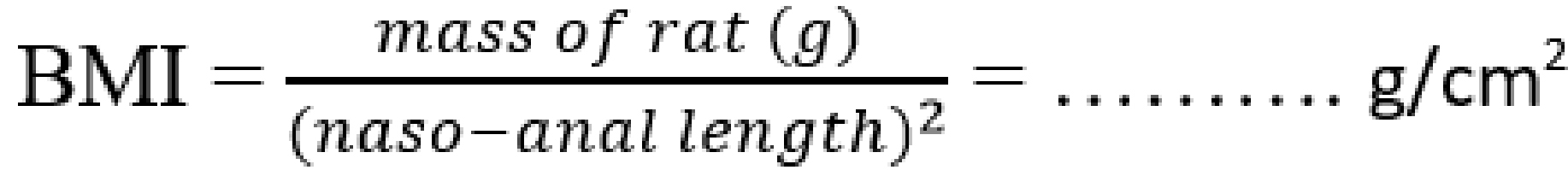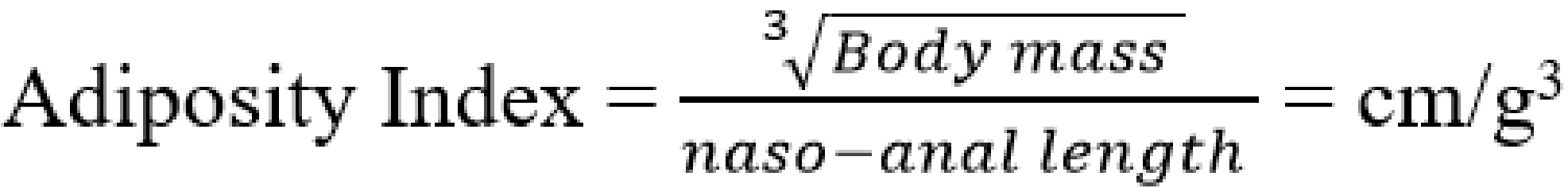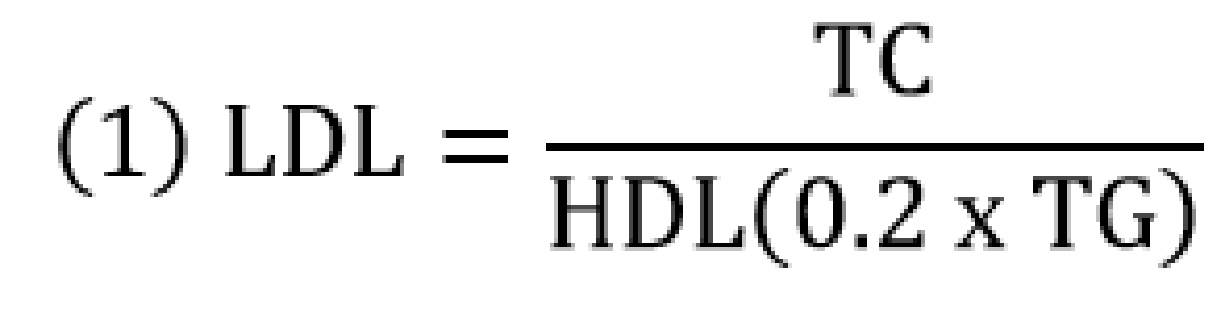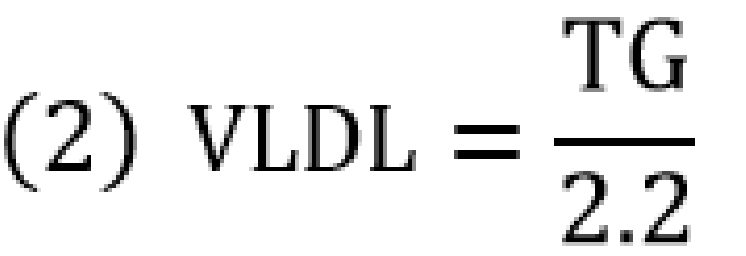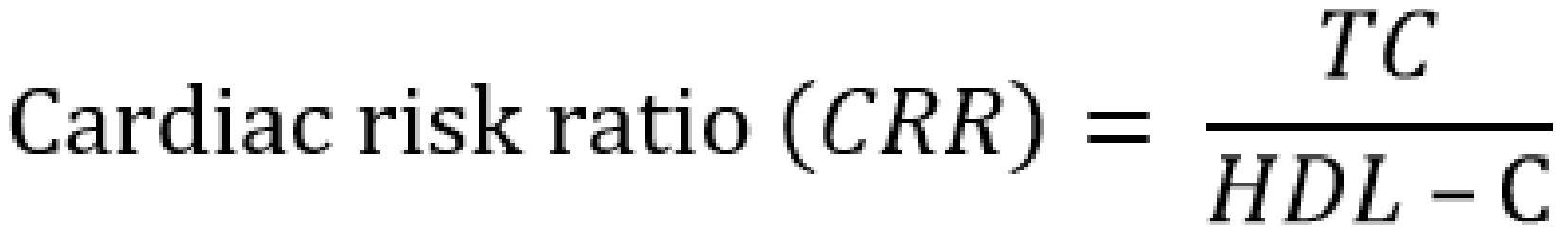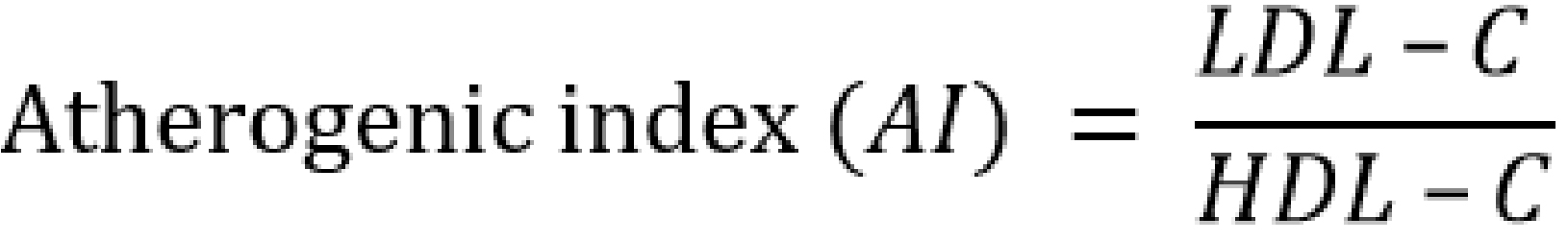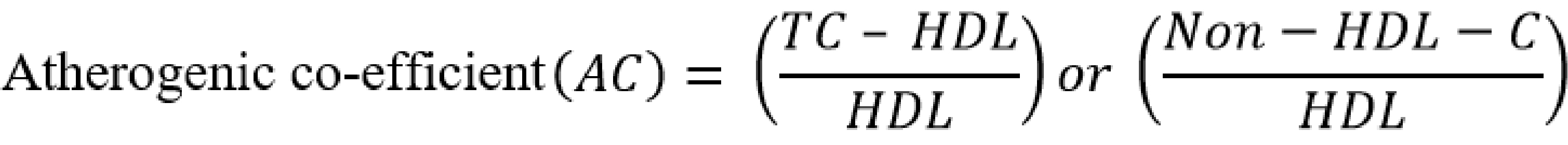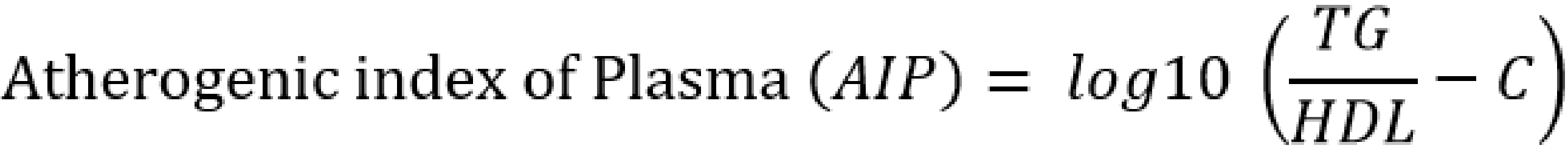Diet-induced obesity influences atherogenic indices and increases risk of cardiovascular disease in male Wistar rats
Abstract
Background: Relationship between obesity and cardiovascular diseases is complex. This study aimed to evaluate the correlation between diet-induced obesity and atherogenic indices.
Material and methods: Two groups of 8-weeks old male Wistar rats (170 ± 15 g) were fed with normal chow and high fat diet (HFD) respectively ad libitum for 10 weeks. Body mass index (BMI), adiposity index, serum lipid profile and atherogenic indices were measured. Associations between lipidemic indices, BMI and atherogenic indices were evaluated.
Results: The group fed with HFD only had significantly increased BMI (0.7630 ± 0.083, p = 0.0065). Serum TG (p < 0.01), LDL-c (p = 0.017), VLDL-c (p < 0.01), and total cholesterol (p = 0.035) were significantly elevated among the animals with relatively higher BMI (BMI > 0.0.71 g/cm2). There was a significant positive linear association of BMI with most of the atherogenic indices investigated: coronary risk ratio (CRR) (OR 48; 95% CI 4.993, 461.50; p < 0.001), atherogenic index (AI) (OR 1.0; 95% CI = 2.56, 229.57; p < 0.001) and atherogenic coefficient (AC) (OR 65.0; 95% CI 6.68, 648.26; p < 0.001).
Conclusions: Consumption of HFD induces hyperlipidemia and increase the risk of coronary artery diseases by increasing the atherogenic indices.
Citation
Kwarteng J K, Laing E. Diet-induced obesity influences atherogenic indices and increases risk of cardiovascular disease in male Wistar rats. Eur J Transl Clin Med. 2025;8(1):59-70Introduction
Inferring from the World Health Organization report (2016), there are about 2 billion overweight adults globally. Of these, over 650 million adults were obese (body mass index, BMI ≥ 30). In 2016, 39% of adults ≥ 18 years (39% of men and 40% of women) were overweight. Overall, about 13% of the world’s adult population (11% of men and 15% of women) was obese in 2016. The worldwide prevalence of obesity nearly tripled over the last five decades [1]. Obesity causes poor health outcomes, reduces quality of life [2], reduces socio-economic productivity and cost nations about 0.7-2.8% of their GDP annually [3]. These staggering statistics presents obesity as a serious global threat that deserves urgent attention.
The principal cause of obesity is due to an organism’s inability to physiologically maintain energy balance in the long term. This may result from over-dependence on high calorie diet, low caloric expenditure, and idiopathic inhibition of substrate oxidation [4]. It substantially increases the risk of myocardial infarction, stroke, and chronic illnesses such as type 2 diabetes mellitus, fatty liver disease, hypertension, dementia, osteoarthritis, obstructive sleep apnea and several cancers [5]. Dyslipidemia occurs early in obesity and becomes marked as the patient advances into the morbid stage of obesity either with or without insulin resistance [6]. This is associated with a relative increase in the levels of pro-atherogenic lipids (triglycerides (TGs) and low density lipoprotein (LDL-C)) as opposed to levels of anti-atherogenic lipids (high density lipoprotein – HDL-C). This phenotypic imbalance in plasma lipid distribution is strongly correlated to increased visceral adiposity, increased inflammation (systemic and vascular) increased risk of atherosclerosis (via vascular endothelial dysfunction), plaque formation, and increased plasma circulation of pro-inflammatory and prothrombotic chemokines together with an overtly increased in vulnerability to oxidative stress [7-14]. The decreased plasma nitric oxide levels in obesity further exacerbates the progression of atherosclerotic lesion formation [14].
Cardiovascular diseases are prominent among the various complications of obesity in humans, albeit they usually take many years to develop. Animal models are therefore an indispensable alternative for studying the probable obesity-associated cardiovascular risks associated with the obese condition. Hitherto, rodent models (including spontaneous single-gene loss-of-function mutation, gain-of-function mutation, transgenic model, polygenic models), and others generated from exposure to different environmental conditions have all been employed in the study of obesity [15-18]. However, neither of these models have been found to truly reflect the sequential order of metabolic and morphometric changes that occur in humans with obesity. The need for a more suitable model for the advancement of the study of obese condition cannot be overemphasized.
The aim of this study therefore was to use a high fat diet (HFD) to induce obesity in male Wistar rats as a model to evaluate the zoometric, morphometric and atherogenic risks associated with obesity condition. In this study, we compared the effect of HFD with that of the standard rat chow (SRC) on male Wistar rat population. We measured morphometric indices (e.g. the Lee/Adiposity index) and several biochemical indices (e.g. lipid profile, atherogenic index of plasma (AIP), coronary risk ratio (CRR), atherogenic coefficient (AC), and the atherogenic index (AI)). Finally, we evaluated possible correlations between measured zoometric and atherogenic indices.
Materials and methods
High fat diet formulation
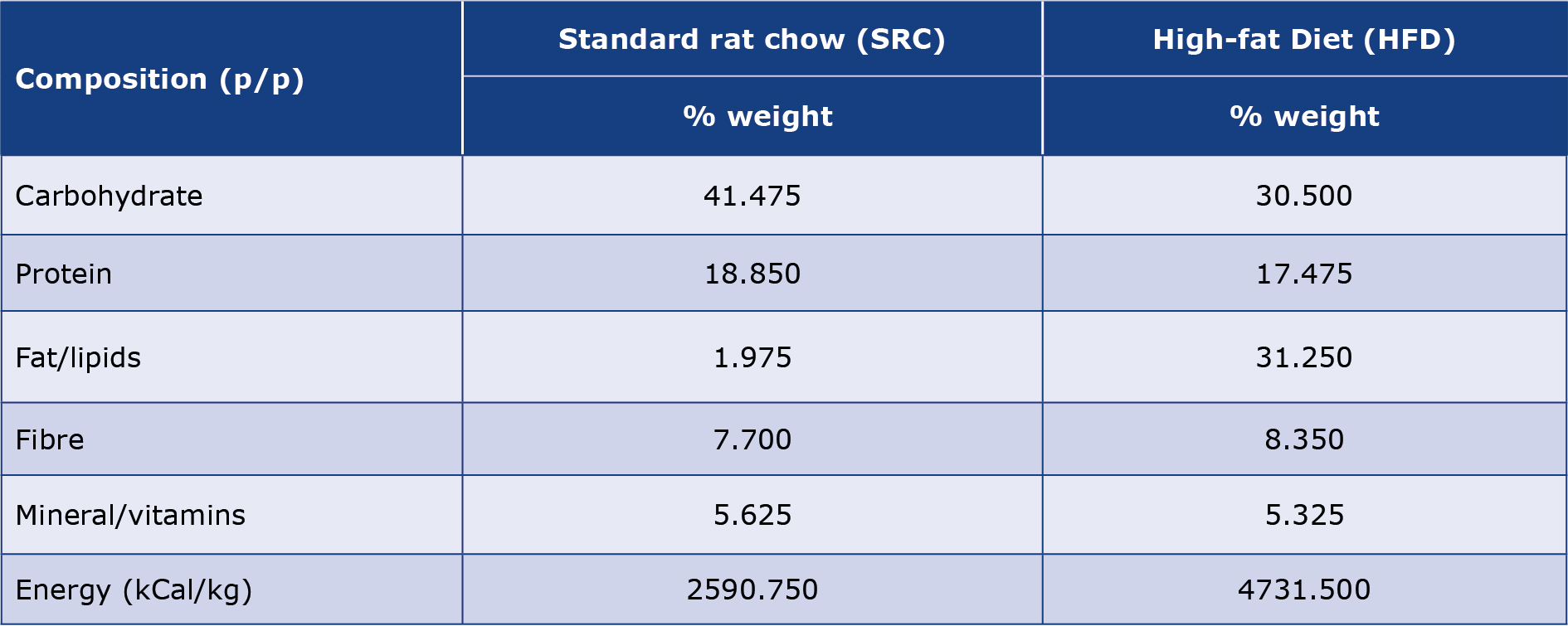
The high fat diet (HFD) was prepared by mixing soya-bean oil and melted pork tallow with commercially-obtained SRC at room temperature to provide 31.25% of the total energy from fat. This mixture was stirred to produce dispersed semi-solid pellets and used for feeding the HFD group. Proximate analysis was undertaken on the feed to determine the proportions of nutrients (proteins, carbohydrate, lipid, and minerals) and energy densities of the HFD and standard chow (Table 1).
Table 1. Nutritional composition and proportions of the experimental rats’ diet obtained from proximate analysis of SRC and HFD
Animal care and ethical considerations
In all, 40 male Wistar rats (170 ± 15 g, 8 weeks old) were obtained from the Department of Animal Science, Kwame Nkrumah University of Science & Technology (KNUST) in Kumasi (Ghana) and were randomly distributed into two equal sized groups (n = 20). The test group was fed HFD, while rats in the control group were fed SRC. The rats were housed in metal cages (5 animals per cage) at 28 ± 2 ˚C, under a cycle of 12 hours of light and 12 hours of darkness, with free access to food and water for 10 weeks. The animals were housed and cared for under conditions in accordance with the current National Institute of Health (NIH) Guidelines for the Care of Laboratory Animals [19].
Zoometric measurements
During the 10 weeks period of dietary treatment, the rat’s body weight, nose-anal length, body mass index (BMI), and adiposity index were measured weekly. Body weight was measured to the nearest 0.01 g using a digital scale (Shanghai Huachao Industrial Co. Ltd, Shanghai, China). The naso-anal length was measured (to the nearest 0.1 cm) using plastic centimeter ruler (Suzhou Chaosheng Stationery Co. Ltd, Anhui, China). The BMI and adiposity index were determined by calculation from the formula:
![]()
![]()
Euthanization
Before the anesthesia, final body weight of animals in each group was recorded. Anesthesia was achieved by intraperitoneal injection of 0.04 mg/g body weight of pentobarbital (Taj Pharmaceutical Ltd, Mumbai, India). About 5 ml of blood was collected by cardiopuncture using disposable syringes (Changzou Standard Medical Devices Co., Xinbei – Changzou, China) into sterile blood sample vacutainers (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA). Samples were kept still for 1hr to clot, then centrifuged at 3000 g for 5minutes and finally the serum aliquoted into clean eppendorf tubes and stored at –80 °C. After successful cardiopuncture, some of the experimental animals were frail and hence were sacrificed and their cadavers were measured.
Morphometric
measurements Following successful dissection, the wet weights of the vital organs (heart, kidney, liver, pancreas), and the abdominal fat were measured to the nearest 0.01 g using a digital scale (Shanghai Huachao Industrial Co. Ltd, Shanghai, China) and recorded.
Biochemical assays
The serum total cholesterol (TC), HDL, and TG concentrations were measured by semi-automated technique using a commercially available reagent (Fortress Diagnostics©, Antrim, N. Ireland) and the Kenza Max BioChemisTry analyzer (Biolabo Diagnostics, Maizy, France). The serum LDL-C, very low density lipoprotein (VLDL-C), and HDL-C/LDL-C ratio were calculated as below;
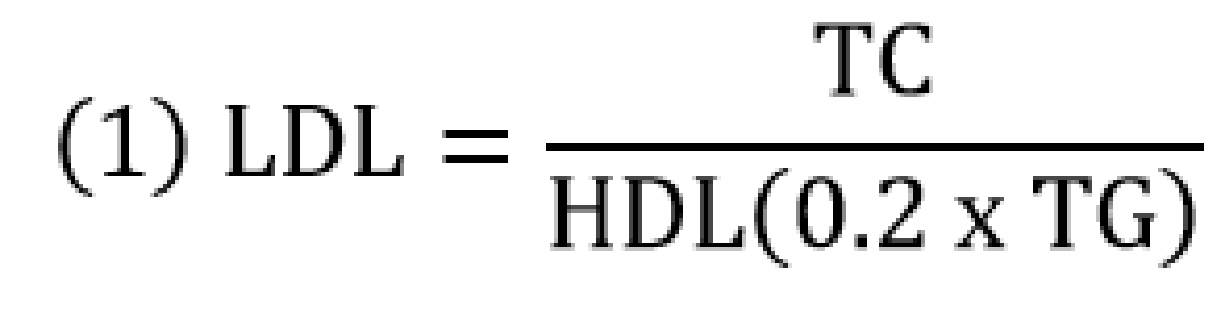
![]()
Nota bene: Equations (1) and (2) are used when TG is measured in mg/dl.
Serum atherogenic indices were calculated as follows [20-24]:
![]()
![]()
![]()
![]()
The serum fasting insulin levels were assayed by enzyme-linked immunosorbent assay (ELISA) technique using a commercially available kit according to the manufacturer’s instructions (Shanghai Chemical Ltd, Shanghai, China) and 10 μl of serum sample.
Statistical analysis
The data from the study were analyzed using the Prism software version 8.0 (GraphPad software, Boston, USA). All continuous and discrete data were expressed as mean ± standard deviation. For continuous variables, group differences were determined using the student t-test or the oneway ANOVA. The Pearson correlation and logistic regression analysis was used to evaluate the correlation and associations respectively between lipidemic indices, BMI, and atherogenic indices among experimental animal population after the dietary treatment. From the analysis, all p values < 0.05 were considered significant.
Results and discussion
Figure 1 and Figure 2 show marked increases in average weight gain (BMI > 40%) and mean adiposity index (0.35) among the animals fed with HFD for 10 weeks, compared to those fed with SRC. The results are presented as mean ± standard error of the mean and n represents the number of animals in each group (SRC group n = 20, HFD group n = 18), (a) p < 0.05 vs SRC group, (aa) p < 0.01 vs SRC group, (bb) p < 0.05 vs SRC.
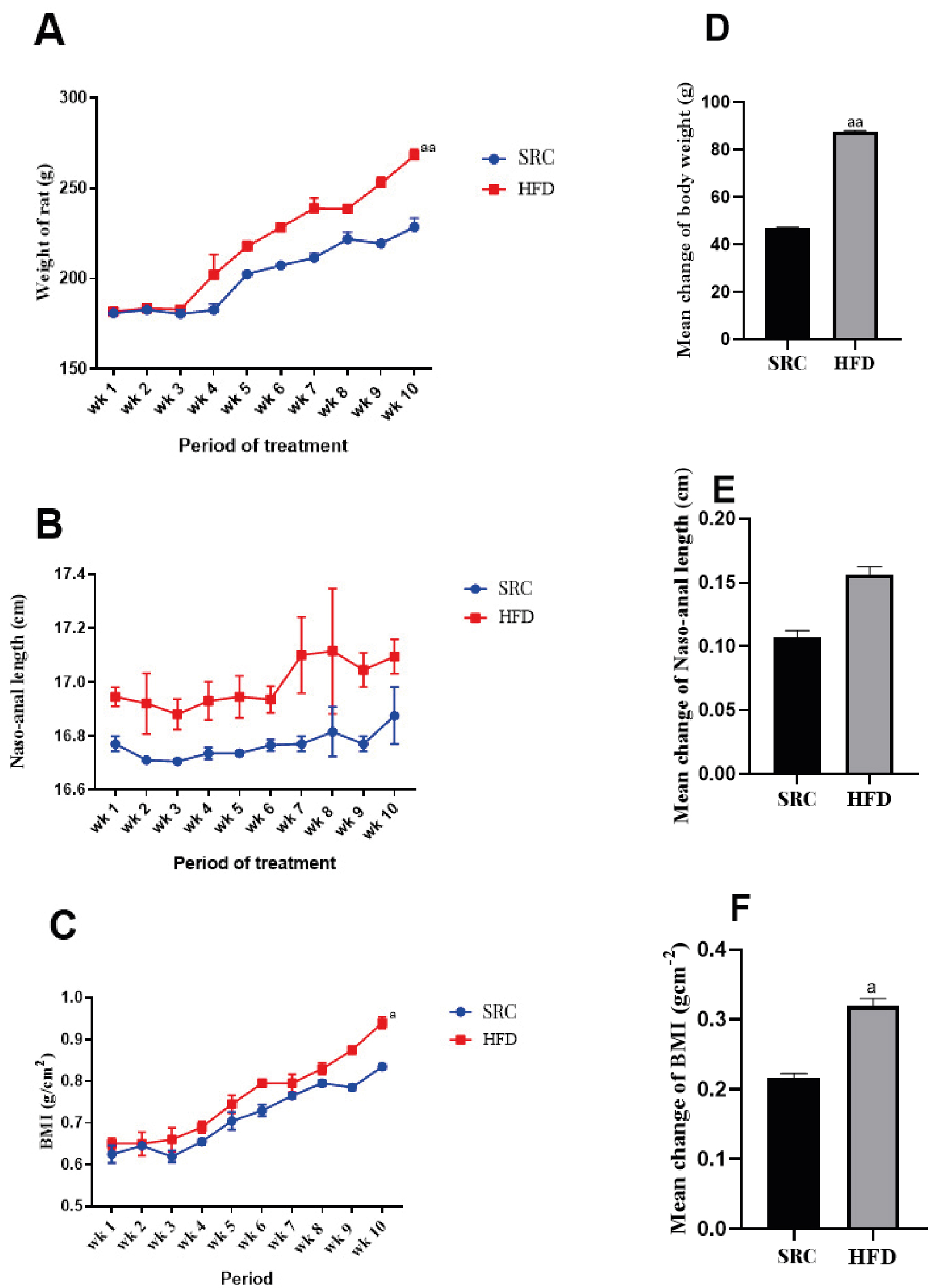
Figure 1. Zoometric measurements of experimental animals
A – body weight evaluation; B – nose-anal length changes; C – body mass index (BMI) evaluation; D – mean change in body weight; E – mean change in nose-anal length; F – mean change in BMI after 10 weeks of dietary treatment; HFD – high fat diet; SRC – standard rat chow
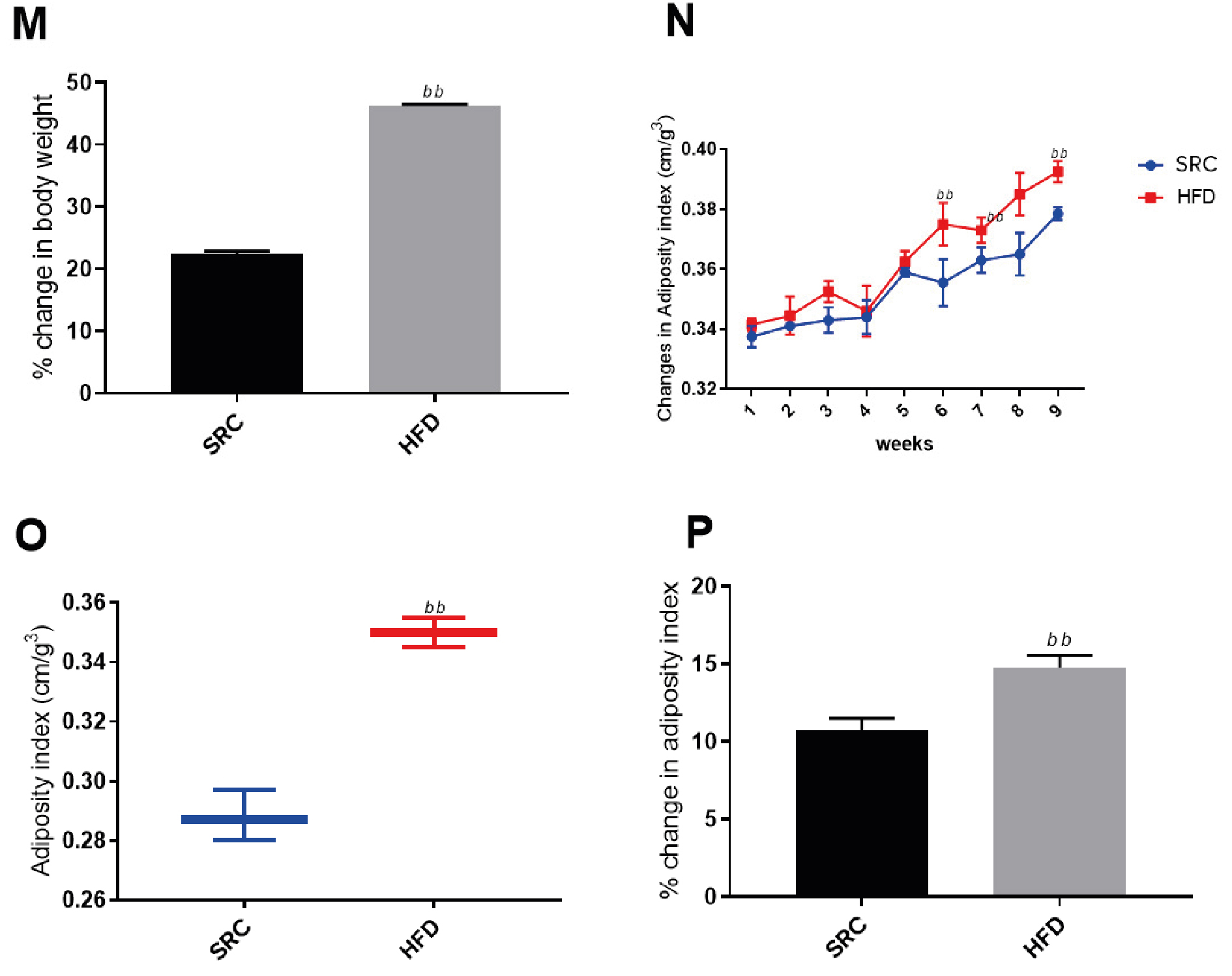
Figure 2. Morphometric measurements of experimental animals
HFD – high fat diet; M – evaluation of percentage body weight; N – change in adiposity index; O – change in adiposity indices; P – percentage change in adiposity index; SRC – standard rat chow
Obesity is one of the common findings from reported in various studies involving the treatment of subjects with high fat diets for extended periods [25-30]. Lozano et al., reported significant weight gain among rat population treated with high fat only and high fat and high fructose (HFHF) diets for two months compared to rats rationed on SRC [24]. Despite the strong association established between genes and obesity [31-35] our findings show overtly the influence of an environmental factor (HFD) on weight gain and increased BMI. In other words, long periods of HFD consumption can result in obesity. As expected, our findings from Figure 2M above suggest an average of 40% weight gain probably due to increased abdominal fat accumulation as showed in the elevated adiposity index for the group that received the HFD compared to the controls fed with SRC. Among our study animals, noticeable weight gains were observed from the 3rd week and through to the 10th week of HFD treatment. Diet induced models of obesity (DIO) have been successfully achieved with cafeteria, western, HFHF and cholesterol-rich diets in non-human primates [36-42]. Additionally, findings from our study indicate that HFD consumption over an extended period could be used as a viable alternative for induction of obesity. However, further research will have to be carried out to compare the robustness (or otherwise) of the above alternative models for induction of obesity in non-human primates and their relative suitability for studies relating to the risk factors associated with obesity.
Obesity is implicated as a viable risk factor to a wide spectrum of diseases such as non-alcoholic fatty liver disease (NAFLD), renal insufficiency, obesity-related cardiomyopathy, cancers, immune-suppression, insulin resistance, metabolic syndrome, and diabetes [43-52] From the pathology point of view, increased visceral, subcutaneous, abdominal, and intra-hepatic fat accumulations are a common effect of obesity [53]. Our study findings in Table 2 and Figure 2 above show increased abdominal fat accumulation and elevated body adiposity among the HFD-treated animals compared to those on SRC respectively. This phenotype is associated with marked increase in plasma circulating pro-inflammatory cytokines such as interleukin – 6 and α-tumor necrosis factor (TNF-α) [54-56] The circulating TNF-α provokes induces mitochondrial damage and induces systemic oxidative stress by inducing increased release of reactive oxygen species (ROS), increased expression of TNF-receptor 2 (TNFR-2) and consequent expression of vascular adhesion factors (VCAM-1 and ICAM-1) downstream increasing recruitment, adhesion and infiltration of inflammatory leucocytes hence increasing risk of CAD [57-60] Again, the weight of the various abdominal organs were significantly higher in the obese rats than in controls. This finding further reveals the possible systemic effect of obesity on the vital organs of an organism owing to the significant increase in the gross weights of liver, kidney, heart, and pancreas in the obese rats. While there is sufficient literature correlating obesity with various diseases associated with these organs [61-67] there is rather a paucity of information relating obesity to the function of the kidney, heart, and pancreas. Our findings shown in Tables 1 and 2 provide a justifiable inference for further study into how this change in weight could affect the gross anatomy and function of these organs in obesity.
Table 2. Morphometric measurements of Wistar rats after 10 weeks of treatment with SRC and HFD
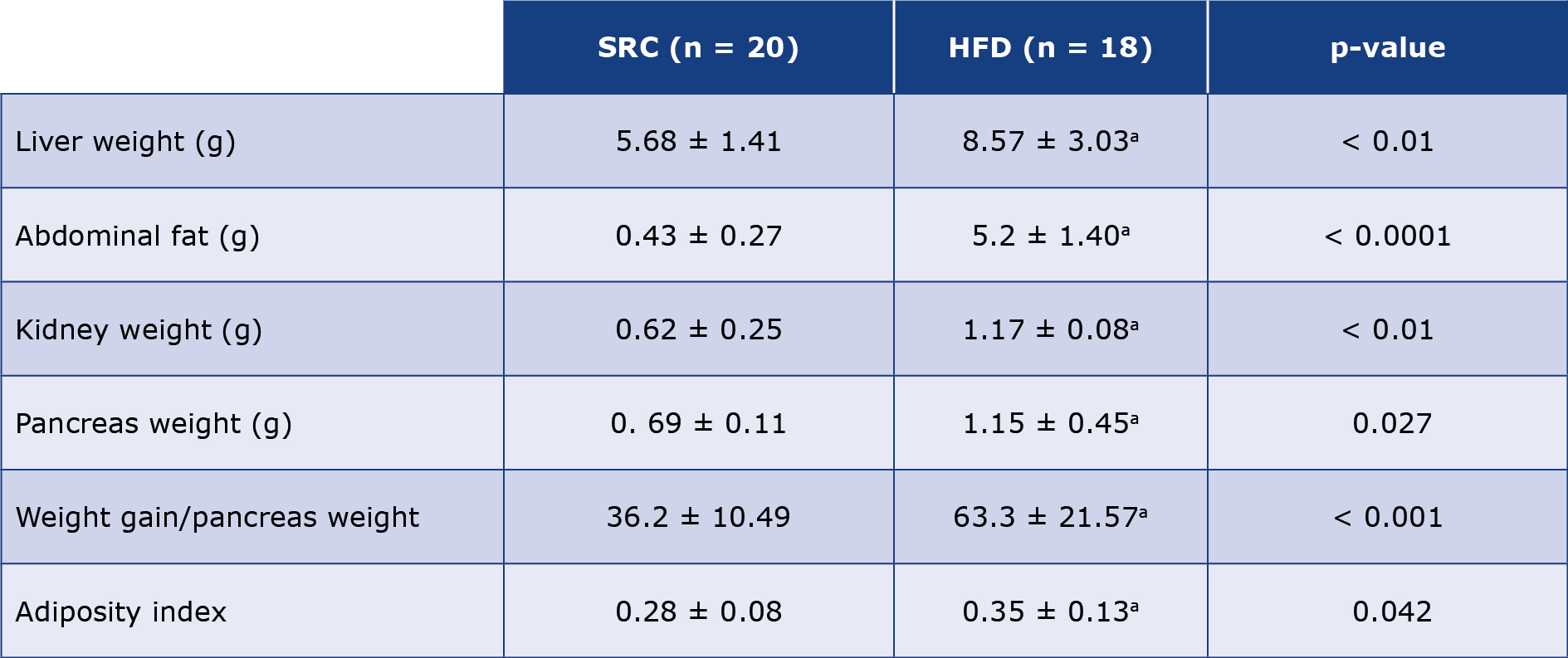
Results are presented as mean ± standard error of the mean and n represents the number of animals in each group; The p-values were determined using the unpaired t-test, SRC versus HFD. (a) p < 0.05 was considered significant when compared against the SRC group; HFD – high fat diet; SRC – standard rat chow.
The atherogenic indices are tools to measure the risk for various forms of coronary artery diseases (CAD)s. Different diseases relate differently to the conventional atherogenic indices. In comprehensive meta-analysis, Wu et al., demonstrated that atherogenic index of plasma (AIP) is independently correlated to CAD among adult population [67]. In another study, AIP, AC, and CRR were implicated in pre-eclampsia among pregnant women [68]. Except for AIP, our findings shown in Table 4 suggest a significant linear relationship between BMI and the principal atherogenic indices namely AC, AI, and CRR. Among them, AI correlated strongly with obesity followed by AC, and CRR with correlation coefficient (r) values of 0.8735 (p < 0.0001), 0.7459 (p < 0.0001), 0.6811 (p < 0.0001) respectively (Figure 3). Notable among our findings is the strong correlation between obesity and the atherogenic indices (Tables 3 and 4). This corroborates the human studies that reported significant increases in atherogenic risks among obese cohorts [69-71] This further underscores the viability of animal models for studies of human diseases.
Table 3. Biochemical characteristics of study animals
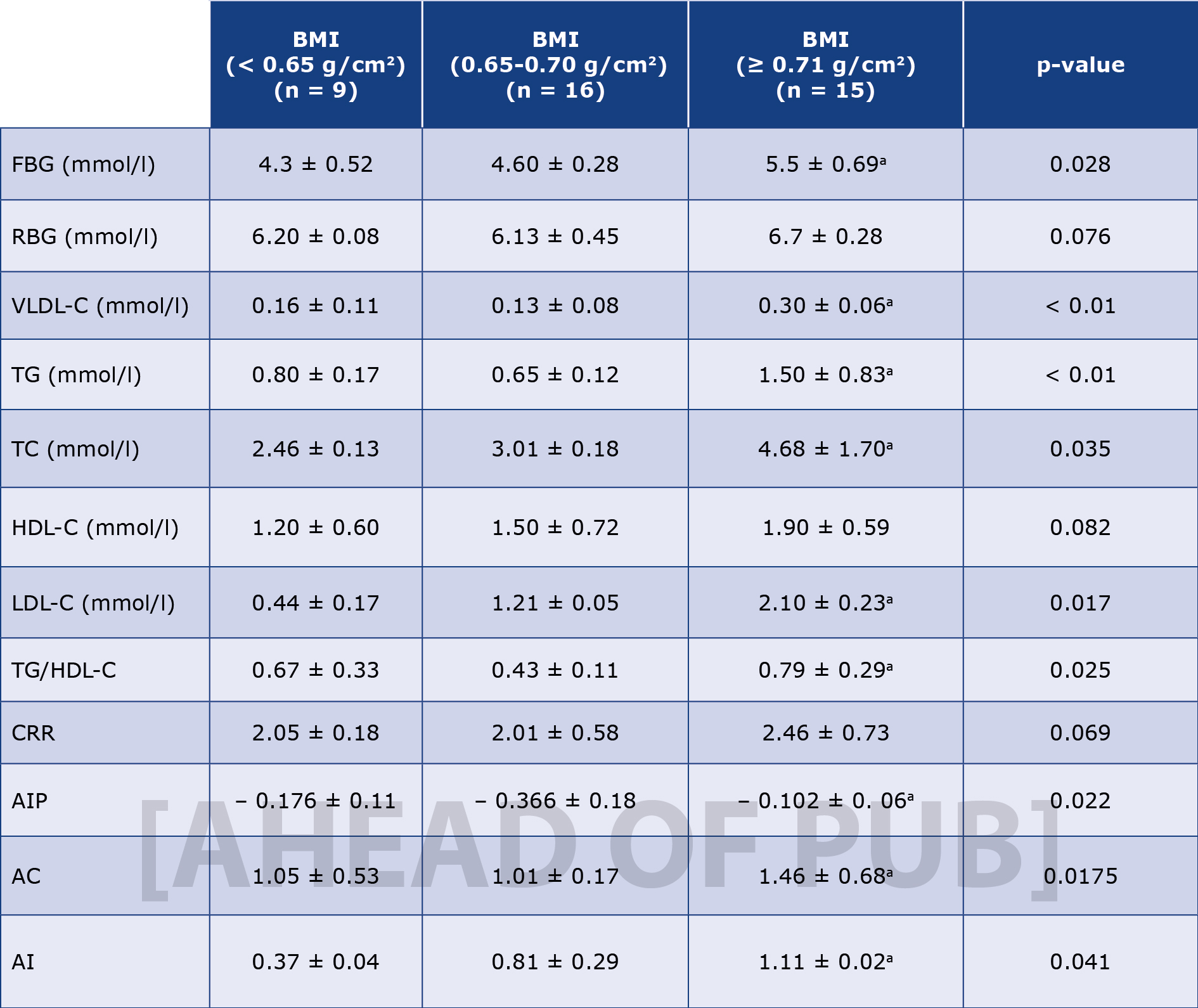
Results are presented as mean ± standard error of the mean and n represents the number of animals used in each group; The p-value was determined by the one-way analysis of variance; p < 0.05 was considered significant; (a) p-value < 0.05; AC – atherogenic coefficient; AI – atherogenic index; AIP – atherogenic index of plasma; BMI – body mass index; CRR – cardiac risk ratio; FBG – fasting blood glucose; HDL-c – high density lipoprotein; LDL-c – low density lipoprotein; RBG – random blood glucose; TC – total cholesterol; TG – triglyceride; VLDL-c – very low density lipoprotein cholesterol.
Table 4. Odds ratio (95% confidence interval) of obesity according to the estimated atherogenic indices
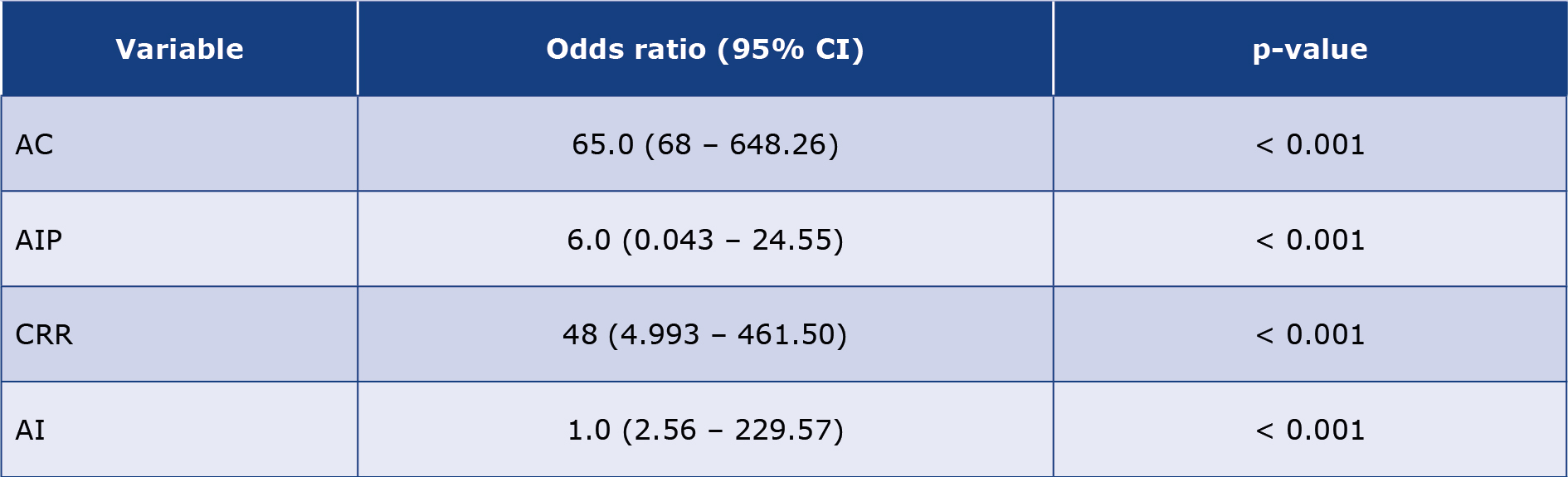
AC – atherogenic coefficient; AI – atherogenic index (LDL-c/HDL-c ratio); AIP – atherogenic index of plasma; CI – confidence interval; CRR – coronary risk index
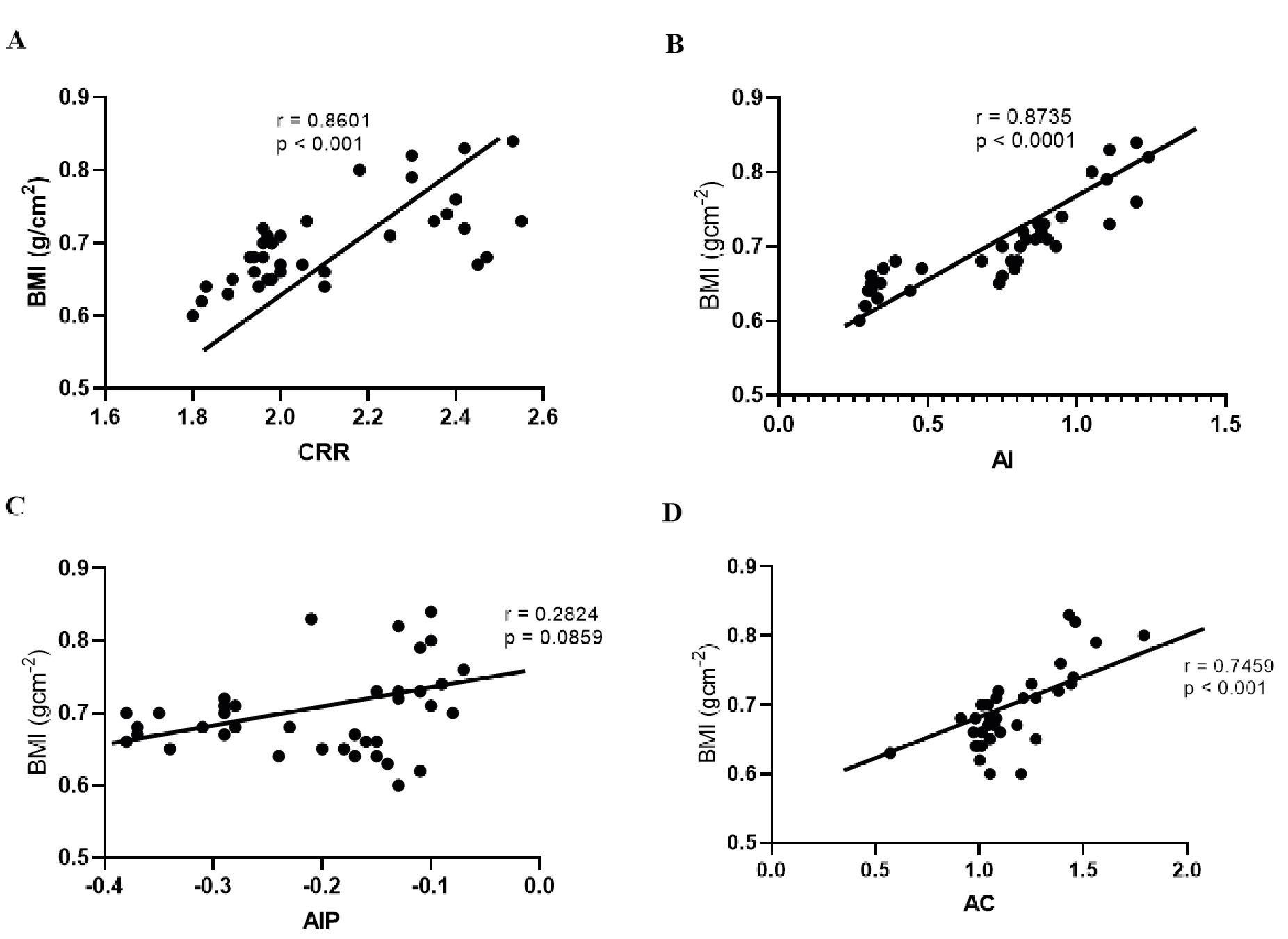
Figure 3. Scatter plot showing significant and positive correlations between different atherogenic indices and increased BMI among experimental animals (r-pearson’s coefficient).
From the above figure, the order of increasing strength of correlation among the atherogenic indices with BMI is AIP, AC, CRR, and AI with the strongest positive association (r = 0.28, 0.74, 0.86, and 0.87) respectively. AC – atherogenic coefficient; AI – atherogenic index; AIP – atherogenic index of plasma; CRR – coronary risk ratio.
Limitations of this study
The animal model we used and the small sample size are a limitation towards extrapolation of our findings to the larger human population. Proximate evaluation of animal diet did not reveal micronutrient distributions which could have played a role in the observed outcomes. Lastly, this experimental study was exploratory and focused primarily on correlational data without exploring the undergirding mechanisms such as oxidative stress, inflammation, insulin resistance among others. Lastly, the 10-week duration of our study was relatively short. Perhaps, a longer duration would have been more appropriate to better explore the effect of diet-induced obesity and coronary artery diseases. These notwithstanding, our findings are consistent with those observed in the human population indicating the usefulness of our model for the further investigations of obesity in humans.
Conclusion
In conclusion, our study corroborates the findings published in various studies that suggest that prolonged consumption of HFD leads to hyperlipidemia and increased cardiovascular risk indicated by increasing several atherogenic indices. This leads to increased vulnerability to heart attack, hypertension, and stroke.
Disclaimer
The findings of this paper are new and solely the responsibility of the authors and do not necessarily represent the official views of any auxiliary agencies.
Conflict of interest
The authors have no conflict of interests regarding the publication of this paper.
Acknowledgements
The authors express their gratitude to the staff of the Animal House, Department of Pharmacology for housing the rats used for this study.
-------------
Image credit: Joanna Servaes; source – wikipedia.com (CC BY-SA 3.0)
References
| 1. |
Obesity and overweight [Internet]. World Health Organization. 2025 [cited 2025 May 27]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
|
| 2. |
Division of Nutrition, Physical Activity, and Obesity [Internet]. National Center for Chronic Disease Prevention and Health Promotion. 2020 [cited 2020 Sep 17]. Available from: https://www.cdc.gov/obesity/adult/causes.html.
|
| 3. |
Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev [Internet]. 2011;12(2):131–41. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2009.00712.x.
|
| 4. |
Sun M, Schutz Y, Maffeis C. Substrate metabolism, nutrient balance and obesity development in children and adolescents: a target for intervention? Obes Rev [Internet]. 2004;5(4):183–8. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2004.00154.x.
|
| 5. |
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol [Internet]. 2019;15(5):288–98. Available from: https://www.nature.com/articles/s41574-019-0176-8.
|
| 6. |
Bamba V, Rader DJ. Obesity and Atherogenic Dyslipidemia. Gastroenterology [Internet]. 2007;132(6):2181–90. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508507005823.
|
| 7. |
Bhardwaj S, Bhattacharjee J, Bhatnagar MK, Tyagi S, Delhi N. Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int J Pharm Biol Sci [Internet]. 2013;3(3):359–64. Available from: http://ijpbs.com/ijpbsadmin/upload/ijpbs_526938e855804.pdf.
|
| 8. |
Bharadwaj R, Mathur K, Sharma D, Sankhla M. The relationship between oxidative stress and atherogenic index (AI) in preeclampsia. Sch J App Med Sci [Internet]. 2014;2(6D):3092–6. Available from: https://saspublishers.com/media/articles/SJAMS_26D3092-3096.pdf.
|
| 9. |
Millán J, Xavier P, Anna M, Manuel Z, Joan R-P, Luis Felipe P, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag [Internet]. 2009;5:757–65. Available from: https://www.tandfonline.com/doi/abs/10.2147/vhrm.s12187457.
|
| 10. |
Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med [Internet]. 2016;26(4):364–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1050173815002492.
|
| 11. |
Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol [Internet]. 2009;6(6):399–409. Available from: https://www.nature.com/articles/nrcardio.2009.55.
|
| 12. |
Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating Levels of Oxidative Stress Markers and Endothelial Adhesion Molecules in Men with Abdominal Obesity. J Clin Endocrinol Metab [Internet]. 2005;90(12):6454–9. Available from: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jc.2004-2438.
|
| 13. |
Engin A. Endothelial Dysfunction in Obesity. In 2017. p. 345–79. Available from: http://link.springer.com/10.1007/978-3-319-48382-5_15.
|
| 14. |
Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2021;143(21):e984–1010. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000973.
|
| 15. |
Wang C-Y, Liao JK. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. In 2012. p. 421–33. Available from: https://link.springer.com/10.1007/978-1-61779-430-8_27.
|
| 16. |
Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, et al. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High‐Fat Diet. Obesity [Internet]. 2011;19(6):1109–17. Available from: https://onlinelibrary.wiley.com/doi/10.1038/oby.2011.18.
|
| 17. |
Buettner R, Schölmerich J, Bollheimer LC. High‐fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity [Internet]. 2007;15(4):798–808. Available from: https://onlinelibrary.wiley.com/doi/10.1038/oby.2007.608.
|
| 18. |
Inui A. Obesity – a chronic health problem in cloned mice? Trends Pharmacol Sci [Internet]. 2003;24(2):77–80. Available from: https://doi.org/10.1016/S0165-6147(02)00051-2.
|
| 19. |
Guide for the Care and Use of Laboratory Animals [Internet]. Washington, D.C.: National Academies Press; 2011. Available from: http://www.nap.edu/catalog/12910.
|
| 20. |
Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between Serum Lipoprotein Ratios and Insulin Resistance in Obesity. Clin Chem [Internet]. 2004;50(12):2316–22. Available from: https://academic.oup.com/clinchem/article/50/12/2316/5639942.
|
| 21. |
Frohlich J, Dobiášová M. Fractional Esterification Rate of Cholesterol and Ratio of Triglycerides to HDL-Cholesterol Are Powerful Predictors of Positive Findings on Coronary Angiography. Clin Chem [Internet]. 2003;49(11):1873–80. Available from: https://academic.oup.com/clinchem/article/49/11/1873/5642132.
|
| 22. |
Dobiášová M. Atherogenic Index of Plasma [Log(Triglycerides/HDL-Cholesterol)]: Theoretical and Practical Implications. Clin Chem [Internet]. 2004;50(7):1113–5. Available from: https://academic.oup.com/clinchem/article/50/7/1113/5639904.
|
| 23. |
Martirosyan DM, Miroshnichenko LA, Kulakova SN, Pogojeva A V, Zoloedov VI. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis [Internet]. 2007;6(1):1. Available from: https://doi.org/10.1186/1476-511X-6-1.
|
| 24. |
Lozano I, Van der Werf R, Bietiger W, Seyfritz E, Peronet C, Pinget M, et al. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab (Lond) [Internet]. 2016;13(1):15. Available from: http://www.nutritionandmetabolism.com/content/13/1/15.
|
| 25. |
RI F. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
|
| 26. |
Ngala RA, Ampong I, Sakyi SA, Anto EO. Effect of dietary vegetable oil consumption on blood glucose levels, lipid profile and weight in diabetic mice: an experimental case—control study. BMC Nutr [Internet]. 2016;2(1):28. Available from: http://bmcnutr.biomedcentral.com/articles/10.1186/s40795-016-0053-y.
|
| 27. |
Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A Controlled High-Fat Diet Induces an Obese Syndrome in Rats. J Nutr [Internet]. 2003;133(4):1081–7. Available from: https://doi.org/10.1093/jn/133.4.1081.
|
| 28. |
Aslani S, Vieira N, Marques F, Costa PS, Sousa N, Palha JA. The effect of high-fat diet on rat’s mood, feeding behavior and response to stress. Transl Psychiatry [Internet]. 2015;5(11):e684–e684. Available from: https://www.nature.com/articles/tp2015178.
|
| 29. |
Marques C, Meireles M, Norberto S, Leite J, Freitas J, Pestana D, et al. High-fat diet-induced obesity Rat model: a comparison between Wistar and Sprague-Dawley Rat. Adipocyte [Internet]. 2016;5(1):11–21. Available from: http://www.tandfonline.com/doi/full/10.1080/21623945.2015.1061723.
|
| 30. |
Lyon HN, Hirschhorn JN. Genetics of common forms of obesity: a brief overview. Am J Clin Nutr [Internet]. 2005;82(1):215S-217S. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002916523295349.
|
| 31. |
Speakman JR. Obesity: The Integrated Roles of Environment and Genetics. J Nutr [Internet]. 2004;134(8):2090S-2105S. Available from: https://doi.org/10.1093/jn/134.8.2090S.
|
| 32. |
Loos RJF. Recent progress in the genetics of common obesity. Br J Clin Pharmacol [Internet]. 2009;68(6):811–29. Available from: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/j.1365-2125.2009.03523.x.
|
| 33. |
Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol [Internet]. 2010;206(3):287–96. Available from: https://joe.bioscientifica.com/view/journals/joe/206/3/287.xml.
|
| 34. |
Amri Z, Ghorbel A, Turki M, Akrout FM, Ayadi F, Elfeki A, et al. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement Altern Med [Internet]. 2017;17(1):339. Available from: http://bmccomplementalternmed.biomedcentral.com/articles/10.1186/s12906-017-1842-9.
|
| 35. |
Lewis AR, Singh S, Youssef FF. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon [Internet]. 2019;5(3). Available from: https://doi.org/10.1016/j.heliyon.2019.e01412.
|
| 36. |
Feijó G dos S, de Oliveira S, Thoen R, Schaab EE, de Moura AC, Franco F, et al. Food Selection of Cafeteria Diet Affects Memory Dysfunction Related to Obesity. Neurochem Res [Internet]. 2019;44(8):1869–77. Available from: http://link.springer.com/10.1007/s11064-019-02821-5.
|
| 37. |
Shively CA, Appt SE, Vitolins MZ, Uberseder B, Michalson KT, Silverstein‐Metzler MG, et al. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity [Internet]. 2019;27(5):777–84. Available from: https://onlinelibrary.wiley.com/doi/10.1002/oby.22436.
|
| 38. |
Varlamov O. Western-style diet, sex steroids and metabolism. Biochim Biophys Acta - Mol Basis Dis [Internet]. 2017;1863(5):1147–55. Available from: https://www.sciencedirect.com/science/article/pii/S0925443916301430.
|
| 39. |
Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J [Internet]. 2014;13(1):61. Available from: http://nutritionj.biomedcentral.com/articles/10.1186/1475-2891-13-61.
|
| 40. |
Dalbøge LS, Pedersen PJ, Hansen G, Fabricius K, Hansen HB, Jelsing J, et al. A Hamster Model of Diet-Induced Obesity for Preclinical Evaluation of Anti-Obesity, Anti-Diabetic and Lipid Modulating Agents. Andrews Z, editor. PLoS One [Internet]. 2015;10(8):e0135634. Available from: https://dx.plos.org/10.1371/journal.pone.0135634.
|
| 41. |
Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism [Internet]. 2019;92:82–97. Available from: https://www.sciencedirect.com/science/article/pii/S0026049518302531.
|
| 42. |
Koppe SWP. Obesity and the liver: nonalcoholic fatty liver disease. Transl Res [Internet]. 2014;164(4):312–22. Available from: https://www.sciencedirect.com/science/article/pii/S1931524414002151.
|
| 43. |
Silva Junior GB da, Bentes ACSN, Daher EDF, Matos SMA de. Obesity and kidney disease. J Bras Nefrol [Internet]. 2017;39(1). Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-28002017000100065.
|
| 44. |
Lakkis JI, Weir MR. Obesity and Kidney Disease. Prog Cardiovasc Dis [Internet]. 2018;61(2):157–67. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0033062018301282.
|
| 45. |
Ayer J, Charakida M, Deanfield JE, Celermajer DS. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J [Internet]. 2015;36(22):1371–6. Available from: https://doi.org/10.1093/eurheartj/ehv089.
|
| 46. |
Tiwari S, Fomusi Ndisang J. The role of obesity in cardiomyopathy and nephropathy. Curr Pharm Des [Internet]. 2014;20(9):1409–17. Available from: https://www.benthamdirect.com/content/journals/cpd/10.2174/13816128113199990562.
|
| 47. |
Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes [Internet]. 2015;22(4):277–82. Available from: https://journals.lww.com/01266029-201508000-00005.
|
| 48. |
Leong KS, Wilding JP. Obesity and diabetes. Best Pract Res Clin Endocrinol Metab [Internet]. 1999;13(2):221–37. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1521690X99900179.
|
| 49. |
Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol [Internet]. 2016;29:77–89. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1471489216300650.
|
| 50. |
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism [Internet]. 2019;92:121–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0026049518302324.
|
| 51. |
Borel A-L, Nazare J-A, Smith J, Aschner P, Barter P, Van Gaal L, et al. Visceral, subcutaneous abdominal adiposity and liver fat content distribution in normal glucose tolerance, impaired fasting glucose and/or impaired glucose tolerance. Int J Obes [Internet]. 2015;39(3):495–501. Available from: https://www.nature.com/articles/ijo2014163.
|
| 52. |
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health [Internet]. 2009;9(1):88. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-9-88.
|
| 53. |
Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and Obesity as Determinants of Cardiovascular Risk. Arch Intern Med [Internet]. 2002;162(16):1867. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.162.16.1867.
|
| 54. |
Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-obese Diabetics: Pre- and Postinsulin Effects. N Am J Med Sci [Internet]. 2012;4(4):180–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22536561.
|
| 55. |
Kern L, Mittenbühler M, Vesting A, Ostermann A, Wunderlich C, Wunderlich F. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers (Basel) [Internet]. 2018;11(1):24. Available from: https://www.mdpi.com/2072-6694/11/1/24.
|
| 56. |
Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L, et al. IL-6 and TNF-α Induced Obesity-Related Inflammatory Response Through Transcriptional Regulation of miR-146b. J Interf Cytokine Res [Internet]. 2014;34(5):342–8. Available from: http://www.liebertpub.com/doi/10.1089/jir.2013.0078.
|
| 57. |
Liu C, Lei S, Cai T, Cheng Y, Bai J, Fu W, et al. Inducible nitric oxide synthase activity mediates TNF-α-induced endothelial cell dysfunction. Am J Physiol Physiol [Internet]. 2023;325(3):C780–95. Available from: https://journals.physiology.org/doi/10.1152/ajpcell.00153.2023.
|
| 58. |
Tran N, Garcia T, Aniqa M, Ali S, Ally A, Nauli SM. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: in Physiology and in Disease States. Am J Biomed Sci Res [Internet]. 2022;15(2):153–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35072089.
|
| 59. |
Król M, Kepinska M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int J Mol Sci [Internet]. 2020;22(1):56. Available from: https://www.mdpi.com/1422-0067/22/1/56.
|
| 60. |
Hwang M, Berceli SA, Tran-Son-Tay R. Modeling and Role of Leukocytes in Inflammation BT - Computational Surgery and Dual Training. In: Garbey M, Bass BL, Collet C, Mathelin M, Tran-Son-Tay R, editors. Boston, MA: Springer US; 2010. p. 221–32. Available from: https://doi.org/10.1007/978-1-4419-1123-0_13.
|
| 61. |
Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd‐Jones D, et al. Obesity‐related hypertension: Pathogenesis, cardiovascular risk, and treatment—A position paper of the The Obesity Society and the American Society of Hypertension. Obesity [Internet]. 2013;21(1):8–24. Available from: https://onlinelibrary.wiley.com/doi/10.1002/oby.20181.
|
| 62. |
Hampel H, Abraham NS, El-Serag HB. Meta-Analysis: Obesity and the Risk for Gastroesophageal Reflux Disease and Its Complications. Ann Intern Med [Internet]. 2005;143(3):199–211. Available from: https://www.acpjournals.org/doi/10.7326/0003-4819-143-3-200508020-00006.
|
| 63. |
Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch Neurol [Internet]. 2005;62(10). Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneur.62.10.1556.
|
| 64. |
Mulnier HE, Seaman HE, Raleigh VS, Soedamah‐Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with Type 2 diabetes in the UK. Diabet Med [Internet]. 2006;23(5):516–21. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1464-5491.2006.01838.x.
|
| 65. |
Câmara NOS, Iseki K, Kramer H, Liu Z-H, Sharma K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol [Internet]. 2017;13(3):181–90. Available from: https://www.nature.com/articles/nrneph.2016.191.
|
| 66. |
Pérez S, Rius‐Pérez S, Finamor I, Martí‐Andrés P, Prieto I, García R, et al. Obesity causes PGC‐1α deficiency in the pancreas leading to marked IL‐6 upregulation via NF‐κB in acute pancreatitis. J Pathol [Internet]. 2019;247(1):48–59. Available from: https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.5166.
|
| 67. |
Wu J, Zhou Q, Wei Z, Wei J, Cui M. Atherogenic Index of Plasma and Coronary Artery Disease in the Adult Population: A Meta-Analysis. Front Cardiovasc Med [Internet]. 2021;8. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2021.817441/full.
|
| 68. |
Agu P, Egbugara M, Ogboi J, Ajah L, Nwagha U, Ugwu E, et al. Atherogenic Index, Cardiovascular Risk Ratio, and Atherogenic Coefficient as Risk Factors for Cardiovascular Disease in Pre-eclampsia in Southeast Nigeria: A Cross-Sectional Study. Niger J Clin Pract [Internet]. 2024;27(2):221–7. Available from: https://journals.lww.com/10.4103/njcp.njcp_633_23.
|
| 69. |
Mallika DK, Manohar SM, Arslan M. Association of atherogenic indices with obesity and as biomarkers of cardiovascular risk. Int J Res Med Sci [Internet]. 2022;10(5):1126. Available from: https://www.msjonline.org/index.php/ijrms/article/view/10690.
|
| 70. |
Wei D, Melgarejo J, Vanassche T, Van Aelst L, Janssens S, Verhamme P, et al. Atherogenic lipoprotein profile associated with anthropometric indices of obesity and their association with cardiometabolic risk markers: a cross-sectional study in community. Eur Heart J [Internet]. 2022;43(Supplement_2). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehac544.2386/6745627.
|
| 71. |
M. MM, M. K. J. Impact of obesity on atherogenic index in young adult females in Gadag, Karnataka. Int J Res Med Sci [Internet]. 2024;12(9):3274–8. Available from: https://www.msjonline.org/index.php/ijrms/article/view/13756.
|