Hormonal profiles and the diagnostic utility of serum dihydrotestosterone in polycystic ovary syndrome: a comparative case-control study
Abstract
Background: Polycystic ovary syndrome (PCOS) is a prevalent endocrine disorder characterized by hyperandrogenism, anovulation, and metabolic disturbances. Dihydrotestosterone (DHT), a potent androgen formed via the 5α-reductase pathway, may play a role in the clinical manifestations of PCOS. This study aimed to compare serum DHT levels in women with PCOS versus healthy controls and to evaluate its diagnostic value.
Methods: In this case-control study, women aged 18-45 years were recruited from outpatient clinics at the Maysan Specialized Surgical Hospital (Al-Amarah, Iraq). PCOS was diagnosed according to the Rotterdam criteria, while age-matched healthy volunteers with regular menstrual cycles served as controls. All participants underwent comprehensive clinical assessments, including anthropometric measurements. Fasting venous blood samples were collected between 8:00 and 10:00 AM, and serum was isolated for biochemical analysis. Serum DHT concentrations were measured using a competitive ELISA. Statistical analyses, including group comparisons and receiver operating characteristic (ROC) curve analysis, were performed using IBM SPSS Statistics.
Results: Participants with PCOS had significantly higher serum DHT levels (4256 ± 233 ng/L) compared to controls (3776 ± 186 ng/L, p < 0.001). ROC curve analysis identified an optimal DHT cutoff value of approximately 408.3 ng/L, yielding a sensitivity of 90% and specificity of 100% for PCOS diagnosis.
Conclusion: Elevated serum DHT is a key feature of PCOS and demonstrates excellent diagnostic accuracy. These findings support the clinical utility of DHT as a reliable biomarker for early PCOS detection and may facilitate targeted therapeutic interventions.
Citation
Hmoud T R, Jawad M M, Alredha R D. Hormonal profiles and the diagnostic utility of serum dihydrotestosterone in polycystic ovary syndrome: a comparative case-control study. Eur J Transl Clin Med. 2025;8(2):17-24Introduction
Polycystic ovary syndrome (PCOS) is one of the most prevalent endocrine disorders affecting women of reproductive age, with a multifaceted presentation that includes hyperandrogenism, chronic anovulation, and polycystic ovarian morphology [1]. Hyperandrogenism is a central feature in PCOS and is typically assessed by measuring serum levels of androgens such as total testosterone and free testosterone. However, dihydrotestosterone (DHT), a potent androgen metabolite of testosterone, has garnered increasing interest due to its higher affinity for androgen receptors and its significant biological activity [2].
DHT is formed from testosterone via the catalytic action of the 5α-reductase enzyme. This conversion is critical because DHT is approximately 2-3 times more potent than testosterone in activating androgen receptors, thereby exerting more pronounced effects on target tissues [3]. Clinically, elevated DHT levels have been linked to manifestations such as hirsutism, acne, and androgenic alopecia – symptoms that are commonly observed in women with PCOS [4]. Despite these observations, the specific role and diagnostic utility of DHT in PCOS remain underexplored compared to other androgens.
The mechanisms leading to increased DHT levels in PCOS are multifactorial. A dysregulated gonadotropin milieu – marked by an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio – stimulates ovarian theca cells to enhance androgen production [4]. Furthermore, insulin resistance and the resultant hyperinsulinemia, which are common in PCOS, can amplify androgen synthesis by upregulating the activity of key steroidogenic enzymes, including 5α-reductase [5]. This hyperinsulinemic state not only accelerates the conversion of testosterone to DHT but also reduces hepatic production of sex hormone-binding globulin (SHBG), thereby increasing the levels of free and bioactive DHT.
Previous research in PCOS has predominantly focused on total and free testosterone measurements, often neglecting the potential diagnostic significance of DHT [6]. Given the potent androgenic properties of DHT, its measurement might provide a more sensitive indicator of hyperandrogenism. Emerging evidence suggests that DHT could serve as a reliable biomarker, offering enhanced diagnostic precision in identifying PCOS compared to traditional androgen assays [7]. Moreover, correlations between elevated DHT levels and the severity of clinical hyperandrogenic symptoms underscore its potential utility in both diagnosis and in assessing disease severity.
The present study is designed to investigate the serum DHT levels in women diagnosed with PCOS relative to healthy controls, and to evaluate its diagnostic performance using receiver operating characteristic (ROC) curve analysis. By focusing on DHT, we aim to clarify its contribution to the pathophysiology of PCOS and to establish its potential role as a diagnostic biomarker. This focus is particularly timely given the clinical challenges in diagnosing PCOS and the need for more precise and reliable biomarkers that can guide therapeutic strategies.
Materials and methods
Study design and ethical considerations
This case-control study was conducted at the Maysan Child and Birth Hospital (Al-Amarah, Iraq) between October 8th, 2024 and January 31st, 2025. The study protocol was approved by the Institutional Review Board (7-37-4480) and adhered to the ethical principles outlined in the Declaration of Helsinki [8]. All participants provided written informed consent prior to enrollment.
Participant recruitment and election
Participants were recruited from the outpatient endocrinology and gynecology clinics at Maysan Child and Birth Hospital. Women aged 18-45 years were considered for inclusion. PCOS diagnosis was made according to the Rotterdam criteria [9], which require the presence of at least two of the following: oligo/anovulation, clinical and/or biochemical hyperandrogenism, and polycystic ovarian morphology on ultrasound. Controls were healthy volunteers with regular menstrual cycles and no clinical signs of hyperandrogenism. Exclusion criteria for all participants included the use of hormonal medications within the preceding three months, pregnancy, and any diagnosed endocrine disorders (e.g. thyroid dysfunction, hyperprolactinemia).
Clinical assessment and anthropometry Participants underwent a detailed clinical evaluation that included a structured interview and physical examination. Anthropometric measurements were recorded following standard protocols (Lohman et al., 1988) [10]. Height was measured to the nearest 0.1 cm using a calibrated stadiometer (Cat. No. ST-100, Seca GmbH, Germany), and body weight was assessed using a digital scale (Cat. No. DS-200, Tanita Corporation, Japan) to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
Sample collection and processing
Venous blood samples were collected between 8:00 and 10:00 AM after an overnight fast. Blood was drawn into plain serum separator tubes (Vacutainer®, BD Biosciences, USA) and allowed to clot at room temperature for 30 minutes. Samples were then centrifuged at 3 000 rpm for 10 minutes, and the serum was aliquoted into polypropylene tubes (Cat. No. PP-500, Eppendorf, Germany). The serum samples were immediately stored at –80°C until further analysis.
Biochemical measurements
Fasting blood glucose (FBG) was measured using the glucose oxidase method on an automated analyzer (Bio Research for Medical Diagnostics, Jordan). Serum insulin was quantified via emiluminescent fluorescence immunoassay on an automated analyzer (A. Menarini Diagnostics S.r.l., Italy), and insulin resistance was evaluated using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) formula:
Reproductive hormones (LH, FSH, testosterone, estradiol, and progesterone) were measured by AFIAS-10 automated fluorescence immunoassay system, on an automated analyzer (AFIAS-10, A. Menarini Diagnostics S.r.l., Italy), ensuring intra- and inter-assay variability below 8% and 10%, respectively. Serum epiregulin levels were quantified using a Human Epiregulin ELISA Kit (R&D Systems, USA), following standard ELISA procedures, with optical density measured at 450 nm using a microplate reader (BioTek ELx800, Agilent, USA). Assay sensitivity and variability were maintained as per manufacturer specifications and all samples were analyzed in duplicate, with necessary dilutions for values exceeding the assay’s dynamic range.
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 25.0 (IBM Corp., USA). Continuous variables are reported as mean ± standard deviation (SD), whereas the categorical variables as frequencies and percentages. The normality of continuous data was evaluated using the Shapiro-Wilk test. For normally distributed data, group comparisons were performed using the Student’s t-test; otherwise, the Mann-Whitney U test was applied. Categorical data were compared using the Chisquare test (χ2 ). Receiver operating characteristic (ROC) curve analysis was employed to assess the diagnostic performance of serum DHT, with sensitivity, specificity, and the area under the curve (AUC) reported. A p-value < 0.05 was considered statistically significant.
Results and discussion
The demographic characteristics of the control and study groups are summarized in Table 1. There was no statistically significant difference between the PCOS and control groups in terms of age or BMI. As shown in Table 2, menstrual irregularity was significantly more prevalent among PCOS patients, while marital status and history of PCOS showed no significant difference between groups (Table 2). The hormonal profile comparisons significantly elevated levels of LH, FSH, and prolactin in the PCOS group (Table 3). Notably, LH levels were approximately threefold higher in PCOS patients. Serum dihydrotestosterone (DHT) levels were significantly elevated in PCOS participants compared to controls (Table 4). The diagnostic performance of DHT is presented in Table 5. ROC analysis demonstrated a high sensitivity (86%) and perfect specificity (100%) at a DHT cutoff value of > 4084.3 ng/L. The ROC curve shows excellent diagnostic accuracy (AUC = 93%, p < 0.001) (Figure 1).
Table 1. Demographic characteristics of the control and PCOS groups
I – independent samples t-test; NS – not significant; SD – standard deviation.
Statistical significance was indicated by ***p < 0.001.
Table 2. Menstrual cycle regularity, marital status and PCOS history in control and PCOS groups
I – independent samples t-test; SD – standard deviation.
Statistical significance was indicated by ***p < 0.001.
Table 3. Mean hormone levels in control and PCOS groups
I – independent samples t-test; SD – standard deviation.
Statistical significance was indicated by ***p < 0.001.
Table 4. Serum dihydrotestosterone levels in control and PCOS groups
I – independent samples t-test; SD – standard deviation.
Statistical significance was indicated by ***p < 0.001.
Table 5. ROC curve analysis for serum dihydrotestosterone in PCOS diagnosis
AUC – area under curve; NPV – negative predictive value; PPV – positive predictive value; Sens – Sensitivity; Spec – Specificity
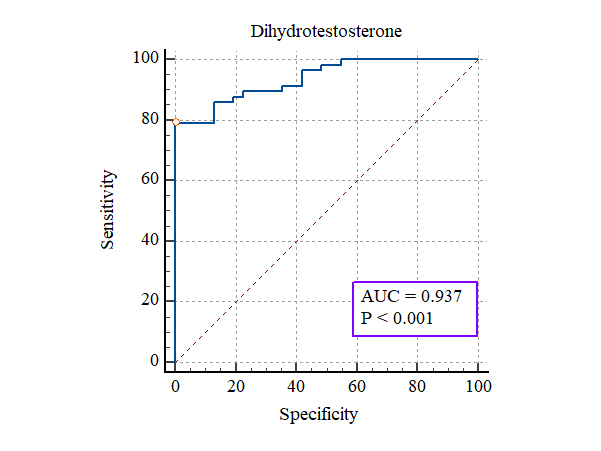
Figure 1. ROC curve analysis for serum dihydrotestosterone in
PCOS diagnosis.
AUC – area under the curve
In this study we aimed to evaluate the hormonal profile of women with polycystic ovary syndrome (PCOS) compared to controls and to evaluate the diagnostic utility of serum dihydrotestosterone (DHT) in this population. Our findings confirm the well‐documented hyperandrogenic state in PCOS and offer compelling evidence for the use of serum DHT as a diagnostic biomarker.
Our demographic analysis revealed that the control and PCOS groups were well‐matched in terms of age and body mass index (BMI), with mean values of 28.8 ± 7.8 years versus 25.3 ± 4.5 years and 27.4 ± 3.6 kg/m2 versus 28.1 ± 4.8 kg/m2 , respectively. These findings are in line with previous research that has shown minimal demographic differences between PCOS and non‐PCOS cohorts when stringent inclusion criteria are applied. However, the markedly higher prevalence of menstrual irregularity in the PCOS group – where only 13% of patients reported regular cycles compared to 85% of controls – confirms the clinical phenotype associated with PCOS as reported by Panidis et al. [11].
The endocrine profile of our PCOS cohort further underscored the syndrome’s characteristic hormonal imbalances. Significantly elevated luteinizing hormone (LH) levels (11.8 ± 5.8 mIU/ml vs. 3.9 ± 1.09 mIU/ml, p < 0.001) and increased follicle-stimulating hormone (FSH) and prolactin levels were observed, which corroborate earlier findings. For example, Legro et al. (1999) reported mean LH levels in PCOS patients of approximately 12.5 ± 5.0 mIU/ml, a value strikingly similar to our results. The modest yet significant increase in FSH (6.09 ± 2.2 mIU/ml vs. 5.11 ± 1.4 mIU/ml, p = 0.03) and prolactin (23.9 ± 7.7 ng/ml vs. 17.6 ± 6.4 ng/ml, p = 0.002) further supports the presence of an altered gonadotropin milieu in PCOS, although the degree of alteration may vary between populations [12].
Of particular note in our study is the substantial increase in serum DHT levels among PCOS patients (4256 ± 233 ng/L) compared to controls (3776 ± 186 ng/L, p < 0.001). we found that women with polycystic ovary syndrome (PCOS) exhibited significantly higher serum DHT levels (4256 ± 233 ng/L) compared to controls (3776 ± 186 ng/L, p < 0.001), reaffirming the hyperandrogenic state characteristic of PCOS. DHT, a potent androgen derived from testosterone through the action of the 5α-reductase enzyme, is increasingly recognized as a key mediator of the clinical manifestations of PCOS, such as hirsutism and acne [13].
The mechanism driving the elevation of DHT in PCOS appears to be multifactorial. One of the central factors is the increased activity of 5α-reductase, which converts testosterone into DHT. In PCOS, ovarian theca cells are hyperstimulated (primarily by elevated LH levels) which upregulates the expression and activity of steroidogenic enzymes, including 5α-reductase [14]. Additionally, insulin resistance (a common feature in PCOS) leads to compensatory hyperinsulinemia. Elevated insulin levels are known to enhance ovarian androgen synthesis by stimulating the activity of enzymes such as CYP17A1 and 5α-reductase, while concurrently reducing hepatic synthesis of sex hormone-binding globulin (SHBG) [15]. This dual action not only boosts the production of androgens but also increases the proportion of free, bioactive androgens available to exert their effects.
Our findings are consistent with previous studies reporting hyperandrogenism in PCOS. For instance, Zeng et al. documented elevated androgen levels in PCOS patients, although most investigations have focused on total testosterone rather than DHT specifically [16]. By concentrating on DHT, our study provides novel insights into the hyperandrogenic profile of PCOS, suggesting that increased 5α-reductase activity and the resultant DHT accumulation may be more directly linked to the clinical severity of the syndrome.
At the molecular level, hyperinsulinemia plays a crucial role by upregulating genes encoding steroidogenic enzymes. This promotes an enhanced conversion of testosterone to DHT, which may further exacerbate the metabolic and reproductive disturbances observed in PCOS [17]. Moreover, the strong correlation between DHT levels and the clinical markers of hyperandrogenism in our cohort underscores the potential of DHT as a reliable biomarker for disease diagnosis and progression.
Clinically, the strong diagnostic performance of serum DHT, as evidenced by our ROC curve analysis (with an optimal cutoff yielding 90% sensitivity and 100% specificity), highlights its utility as a non-invasive diagnostic tool for PCOS. Early detection of hyperandrogenism using DHT measurements could facilitate timely intervention, potentially mitigating long-term complications such as metabolic syndrome and infertility. Furthermore, our study suggests that targeting insulin resistance (through lifestyle interventions or pharmacotherapy) may reduce DHT levels and ameliorate hyperandrogenic symptoms, thereby offering a promising therapeutic avenue. The novelty of our study lies in the focused evaluation of DHT, rather than the more commonly assessed total testosterone, providing additional insight into the androgenic profile of PCOS.
Despite its strengths, this study has several limitations. The case-control design precludes any inference of causality. The sample size, while adequate for initial comparisons, may limit the generalizability of our findings. Furthermore, immunoassays for DHT quantification are practical, yet may be less precise than liquid chromatography-tandem mass spectrometry (LC-MS/MS) [18]. Future studies should include longitudinal design and largger, more diverse populations to validate these findings.
Conclusions
In conclusion, our study confirms that women with PCOS exhibit a distinct hormonal profile characterized by elevated LH, FSH, prolactin, and particularly DHT levels. The diagnostic performance of serum DHT is promising, offering a novel tool for the accurate identification of PCOS. These results not only align with previous literature but also contribute new insights into the pathophysiology and potential diagnostic strategies for PCOS.
Acknowledgments
We sincerely appreciate all the participants who volunteered for this study, as their contribution was essential to this research. We are also grateful to the medical and nursing staff for their invaluable support in patient recruitment and sample collection.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
No funds were received to complete this work.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
References
| 1. |
Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol [Internet]. 2018;132(2):321–36. Available from: https://journals.lww.com/00006250-201808000-00009.
|
| 2. |
Pfaff DW, Baum MJ. Hormone-dependent medial preoptic/lumbar spinal cord/autonomic coordination supporting male sexual behaviors. Mol Cell Endocrinol [Internet]. 2018;467:21–30. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0303720717305646.
|
| 3. |
Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab [Internet]. 2022;36(4):101665. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1521690X22000525.
|
| 4. |
Taieb A, Feryel A. Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review. Diagnostics [Internet]. 2024;14(22):2578. Available from: https://www.mdpi.com/2075-4418/14/22/2578.
|
| 5. |
Moghetti P. Insulin resistance and polycystic ovary syndrome. Curr Pharm Des [Internet]. 2016;22(36):5526–34. Available from: https://www.ingentaconnect.com/content/ben/cpd/2016/00000022/00000036/art00004.
|
| 6. |
Abdelazim I, Alanwar A, AbuFaza M, Amer O, Bekmukhambetov Y, Zhurabekova G, et al. Elevated and diagnostic androgens of polycystic ovary syndrome. Menopausal Rev [Internet]. 2020;19(1):1–5. Available from: https://www.termedia.pl/doi/10.5114/pm.2020.95293.
|
| 7. |
Bizuneh AD, Joham AE, Teede H, Mousa A, Earnest A, Hawley JM, et al. Evaluating the diagnostic accuracy of androgen measurement in polycystic ovary syndrome: a systematic review and diagnostic meta-analysis to inform evidence-based guidelines. Hum Reprod Update [Internet]. 2025;31(1):48–63. Available from: https://academic.oup.com/humupd/article/31/1/48/7762932.
|
| 8. |
Goodyear MDE, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ [Internet]. 2007;335(7621):624–5. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.39339.610000.BE.
|
| 9. |
Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J Clin Endocrinol Metab [Internet]. 2023;108(10):2447–69. Available from: https://academic.oup.com/jcem/article/108/10/2447/7242360.
|
| 10. |
Panidis D, Tziomalos K, Papadakis E, Chatzis P, Kandaraki EA, Tsourdi EA, et al. Associations of menstrual cycle irregularities with age, obesity and phenotype in patients with polycystic ovary syndrome. Hormones [Internet]. 2015;14(3):431–7. Available from: https://link.springer.com/10.14310/horm.2002.1593.
|
| 11. |
Saei Ghare Naz M, Mousavi M, Mahboobifard F, Niknam A, Ramezani Tehrani F. A Meta-Analysis of Observational Studies on Prolactin Levels in Women with Polycystic Ovary Syndrome. Diagnostics [Internet]. 2022;12(12):2924. Available from: https://www.mdpi.com/2075-4418/12/12/2924.
|
| 12. |
Housman E, Reynolds R V. Polycystic ovary syndrome: A review for dermatologists. J Am Acad Dermatol [Internet]. 2014;71(5):847.e1-847.e10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0190962214014340.
|
| 13. |
Chang WY, Azziz R. Pathogenesis of Hyperandrogenism in Polycystic Ovary Syndrome. In: Polycystic Ovary Syndrome [Internet]. Totowa, NJ: Humana Press; p. 281–94. Available from: http://link.springer.com/10.1007/978-1-59745-108-6_17.
|
| 14. |
Zhu J, Chen Z, Feng W, Long S, Mo Z-C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta [Internet]. 2019;499:142–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S000989811932039X.
|
| 15. |
Zeng X, Xie Y, Liu Y, Long S, Mo Z. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta [Internet]. 2020;502:214–21. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009898119321187.
|
| 16. |
Ressler IB, Grayson BE, Ulrich-Lai YM, Seeley RJ. Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. Am J Physiol Metab [Internet]. 2015;308(12):E1076–84. Available from: https://www.physiology.org/doi/10.1152/ajpendo.00182.2014.
|
| 17. |
Greaves RF, Jolly L, Hartmann MF, Ho CS, Kam RKT, Joseph J, et al. Harmonisation of serum dihydrotestosterone analysis: establishment of an external quality assurance program. Clin Chem Lab Med [Internet]. 2017;55(4). Available from: https://www.degruyter.com/document/doi/10.1515/cclm-2016-0394/html.
|








