of cancer recurrence, which is reported in 2.6% - 12.1% of patients [4, 12]. An additional condition is the cessation of immunosuppressive treatment [1-2]. A shorter deferment period lasting a few months post-oncological therapy may be considered in patients treated with chemotherapy only [12]. In cases of exclusively surgical treatment, immediate commencement of orthodontic treatment is permissible without a 24-month latency period, provided the tumour has been completely excised and the lymph nodes are normal [2, 5, 10].
Treatment method and type of orthodontic appliance should be chosen carefully. The orthodontic forces should be low, (20 to 150 g/tooth) and the mechanics used as simple as possible to reduce the risk of root resorption [1-2, 4-5, 7-11, 19]. Points of force application must be carefully considered, anchorage and methods of affixing of the appliance must be closely monitored [2, 7]. Additionally, it is advisable to shorten the duration of orthodontic treatment as much as possible and finish earlier than usual [1-3, 5, 8-11, 19]. Due to the increased risk of osteoradionecrosis, tooth extraction (if required) should be postponed until 2 years after the completion of cancer therapy and performed atraumatically with precise wound management [2, 5, 10-11]. Oncological patients often have short, narrow roots that are particularly susceptible to resorption during movement [11-12]. This increases demands on anchorage and limits possible orthodontic movements [9-10]. Routine X-ray radiographs are necessary every 12-18 months to detect any changes in the tooth crown-to-root length ratio [10]. Additionally, Levander et al. recommend performing a panoramic radiograph after the first 6 months of active orthodontic treatment [32]. Observations suggest that rootless teeth can function in the oral cavity for some time (in most cases correctly), despite increased mobility [10]. Mituś-Kenig highlight the need for a pause in orthodontic treatment when signs of root resorption are observed [4]. There is no need to remove braces and it is recommended to use passive arch wires for 2-3 months [4]. Levander et al. have shown that thanks to a routine 2-3 month break in active orthodontic treatment and the use of passive arch wires after the first 6 months of treatment, the risk of advanced root resorption can be significantly reduced [32].
It is advisable to use protective orthodontic waxes and silicones along with appliances that irritate the mucous membrane as little as possible due to the patients’ reduced resistance to infections, decreased saliva secretion and increased sensitivity [2, 4-5, 10-11]. Nickel-containing steel brackets should be avoided due to the possibility of increased generation of free radicals that lead to cytotoxicity [7, 11]. If possible, it is better to choose aligners or ceramic brackets, which also cause significantly fewer artifacts in imaging tests [4, 10]. Before such tests, removable elements of the appliance should be removed and the quality of adhesion of the remaining elements should be checked [4].
Patient should maintain perfect oral hygiene to limit the development of caries in the course of the already reduced saliva production [4-5, 7, 11]. Severe xerostomia may constitute a contraindication to undertaking orthodontic treatment [7]. It is good to eliminate elastic ligatures in favour of metal ones and repeat hygiene instructions [4, 12]. A paedodontist (paediatric dentist) should also concurrently supervise such a patient and implement an individual fluoride prophylaxis plan [4-5, 7, 9, 11].
Radiotherapy of the head and neck region in a growing patient significantly worsens the prognosis of orthodontic treatment [5]. Due to shortened puberty and inhibited mandible growth, orthodontic treatment is suggested only in the upper dental arch, which additionally accelerates the orthodontic therapy [1, 4-5]. Treatment of Class II malocclusions is exceptionally challenging and modification of growth may be ineffective or not even possible [4, 10-11]. During functional orthodontic treatment, growth hormone therapy may be necessary to normalize the patient’s craniofacial growth [2, 5]. After completing growth hormone therapy, good effects of functional treatment can no longer be expected [2].
Michalak et al. [5] remind of the potential need for prosthetic reconstruction in patients after cancer treatment, which may be due to the high risk of tooth loss with aplastic roots or the absence of permanent tooth buds. Paediatric dentures used in such cases restore the ability to chew and improve speech and general facial aesthetics. However, prosthesizing conditions may often be unfavourable due to underdevelopment of alveolar bone. After growth cessation, dental implants can be placed [5]. Additionally, Deshpande et al. recommend the use of removable dentures whenever deemed important [7]. Regular check-ups are necessary as they can be a source of potential mucosal irritations. Proper hygiene of both oral cavity dentures must be maintained [7].
Hernandez et al. point out contraindications to orthodontic treatment in case of underdeveloped, too short permanent tooth roots due to a strong risk of their resorption [21]. It is necessary to monitor the eruption of such teeth, assess their mobility and try to keep the present primary teeth in the oral cavity as long as possible. In the case of failure, prosthetic treatment should be applied [21]. Radej et al. note that in the case of shortened, V-shaped roots of lower incisors, their intrusion is ill-advised, therefore, an orthodontic appliance cannot be used to level a deepened Spee’s curve [2]. Additionally, in the case of chronic gingivitis and decreased teeth mineralization, it is necessary to refrain from using fixed appliances. If there is a need for distalization of teeth example (e.g. to recreate space in the arch for canines), extractions should be chosen as a simpler treatment method [2]. In the case of treatment with removable appliances, they should be frequently checked, sharp areas should be smoothed and adjusted to current occlusal conditions to minimize the risk of mucosal irritation [2, 7]. Patients should frequently disinfect them by soaking in disinfectant solutions to limit microbial growth and the possibility of infections [19].
Mituś-Kenig et al. point out, that the results of orthodontic treatment in oncological patients do not significantly differ from healthy individuals [1, 4]. They reported no serious complications of orthodontic treatment and in most cases proper occlusion was achieved [1, 4]. However, patients after cancer treatment had mucositis and gingivitis while wearing braces more frequently and had root resorption slightly more often than healthy individuals. They also experienced a higher discomfort during the first 3 months of orthodontic treatment [1]. Mituś-Kenig et al. noted a significant decline in stability of orthodontic treatment effects among patients post-cancer treatment over a 3-year retention period compared to a healthy group [8]. Therefore, they require intensified observation to maintain the stability of orthodontic treatment effects and should be warned before starting treatment about the increased risk of relapse. It should also be considered that the stability of orthodontic treatment results also depends on factors related to the periodontal tissues and pressure from soft tissues, which were not considered in this study [8]. Retention after orthodontic treatment should be well planned. The retention using well-fitted appliances that do not irritate the mucous membrane (to prevent wounds and ulcers) [11]. The patient must constantly monitor them and maintain precise hygiene in their area. Retention splints can additionally be disinfected in a chlorhexidine solution [12].
Summary of guidelines for orthodontic treatment in patients after childhood cancer treatment is presented in Table 5.
Table 5. Summary of guidelines for orthodontic treatment in patients after childhood cancer treatment
Recurrence of cancer requires an immediate cessation of active orthodontic treatment and removal of fixed appliances, including space maintainers and bands, if cancer therapy may lead to mucositis and when oral hygiene is not adequate [7, 9-11, 19-21]. Removable appliances can be worn as long as they do not irritate mucous membrane and the patient can tolerate them [9, 16, 19-20]. The same approach should be taken if the patient develops cancer for the first time during orthodontic treatment [12]. Patients should be provided with a removable retainer [9-10, 19-20]. Resuming the original orthodontic treatment can be considered after achieving remission lasting at least 2 years [9-11, 16, 19].
The American Academy of Pediatric Dentistry reminds of the possibility of secondary cancers within the head and neck area, therefore, for the orthodontist it is very important to maintain oncological vigilance on the part of [19]. During orthodontic treatment, the orthodontist should pay attention to health status of the oral cavity at each check-up visit and, in case of any suspicious lesions, refer for further diagnostics to a specialist in mucosal diseases and oral surgeons [9, 16, 20].
Orthodontic treatment of individuals who have survived childhood cancer should be planned on an individual basis. The tissue response to the same treatment can vary, consequently various treatment results can be achieved. Additionally, each patient will perceive their new bite and the aesthetics of their teeth differently, just as there are various standards of beauty around the world. The outcome of orthodontic treatment may also be assessed differently by the orthodontists themselves. The stability of orthodontic treatment or the achieved bite is difficult to evaluate, as it requires close cooperation from the patient, who should attend regular check-ups after completing treatment with orthodontic appliances. For this reason, retrospective studies often do not include longterm follow-ups, which is a limitation of this review.
An unfortunate limitation of this review is the fact that none of the analyzed articles included information about new orthodontic techniques. Nowadays, with the help of intraoral scanners, it is possible to digitally record teeth and bite. Additionally, access to cone beam computed tomography (CBCT) scans enables a precise understanding of the dimensions and shapes of tooth roots along with the surrounding bone of the alveolar processes. This allows for the digital planning of favourable tooth movements and the installation of appliance components, as well as the prediction of potential adverse effects of orthodontic treatment. Based on this information, templates for appliance mounting can be made using 3D printers. Moreover, the use of skeletal anchorage systems temporary anchorage devices (TADs) or Bollard plates can significantly reduce the negative impact on tooth roots by applying forces directly to the bone, and more effectively modify growth in cases of jaw deformities, such as prognathism of mandible or constricted maxilla, even when there are no developed permanent tooth buds or when there are compromised teeth (e.g. those with shortened roots), making traditional braces unsuitable. In the future, it will likely be possible to treat orthodontic patients even more effectively due to the developments in AI technology, e.g. assisting orthodontists in the digital planning of orthodontic treatment, which may lead to potentially better outcomes with simultaneously fewer adverse effects.
Conclusions
Cancer treatment during childhood contributes to the development of a range of dental and skeletal abnormalities, including those of the craniofacial complex. Risk of their occurrence increases with use of combined chemotherapy and radiotherapy, particularly of the head and neck area, which significantly worsens orthodontic prognosis. Among the most commonly encountered developmental defects of teeth are abnormalities in root structure, lack of tooth buds and microdontia are. Orthodontists must be aware of the patient’s full medical history and take it into consideration each time when planning their treatment in order to prevent or at least minimize possible adverse outcomes. Orthodontic treatment should be modified using simple methods and appliances that do not irritate the mucous membrane, omit the mandible, employ lower forces, and ideally shorten the duration of orthodontic treatment. There is a need to introduce education about patients with a cancer history during the course of undergraduate dental education.
Acknowledgements
All figures are courtesy of the Department of Orthodontics, University Dental Centre of the Medical University of Gdańsk.
Funding
None.
Conflicts of interests
None.
References
| 1. |
Mitus-Kenig M, Derwich M, Czochrowska E, Pawlowska E. Quality of Life in Orthodontic Cancer Survivor Patients —A Prospective Case – Control Study. Int J Environ Res Public Health [Internet]. 2020 Aug 12;17(16):5824. Available from: https://www.mdpi.com/1660-4601/17/16/5824.
|
| 2. |
Radej I, Bugała-Musiatowicz B, Szarmach I, Grodzka I. Planning of orthodontic treatment in patients with a history of neoplastic disease – case reports. Orthod Forum [Internet]. 2013;9(40):267–83. Available from: https://publisherspanel.com/api/files/view/31320.pdf.
|
| 3. |
Mitus-Kenig M, Derwich M, Czochrowska E, Pawlowska E. Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study. Int J Environ Res Public Health [Internet]. 2020 Dec 4;17(23):9068. Available from: https://www.mdpi.com/1660-4601/17/23/9068.
|
| 4. |
Mituś-Kenig M, Łoboda M, Marcinkowska-Mituś A, Durka-Zajac M, Pawłowska E. Orthodontic treatment in oncological patients. Przegla̧d Lek [Internet]. 2015;72(5):243–5. Available from: https://ruj.uj.edu.pl/xmlui/bitstream/handle/item/136066/mitus-kenig_et-al_orthodontic_treatment_in_oncological_2015.pdf?sequence=1&isAllowed=y.
|
| 5. |
Michalak I, Kuśmierczyk D, Zadurska M. Radiological imaging and orthodontic treatment in the case of growing patients after oncological treatment: Case reports. Dent Med Probl [Internet]. 2019 May 10;56(2):209–15. Available from: https://dmp.umw.edu.pl/en/article/2019/56/2/209/.
|
| 6. |
Neill CC, Migliorati C, Trojan T, Kaste S, Karydis A, Rowland C, et al. Experience and expertise regarding orthodontic management of childhood and adolescent cancer survivors. Am J Orthod Dentofac Orthop [Internet]. 2015 Nov;148(5):765–70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889540615008938.
|
| 7. |
Deshpande M, Jain D, Mehta S, Bhat D, Gambhir L, Patel R. Orthodontic Care in Pediatric Cancer Survivors: A Review. J Dent Oral Sci [Internet]. 2020 Apr 3;2(3):1–6. Available from: https://maplespub.com/article/Orthodontic-Care-in-Pediatric-Cancer-Survivors-A-Review.
|
| 8. |
Mitus-Kenig M, Derwich M, Czochrowska E, Pawlowska E. Cancer survivors present significantly lower long-term stability of orthodontic treatment: a prospective case–control study. Eur J Orthod [Internet]. 2021 Dec 1;43(6):631–8. Available from: https://academic.oup.com/ejo/article/43/6/631/6088062.
|
| 9. |
Ritwik P. Dental Care for Patients With Childhood Cancers. Ochsner J [Internet]. 2018 Dec 13;18(4):351–7. Available from: http://www.ochsnerjournal.org/lookup/doi/10.31486/toj.18.0061.
|
| 10. |
Radej I, Bugała-Musiatowicz B, Szarmach I, Grycz M. Orthodontic treatment for oncological patients – literature review. J Stoma. 2013;66(4):517–33. Available from: https://www.researchgate.net/publication/273376720_Orthodontic_treatment_for_oncological_patients_-_Literature_review.
|
| 11. |
Sumita M. Orthodontic Therapy for Paediatric Cancer Survivors: A Review. J Clin DIAGNOSTIC Res [Internet]. 2017;11(3):ZE01–4. Available from: http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2017&volume=11&issue=3&page=ZE01&issn=0973-709x&id=9404.
|
| 12. |
Boyer É, Robert G, Gandemer V, Bonnaure-Mallet M. Orthodontic strategies in pediatric oncology. J Dentofac Anomalies Orthod [Internet]. 2017 Apr 23;20(1):104. Available from: https://www.jdao-journal.org/10.1051/odfen/2016035.
|
| 13. |
Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev [Internet]. 2019 Dec 26;4(1):5. Available from: https://researchintegrityjournal.biomedcentral.com/articles/10.1186/s41073-019-0064-8.
|
| 14. |
Carrillo CM, Corrêa FNP, Lopes NNF, Fava M, Filho VO. Dental anomalies in children submitted to antineoplastic therapy. Clinics [Internet]. 2014 Jun;69(6):433–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1807593222009073.
|
| 15. |
Halperson E, Matalon V, Goldstein G, Saieg Spilberg S, Herzog K, Fux-Noy A, et al. The prevalence of dental developmental anomalies among childhood cancer survivors according to types of anticancer treatment. Sci Rep [Internet]. 2022 Mar 16;12(1):4485. Available from: https://www.nature.com/articles/s41598-022-08266-1.
|
| 16. |
Ritwik P, Chrisentery-Singleton TE. Oral and dental considerations in pediatric cancers. Cancer Metastasis Rev [Internet]. 2020 Mar 27;39(1):43–53. Available from: http://link.springer.com/10.1007/s10555-020-09842-5.
|
| 17. |
Krasuska-Sławińska E, Brożyna A, Dembowska-Bagińska B, Olczak-Kowalczyk D. Antineoplastic chemotherapy and congenital tooth abnormalities in children and adolescents. Współczesna Onkol [Internet]. 2016;5:394–401. Available from: http://www.termedia.pl/doi/10.5114/wo.2016.64602.
|
| 18. |
Román J, Villaizán CJ, García-Foncillas J, Salvador J, Sierrasesúmaga L. Growth and growth hormone secretion in children with cancer treated with chemotherapy. J Pediatr [Internet]. 1997 Jul;131(1):105–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S002234769770132X.
|
| 19. |
Dental Management of Pediatric Patients Receiving Immunosuppressive Therapy and/or Radiation Therapy. Pediatr Dent [Internet]. 2018 Oct 15;40(6):392–400. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32074911.
|
| 20. |
Ali Hassan S, Bhateja S, Arora G, Prathyusha F. Dental care of pediatric cancer patient. IP Int J Med Paediatr Oncol [Internet]. 2020 Jul 28;6(2):43–7. Available from: https://ijmpo.com/article-details/11697.
|
| 21. |
Hernandez M, Pochon C, Chastagner P, Droz D. Long-term Adverse Effects of Acute Myeloid Leukemia Treatment on Odontogenesis in a Child. Int J Clin Pediatr Dent [Internet]. 2019 Jun;12(3):243–6. Available from: https://www.ijcpd.com/doi/10.5005/jp-journals-10005-1614.
|
| 22. |
Dahllöf G, Huggare J. Orthodontic considerations in the pediatric cancer patient: A review. Semin Orthod [Internet]. 2004 Dec;10(4):266–76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1073874604000520.
|
| 23. |
Geenen MM, Cardous-Ubbink MC, Kremer LCM, van den Bos C, van der Pal HJH, Heinen RC, et al. Medical Assessment of Adverse Health Outcomes in Long-term Survivors of Childhood Cancer. JAMA [Internet]. 2007 Jun 27;297(24):2705. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.297.24.2705.
|
| 24. |
Sonis AL, Tarbell N, Valachovic RW, Gelber R, Schwenn M, Sallan S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia: A comparison of three treatment modalities. Cancer [Internet]. 1990 Dec 15;66(12):2645–52. Available from: https://onlinelibrary.wiley.com/doi/10.1002/1097-0142(19901215)66:12%3C2645::AID-CNCR2820661230%3E3.0.CO;2-S.
|
| 25. |
Nemeth O, Hermann P, Kivovics P, Garami M. Long-term Effects of Chemotherapy on Dental Status of Children Cancer Survivors. Pediatr Hematol Oncol [Internet]. 2013 Mar 13;30(3):208–15. Available from: http://www.tandfonline.com/doi/full/10.3109/08880018.2013.763391.
|
| 26. |
Proc P, Szczepańska J, Skiba A, Zubowska M, Fendler W, Młynarski W. Dental Anomalies as Late Adverse Effect among Young Children Treated for Cancer. Cancer Res Treat [Internet]. 2016 Apr 15;48(2):658–67. Available from: http://e-crt.org/journal/view.php?doi=10.4143/crt.2015.193.
|
| 27. |
Dahllöf G, Rozell B, Forsberg C-M, Borgström B. Histologic changes in dental morphology induced by high dose chemotherapy and total body irradiation. Oral Surgery, Oral Med Oral Pathol [Internet]. 1994 Jan;77(1):56–60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0030422006801076.
|
| 28. |
Nishimura S, Inada H, Sawa Y, Ishikawa H. Risk factors to cause tooth formation anomalies in chemotherapy of paediatric cancers. Eur J Cancer Care (Engl) [Internet]. 2013 May 21;22(3):353–60. Available from: https://onlinelibrary.wiley.com/doi/10.1111/ecc.12038.
|
| 29. |
Cubukcu CE, Sevinir B, Ercan İ. Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr Blood Cancer [Internet]. 2012 Jan 19;58(1):80–4. Available from: https://onlinelibrary.wiley.com/doi/10.1002/pbc.22902.
|
| 30. |
Akharzouz C, Chauty S, Bodard A-G. Enfants ayant reçu une irradiation de la région cranio-cervico-faciale : évaluation du besoin de traitement orthodontique. L’Orthodontie Française [Internet]. 2013 Jun 30;84(2):157–68. Available from: https://www.jle.com/10.1051/orthodfr/2013047.
|
| 31. |
Kim J-H, Jih M. An orthodontic approach for Class III malocclusion in a pediatric cancer patient: A case report. Oral Biol Res [Internet]. 2019 Mar 31;43(1):103–9. Available from: http://www.chosunobr.org/journal/view.html?doi=10.21851/obr.43.01.201903.103.
|
| 32. |
Levander E, Malmgren O, Eliasson S. Evaluation of root resorption in relation to two orthodontic treatment regimes. A clinical experimental study. Eur J Orthod [Internet]. 1994 Jun 1;16(3):223–8. Available from: https://academic.oup.com/ejo/article-lookup/doi/10.1093/ejo/16.3.223.
|














