Abstract
Background: Diabetes mellitus (DM) manifests itself in consistently high blood glucose concentrations as a result of insufficient insulin secretion, action, or both. This can result in long-term complications, including diabetic nephropathy (DN). The aim of this study was to examine the impact of a four-week administration of zinc sulfate (Zn) and vitamin D (Vit D) in controlling glycemia and preventing renal damage in an experimental model of DM.
Material and methods: A total of 55 rats, each weighing between 100 and 150 grams, were grouped into 11 groups: diabetic control rats, healthy control rats, and diabetic rats that had received Zn, Vit D and metformin as reference medication before and after alloxan injection.
Results: DM induced by alloxan led to notable rises in blood urea nitrogen, creatinine and uric acid concentrations. Moreover, it caused damage to kidney tissues, with severe morphological damage in the renal tissues of diabetic rats. Elevated levels of kidney function markers and other biochemical parameters were decreased by administering zinc and Vit D, but Zn produced the greatest reduction. Additionally, histopathological examination of the excised kidneys revealed increased protection following supplementation.
Conclusions: The findings of this study suggest that Zn and Vit D are effective in managing DM and protecting against DN.
Citation
Bakr M. F., Elrehany M. A., Othman O. A. Role of zinc and vitamin D in modulating diabetic nephropathy in experimental models Eur J Transl Clin MedIntroduction
Diabetes mellitus (DM) is a complicated, multi-causal and chronic condition manifested by increased levels of blood sugar (hyperglycemia). It is characterized by a total or partial decrease of insulin synthesis or secretion by β-cells of the pancreas, in addition to its inability to produce normal physiological effects [1].
By specifically inhibiting glucokinase, the β-cells’ sensor of glucose, insulin release induced by glucose is selectively inhibited by alloxan. Additionally, alloxan causes reactive oxygen species (ROS) to generate, which leads selective necrosis of β-cells and the development of IDDM. The preferential cellular uptake and alloxan accumulation by β-cells is the common denominator between these two pathways. This is explained by the unique chemical properties of alloxan [2].
Hyperglycemia induces oxidative and nitrosative stress in various cell types, leading to the generation of reactive species like superoxide, nitric oxide, and peroxynitrite. These ROS contribute to apoptotic cell death, which is associated with diabetes mellitus complications such as nephropathy, neuropathy, and cardiovascular disease. Key proteins involved in this process include caspases and BCL-2 family members [3].
Diabetic nephropathy (DN) is a microvascular complication of diabetes that ultimately results in end-stage renal disease [4]. Because DN is a common cause of chronic kidney disease (CKD), dialysis patients with DM have a greater death rate than patients without the disease [5]. Thus, effective antioxidants might be effective in the treatment of disorders associated with DM [6].
DN is caused by the interplay of metabolic and hemodynamic variables [7]. Increased intraglomerular and systemic pressure, as well as the activation of vasoactive hormone pathways, such as the renin-angiotensin system (RAS) and endothelin, are hemodynamic factors that lead to the development of DN [8-9]. In the diabetic kidney, glucose-dependent pathways are also triggered, leading to increased oxidative stress, the production of renal polyol [10], and the buildup of advanced glycation end products (AGEs) [11]. When these mechanisms work together, they eventually result in increased extracellular matrix accumulation and renal albumin permeability, which leads to proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis.
Zinc sulfate (Zn) is a necessary trace element that maintains many of the body’s cellular processes. Zn plays a vital role in facilitating the transport of glucose into cells, as well as in improving glucose storage, insulin crystallization and signaling, which is necessary for glucose metabolism. When Zn supplementation was administered to rats with DN, proteinuria, fibrosis, and renal oxidative stress were all reduced, protecting kidney structure and function [12-15].
Vitamin D (Vit D) is one of the fat-soluble vitamins [16]. There is a long history of the use of various Vit D derivatives in the management of renal disorders and some research studies have indicated that Vit D compounds may reduce the death rate in CKD [17]. Furthermore, Vit D helps maintain low resting levels of free radicals and ROS, normalizes Ca2+ signaling, reduces the expression of pro-inflammatory cytokines, and increases the production of anti-inflammatory cytokines [18].
Numerous studies have shown that oxidative stress (excess free radicals) contributes to the development of DM, reduces insulin function, and increases the risk of DM complications, therefore antioxidants have already demonstrated promise in the management of T1D and T2D [19]. The aim of this study was to examine the impact of a 4-week administration of Zn and Vit D in controlling glucose levels and preventing renal damage.
Material and methods
Animals
Adult male albino rats, weighing 100 to 150 g, were obtained from the National Research Center in Giza (Egypt) and kept in the animal house of the Faculty of Pharmacy in Deraya University (El-Minia, Egypt). A 12-hour cycle of light and dark and constant environmental factors (humidity 50% ± 10%, temperature 23 °C ± 3 °C) were provided. During the experiment, the rats were allowed free access to water and a standard chow diet, unless otherwise stated. For two weeks before the experimental procedures, the animals were kept in separate aerated cages to acclimate to the new environment and ensure they were disease-free.
Experimental design
The current study was performed in accordance with the international guidelines regarding animal experiments [20]. A total of 55 adult male albino rats were divided randomly into eleven groups (n = 5 for each) as follows:
- Group 1: healthy controls receiving normal saline only.
- Group 2: diabetic controls receiving a solution of alloxan monohydrate.
- Group 3: healthy controls treated with Zn only.
- Group 4: healthy controls treated with Vit D only.
- Group 5: healthy controls treated with metformin only.
- Group 6: diabetic post-treated with Zn.
- Group 7: diabetic post-treated with Vit D.
- Group 8: diabetic post-treated with metformin.
- Group 9: diabetic pre-treated with Zn.
- Group 10: diabetic pre-treated with Vit D.
- Group 11: diabetic pre-treated with metformin.
Chemicals and drugs
Alloxan monohydrate, metformin (Glucophage 500 mg/ tablet), Zinc sulfate (Octozinc 110 mg/capsule) and vitamin D (Vi drop 2800 unit/ml) were obtained from Loba Chemie Pvt Ltd (India), Mina Pharm (Egypt), October Pharma S.A.E and Medical Union Pharmaceuticals (Egypt), respectively. The administered drugs were freshly prepared daily for 28 days except for alloxan, which was freshly prepared once before and after the experiment. Metformin (120 mg/kg BW), Zn (100 mg/kg BW) and Vit D solution (10 IU/kg BW) were administered orally using an oral gavage feeding needle, whereas the alloxan solution was injected intraperitoneally with an insulin syringe, while the rat was held securely to avoid harming it [21-23].
Induction of diabetes
Since alloxan is less stable when dissolved and its half-life is short (1.5 minutes at pH 7.4 and 37 °C), the rats (apart from the control groups) underwent overnight fasting and were given a single dose (150 mg/kg BW) of freshly prepared alloxan immediately after dissolving in 0.9% saline solution [24]. Then, in order to overcome the alloxan-induced hypoglycemia (due to the large pancreatic insulin release following beta cell damage), the rats were given free access to a glucose solution (5%) for 24 hours [21, 25]. A glucometer (PreciChek Autocode, AC-302, Germany) and compatible test strips of blood glucose were used to measure the rats’ fasting glycemia after 3 days in order to confirm that DM had been successfully induced. Blood was collected from the tail vein. In this study, only rats with fasting blood glucose levels exceeding 250 mg/dl were selected as diabetics [26].
Blood sample collection and dissection
Four weeks following treatment, the rats were decapitated after fasting overnight. Their blood was then collected in sterile centrifuge tubes, allowed to coagulate for 30 min at room temperature, and then centrifuged at 4000 rpm for 10 min (Z 200 A, Hermle LaborTechnik GmbH, Germany). The obtained serum (supernatant) was then stored at -80° C immediately after separation for biochemical analysis. Finally, each rat’s abdomen was dissected, the right kidney was rapidly excised, weighed, and subsequently stored in formaline solution for histopathological examinations.
Biochemical analysis
- Glucose and Kidney function markers
Glucose, creatinine, blood urea nitrogen (BUN) and uric acid were assessed using colorimetric assay kits (purchased from Spectrum Diagnostics in Cairo, Egypt) by a semi-automated chemistry analyzer (SK3002B, Sinothinker Technology Co. Limited, Shenzen, China).
- Glucose
In the presence of glucose oxidase, glucose is measured following enzymatic oxidation. Under the catalytic action of peroxidase (PAP), the generated hydrogen peroxide combines with phenol and 4-aminoantipyrine to generate a red violet quinoneimine dye that serves as an indicator.
- Creatinine
Creatinine reacts with picric acid in alkaline solution to form a colored complex.
- Blood urea nitrogen
Urea hydrolyzes in water to release carbon dioxide and ammonia. In an alkaline pH, free ammonia forms a colored complex that is proportionate to the specimen’s urea concentration when an indicator is present.
- Uric Acid
Uric acid is oxidized to allantoin by uricase with the production of hydrogen peroxide. The peroxide reacts with 4-amino-antipyrine and (DCHB) in the presence of peroxidase to yield a quinoneimine dye.
Apoptotic markers
B-cell lymphoma 2 (BCL-2) and caspase 3 (CASP-3) ELISA kits were purchased from Elabscience, Texas, USA. These markers were determined using an ELISA System (ChroMate SF 4300 Microplate Reader, Awareness Technology, Palm City, FL, USA). Both ELISA kits use the Sandwich-ELISA method. The microplate is pre-coated with an antibody specific to BCL-2 or CASP-3, and samples or standards were added to the wells. A biotinylated detection antibody and Avidin-HRP conjugate were then added, followed by incubation. After washing away unbound components, a substrate solution was added, resulting in a color change in the wells containing BCL-2 or CASP-3. The optical density (OD) at 450 nm was measured, which is proportional to the BCL-2 or CASP-3 concentration.
Histopathological examination
The right kidney from each experimental rat was washed with 0.9% saline solution and instantly fixed in a solution of formalin (10%) prepared in saline for 3 days. Then the organ was washed, dehydrated in increasing ethanol grades from 70% to absolute, cleared in xylene, impregnated and embedded in paraffin wax. Serial sections (5 μm thick) were obtained using a microtome and stained with hematoxylin and eosin (H&E) for histopathological examination using a light microscope equipped with a digital camera (Olympus, Japan) [27].
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) software (version 26.0, IBM, Armonk, NY, United States) was used to perform the statistical analyses. The mean of the results ± standard deviation (SD) was presented. Differences of p < 0.05, p < 0.01 or p < 0.001 were accepted as significant, moderately significant or highly significant, respectively. Insignificant is denoted by the symbol (#), significant by the symbol (*), moderately significant by the symbol (**), and highly significant by the symbol (***).
Results
Effects of zinc, vitamin D and metformin administration on blood glucose levels
The effects of the oral administration of Zn and Vit D on fasting blood glucose are presented in Table 1. The experimentally induced DM caused a highly significant (p > 0.001) increase in the level of fasting glucose in the diabetic control group (2) compared to the control levels of healthy groups. However, post and pre-treatment with Zn and Vit D caused a highly significant (p >0.001) reduction in the fasting glucose levels of the alloxan-diabetic rats of groups (6, 7, 9 and 10) compared with the diabetic control group (2), as shown in Figure 1A. However, the rats in the post-treated groups (6 and 9) showed a higher reduction of glycemia than the pre-treated groups (7 and 10).
Table 1. Mean glycemia before & after the experiment and mean of selected kidney function markers concentrations in experimental rats after administration of alloxan and test drugs.
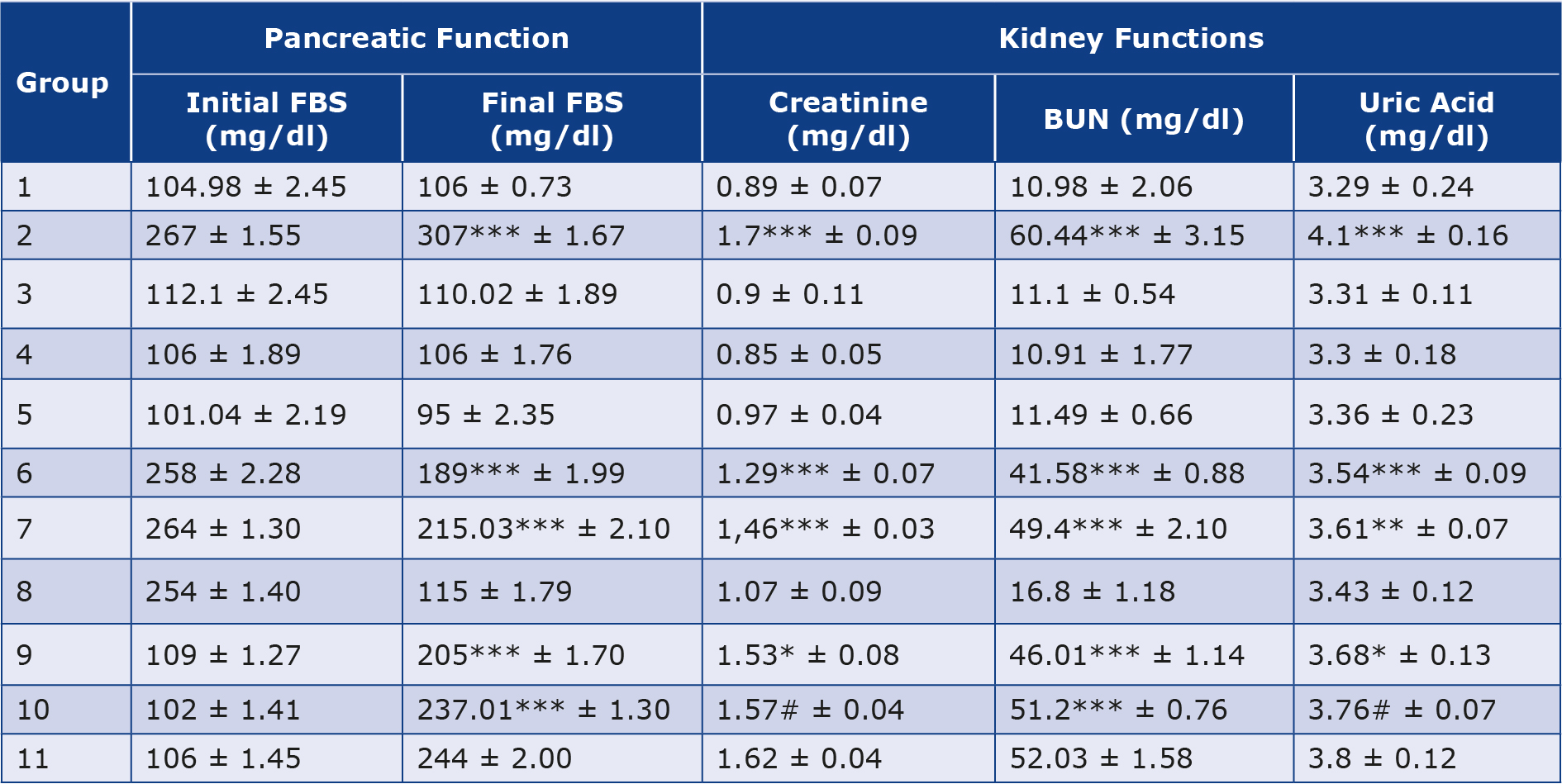
All values are expressed as mean ± standard deviation of 5 rats from each group.
BUN – blood urea nitrogen, FBS – fasting blood sugar
Effects of zinc, vitamin D and metformin administration on selected kidney function markers
The experimental rats’ serum kidney function marker concentrations were measured and summarized in Table 1. In alloxan-diabetic rats of group (2), the concentrations of serum creatinine, blood urea nitrogen (BUN) and uric acid underwent a highly significant (p > 0.001) increase compared to the healthy control groups (Figure 1 B-D). On the other hand, post-treatment of the diabetic rats with Zn and Vit D caused a highly significant (p > 0.001) reduction in the concentrations of these markers in the serum of groups (6 and 7) compared to the mean values of the diabetic control group (2), except for uric acid levels in rats of group (7), which underwent a moderately significant (p > 0.01) reduction. However, pre-treatment with Zn and Vit D resulted in a highly significant (p > 0.01) reduction in the BUN level of groups (9 and 10), a significant (p > 0.05) reduction in the creatinine and uric acid levels of group (9) and an insignificant (p > 0.05) reduction in the creatinine and uric acid levels of group (10).
Effects of zinc, vitamin D and metformin administration on apoptotic markers
As shown in Table 2 & Figure 1E-F, the 2 apoptotic markers we investigated were affected by the alloxan injection. The diabetic control group (2) showed a highly significant (p < 0.001) increase in levels of CASP-3 and decrease in levels of BCL-2 compared to the healthy control groups (1, 3, 4 and 5). However, post and pre-treatment with Zn and Vit D insignificantly (p< 0.05) increased the levels of BCL-2, significantly (p < 0.05) decreased the levels of CASP-3 in groups (6, 9 and 10) and insignificantly (p > 0.05) decreased the CASP-3 levels in group (7). Thus, the administration of Zn and Vit D reversed the levels of apoptotic makers compared to the diabetic control group (2).
Table 2. Mean of some apoptotic markers concentrations in experimental rats after administration of alloxan and test drugs.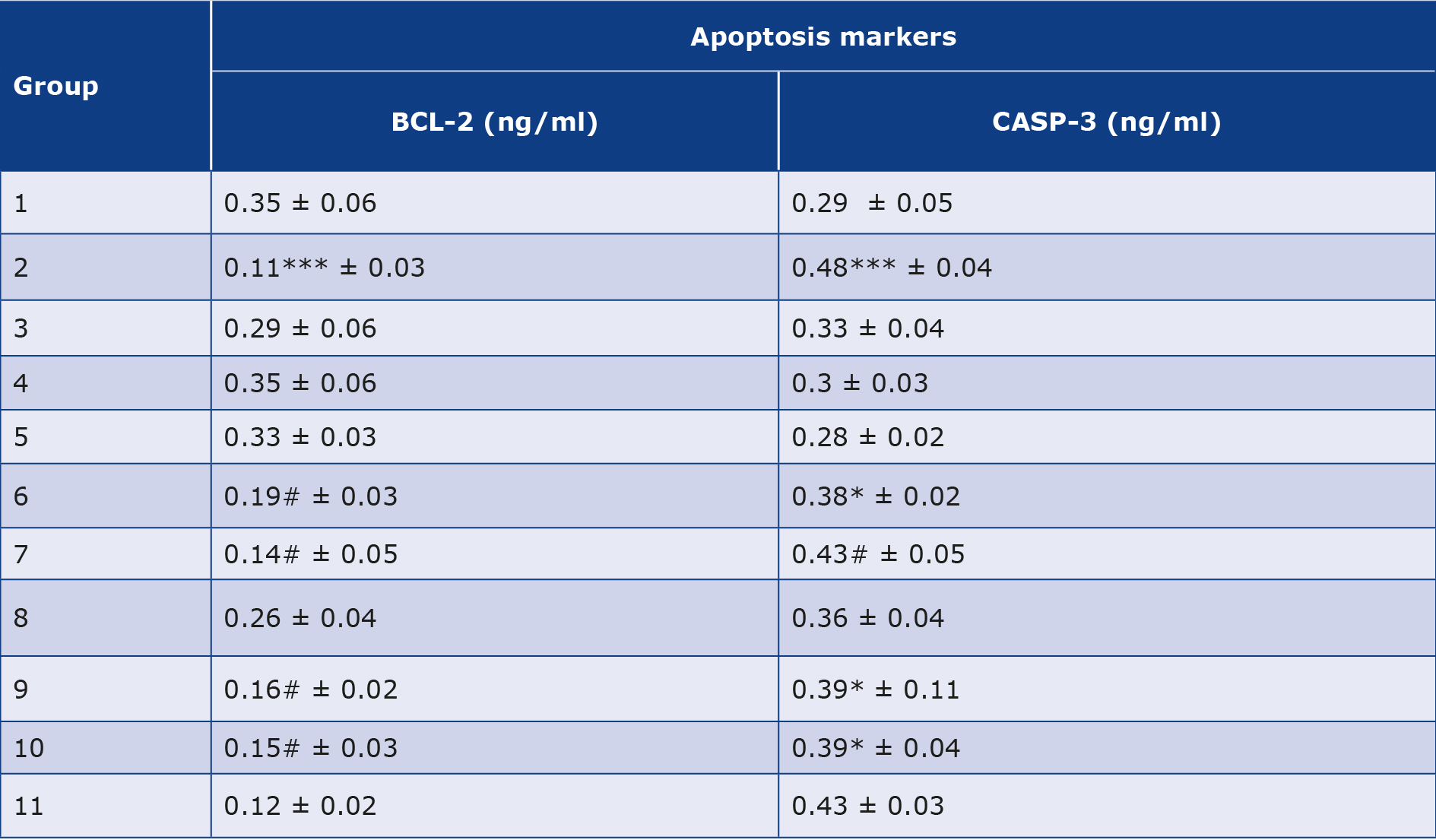
All values are expressed as mean ± standard deviation of 5 rats from each group.
BCL-2 – β-cell lymphoma 2, CASP-3 – caspase 3
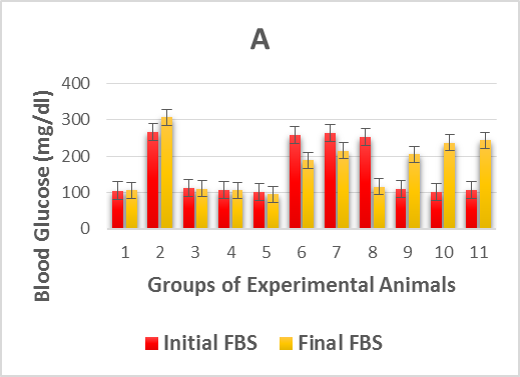
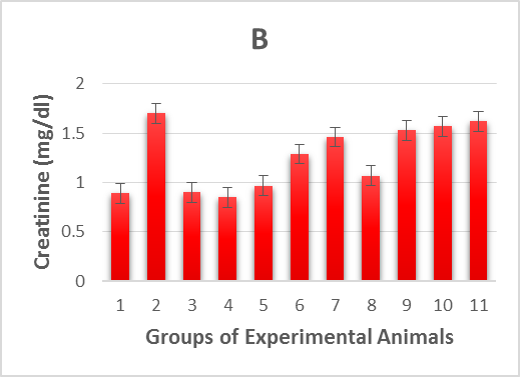
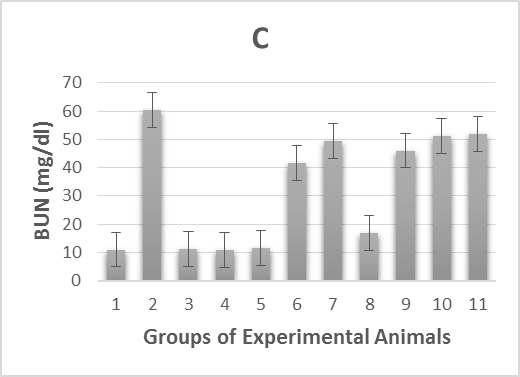
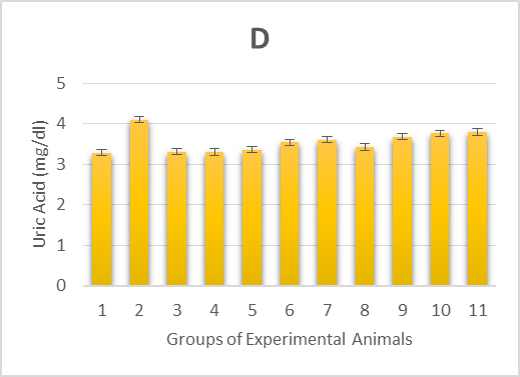
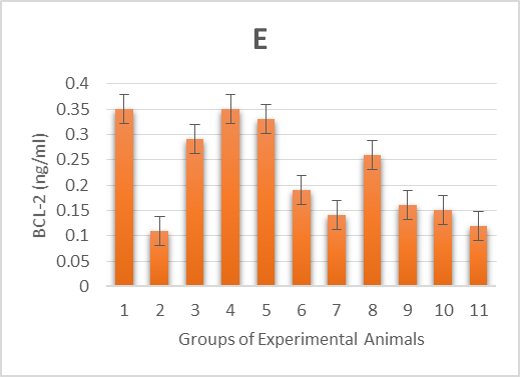
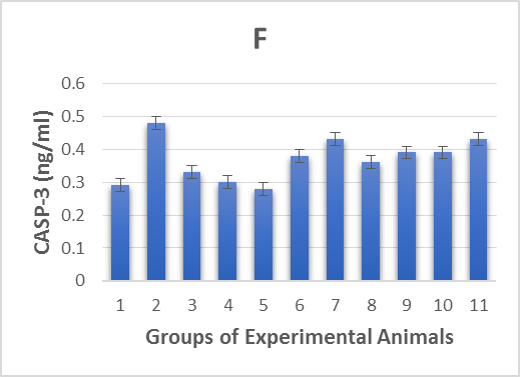
Figure 1. Illustrates effect of metformin, zinc and vitamin D administration either before alloxan injection (prophylactic) or after injection (therapeutic) on:
1A. the initial fasting blood sugar (3 days after alloxan injection) and final fasting blood sugar (28 days after test drugs administration) of alloxan-induced diabetic rats,
1B. creatinine levels of alloxan-induced diabetic rats,
1C. blood urea nitrogen (BUN) levels of alloxan-induced diabetic rats,
1D. uric acid levels of alloxan-induced diabetic rats,
1E. β-cell lymphoma 2 (BCL-2) levels of alloxan-induced diabetic rats,
1F. caspase 3 levels of alloxan-induced diabetic rats.
Effects of zinc, vitamin D and metformin administration on renal tissues
Our study also showed that alloxan-induced DM in the rats of group (2) caused damage to the renal structures while the healthy control groups (1, 3, 4 and 5) showed no significant pathological changes. Histological sections of the kidney excised from the diabetic group (2) showed congested glomerular capillary tuft, congested blood vessels and interstitial haemorrhage with signs of renal tubular injury (Figure 2B). In contrast, sections of healthy groups’ kidneys (1, 3, 4 and 5) showed normal glomeruli and normal renal tubules (Figure 2A, Figure 2C-E). However, sections obtained from post and pre-treated diabetic rats of groups (6, 7, 9 and 10) with Zn and Vit D showed reduced glomeruli congestion and necrosis, normal tubules, dilated Bowman’s space, and some had signs of renal tubular injury (Figure 2F-G, 2I-K), compared to the diabetic control group (2).
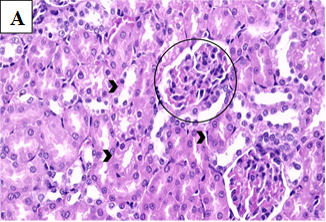
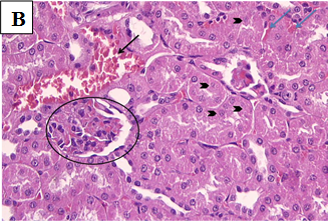
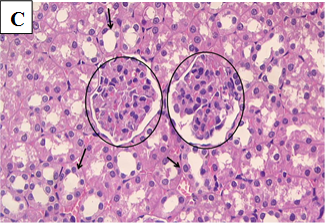
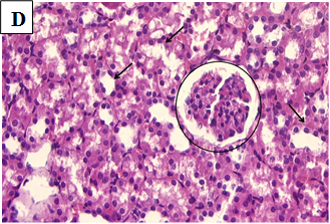
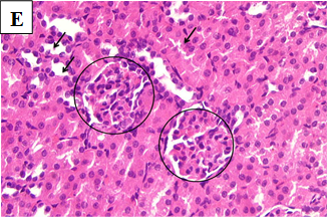
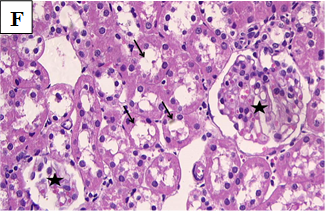
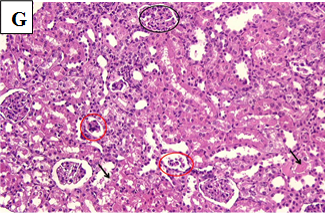
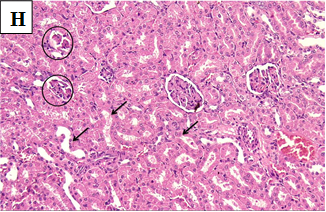
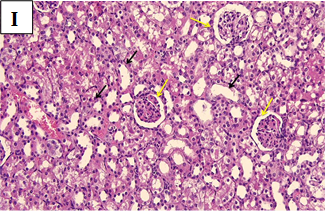
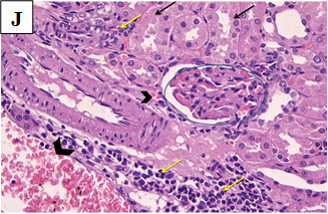
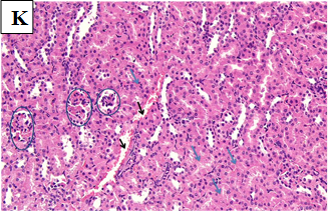
Figure 2A. Photomicrographs of rat renal tissues of healthy control group showing normal glomeruli (black circles) and normal renal tubules (black arrow head) × 400.
Figure 2B. Photomicrographs of rat renal tissues of diabetic control group showing congested glomerular capillary tuft (black circle), congested blood vessels (black arrow), interstitial haemorrhage (blue arrows) with signs of renal tubular injury and necrosis (arrow head) × 400.
Figure 2C. Photomicrographs of rat renal tissues of healthy control group treated with zinc sulfate only showing normal glomeruli (circle) and normal renal tubules (arrows) × 400.
Figure 2D. Photomicrographs of rat renal tissues of healthy control group treated with vitamin D only showing normal glomeruli (circle) and normal renal tubules (black arrows) × 400.
Figure 2E. Photomicrographs of rat renal tissues of healthy control group treated with metformin only showing normal looking glomeruli (circles) and tubules (arrows) × 400.
Figure 2F. Photomicrographs of rat renal tissues of diabetic group post-treated with zinc sulfate showing normal glomeruli (star) and normal tubules (arrows) × 400.
Figure 2G. Photomicrographs of rat renal tissues of diabetic group post-treated with vitamin D showing some glomeruli are normal (black circle) while others are necrotic or sclerotic (red circles) with dilated Bowman’s space, some tubules show signs of renal tubular injury (black arrow) × 200.
Figure 2H. Photomicrographs of rat renal tissues of diabetic group post-treated with metformin showing some atrophic glomeruli (black circle), mild cloudy swelling of renal tubular epithelium (black arrow) × 200.
Figure 2I. Photomicrographs of rat renal tissues of diabetic group pre-treated with zinc sulfate showing normal glomeruli (yellow arrows) and renal tubules (black arrows) × 200.
Figure 2J. Photomicrographs of rat renal tissues of diabetic group pre-treated with vitamin D showing congested glomerular tuft (blue circle), signs of renal tubular injury and cloudy swelling (black arrows), interstitial haemorrhage (arrow head) and chronic inflammatory cellular infiltrate (yellow arrows) × 400.
Figure 2K. Photomicrographs of rat renal tissues of diabetic group pre-treated with metformin showing congested or atrophic renal glomeruli (blue circles), interstitial haemorrhage (black arrows), moderate cloud swelling of renal tubular epithelium (blue arrows) × 200.
Discussion
DN is caused by renal tissue destruction resulting from toxic levels of elevated blood sugar. Patients with DM experience impaired kidney function due to hemodynamic alterations in renal tissue caused by hyperglycemia and glycosylated proteins, linked to increased oxidative stress [28]. In our study, injection of alloxan in Group 2 induced hyperglycemia and significantly elevated serum glucose levels compared to the healthy control (Group 1), supporting previous findings that chronic hyperglycemia leads to pathological renal changes such as tubulointerstitial alterations and thickening of the tubular basement membrane, which is further characterized by the accumulation of matrix protein, tubular and glomerular hypertrophy, and renal hypertrophy [29].
Numerous complications of DM, including DN, are linked to oxidative stress triggered by hyperglycemia [30]. Chronic hyperglycemia in renal tissues leads to oxidative stress and release of ROS, which activate the nuclear transcription factor (NF-κB), thereby promoting kidney inflammation [31]. In agreement with these mechanisms, our diabetic control group (Group 2) exhibited significant increases in markers of renal damage, such as creatinine, blood urea nitrogen (BUN), uric acid, and CASP-3, while BCL-2 was reduced, indicating enhanced apoptosis due to oxidative damage. These findings align with the established role of ROS and oxidative stress in promoting renal fibrosis and end-stage renal disease (ESRD) through extracellular matrix (ECM) accumulation and glomerulosclerosis which is associated with deteriorating renal function [32-33].
Sustained hyperglycemia contributes to nephropathy through multiple cellular mechanisms, such as PKC pathway activation, the generation of cytokines, enhanced polyol pathways, the increased formation of AGEs, increased oxidative stress, and the hexosamine pathway [35]. In our study, the deleterious renal effects observed in Group 2 further confirm the damaging impact of these pathways, as shown by significant biochemical and apoptotic alterations. Furthermore, DM-related hyperglycemia damages mitochondria and raises reactive free radicals, which in turn damages DNA and triggers the apoptosis process. Additionally, hyperglycemia raises lipid peroxidation, glutathione (GSH) oxidation, and oxidative stress. Lastly, hyperglycemia causes diabetic nephrons to experience oxidative stress, which stimulates a number of biochemical mechanisms that result in the death of renal cells, elevated albuminuria, and kidney failure that were likely contributors to the observed elevation of apoptotic markers such as CASP-3 [35].
Among diabetic patients, DN remains a leading cause of renal failure [36]. Chronic low-grade inflammation and activation of the innate immune system play critical roles in DN progression [37]. Our findings, showing elevated CASP-3 and reduced BCL-2 in diabetic controls, are consistent with reports that TNF-α and other pro-inflammatory cytokines promote renal injury by inducing apoptosis and necrotic cell death [38-40].
DN has a complicated etiology involves direct effects of extracellular glucose on renal cells, activating growth factors and cytokines like monocyte chemoattractant protein (MCP)- 1, transforming growth factor-b (TGF-b), and angiotensin II (Ang II), which further mediate development of DN [41-43]. The role of the renin-angiotensin system (RAS) in DN is well established, with intrarenal Ang II levels contributing significantly to glomerulosclerosis and fibrosis. So, It is thought that intrarenal RAS plays a primary injurious role in causing kidney damage [44-45]. Our study supports these concepts indirectly by demonstrating that unregulated diabetes induced by alloxan led to substantial renal damage, which could be associated with the known effects of elevated Ang II on promoting inflammation, cell proliferation, and fibrosis [41, 46].
Given the critical role of oxidative stress and inflammation in DN, attention has been directed towards potential nephroprotective agents, such as zinc (Zn). Zn is a vital cofactor for over 300 enzymes and plays a crucial role in antioxidant defense [48]. Zn deficiency has been linked to increased susceptibility to infections and elevated ROS generation [49], which aligns with the observed exacerbation of oxidative damage in the diabetic group of our study.
Necessity of Zn for superoxide dismutase activation, a potent antioxidant enzyme [49], underlines its antioxidant role. Low serum Zn concentrations have been associated with higher incidences of DM, glucose tolerance and cardiovascular diseases [50]. The administration of Zn in our study, both as treatment (Group 6) and prophylaxis (Group 9), significantly improved glycemic control and reduced markers of renal dysfunction compared to the diabetic group. These results are consistent with previous findings that restoring Zn status mitigates oxidative stress and prevents complications related to DM [51].
Zn facilitates glucose transporter 4 (GLUT4) translocation and insulin receptor β-subunit phosphorylation [52-53], both crucial for glucose metabolism. Furthermore, Zn ions are vital for insulin hexamer formation, while insulin combines with two Zn ions to form a hexameric structure, which is required for insulin to mature inside pancreatic β-cell secretory granules and release insulin [54-55]. Certain Zn carrier proteins that are essential for insulin release are expressed by pancreatic β-cells [56-58]. One such important protein is ZnT8, which is necessary for the general metabolism of glucose as well as the crystallization, processing, storage and secretion of insulin [59-62]. Moreover, the Zn transporter protein ZnT7 is in charge of delivering Zn to the pancreatic β-cells’ Golgi apparatus, which is a necessary step for the correct synthesis of insulin [63-64]. Our observation that Zn supplementation improved glycemic parameters and renal function supports the established role of Zn in enhancing insulin secretion and action.
Supplementing with Zn decreased the rate of renal damage in comparison to the control groups by activating metallothionein, a cysteine-rich protein that interacts with Zn and iron to reduce ROS and, in turn, the oxidation process [65-67]. Another key mechanism by which Zn can protect the kidneys from chronic hyperglycemia-induced damage is through apoptotic regulation. Zn supplementation in our study reduced serum CASP-3 and elevated BCL-2 levels compared to the diabetic group, indicating its anti-apoptotic properties. Previous studies have demonstrated that Zn reduces apoptosis by the inhibition of CASP-3 and promoting BCL-2 expression [68].
Similar protective effects were observed with vitamin D (Vit D) administration. Vit D, acting primarily through its receptor in renal tissues, has been reported to exert nephroprotective effects in diabetic models [69]. In our study, both treatment (Group 7) and prophylaxis (Group 10) with Vit D improved serum glucose levels and renal function markers, consistent with studies linking optimal Vit D levels to better glucose homeostasis and insulin sensitivity [70-71].
Vit D’s nephroprotective mechanisms include enhancing glucose metabolism, inhibiting the renin-angiotensin system, blocking oxidative stress pathways, and reducing the production of inflammatory mediators like TNF-α and IL-6 [72-73]. Our observation of reduced renal injury with Vit D treatment supports previous findings that Vit D modulates RAS activity and protects against DN by regulating BCL-2 and CASP-3 expression [74].
The nephroprotective properties of Vit D may be attributed to several mechanisms, including suppressing the RAS and inhibiting inflammatory cytokines and profibrotic growth factors. First, TGF-β and CTGF, which are crucial mediators for the formation of sclerosis in DN, may be targeted by 1,25(OH)2D3 [45, 77-78]. Second, the nephroprotective effects of Vit D may also be linked to its control of the RAS, specifically renin [77]. DM causes an increase in the intrarenal RAS activity, which is crucial for the onset of diabetic nephropathy [78-79]. Ang II causes mesangial and tubular cells to produce more ECM proteins and express TGF-β [80]. Additionally, Ang II causes glomerular damage and increases glomerular permeability, which results in proteinuria. It has been noted that diabetic nephropathy can be improved by inhibiting renin or Ang II activity [81-82].
Thus, both Zn and Vit D demonstrated significant protective effects against alloxan-induced diabetic nephropathy by reducing oxidative stress, improving glycemic control, modulating apoptotic pathways, and preserving renal function, as reflected by the improvement of biochemical markers and apoptotic regulators compared to untreated diabetic rats.
Conclusion
Zn is an important microelement that plays a role in vital processes that control body homeostasis. Supplementing with Zn appears to have a positive impact on DN risk factors as well as slowing the development of the disease. The potential of supplemental Zn in reducing renal damage in DN was investigated in this study. External administration of Zn repaired the pathological changes in the renal tissues of rats with DM. By reversing proinflammatory and profibrotic markers implicated in the pathophysiology of DN, Vit D therapy has been shown to protect the kidneys from damage. This can happen through the expression of the Vit D receptor and by preventing the compensatory activation of renin-angiotensin II. Therefore, supplementation with both Zn and Vit D needs to be regarded as key targets in the prevention and treatment of DM.
Acknowledgements
We extend our gratitude to Dr. Dina Moustafa Thabit (Lecturer of Pathology, Faculty of Medicine, Minia University) for her valuable contributions in conducting the histological examinations in this study.
Funding
There is no source of funding for this study.
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study and the handling of rats were carried out as stated by the guidelines of the Committee of Research Ethics of the Faculty of Pharmacy, Minia University, Egypt (MPEC230202), and following the principles outlined in the Guide for the Care and Use of Lab Animals [20]. Every attempt was made to reduce animal suffering and use as few animals as possible.
References
| 1. |
Ke C, Narayan KV, Chan JC, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18(7):413-432. Available from: https://www.nature.com/articles/s41574-022-00669-4.
|
| 2. |
Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008;51(2):216-226. Available from: https://link.springer.com/article/10.1007/S00125-007-0886-7.
|
| 3. |
Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16(12):705-713. Available from: https://doi.org/10.1016/j.jnutbio.2005.06.007.
|
| 4. |
Gomes IB, Porto ML, Santos MCL, Campagnaro BP, Pereira TM, Meyrelles SS, Vasquez EC. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014;13:1-10. Available from: https://link.springer.com/article/10.1186/1476-511x-13-184.
|
| 5. |
Zhang G, Zhou L, Xu Z, Pronyuk K, Chen X, Wang H. Diabetic nephropathy: causative and protective factors. Glob J Med Res. 2015;15(2):17-26. Available from: https://medicalresearchjournal.org/index.php/GJMR/article/view/901/3-Diabetic-Nephropathy_html.
|
| 6. |
Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life sci. 2014;109(1):8-14. Available from: https://doi.org/10.1016/j.lfs.2014.06.005.
|
| 7. |
Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001;44(11):1957-72. Available from: https://doi.org/10.1007/s001250100000.
|
| 8. |
Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77(6):1925-30. Available from: https://doi.org/10.1172/jci112521.
|
| 9. |
Hargrove GM, Dufresne J, Whiteside C, Muruve DA, Wong NC. Diabetes mellitus increases endothelin-1 gene transcription in rat kidney. Kidney Int. 2000;58(4):1534-45. Available from: https://doi.org/10.1046/j.1523-1755.2000.00315.x.
|
| 10. |
Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S3-12. Available from: https://doi.org/10.1046/j.1523-1755.2000.07702.x.
|
| 11. |
Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes 1991;40(10):1328-34. Available from: https://doi.org/10.2337/diab.40.10.1328.
|
| 12. |
Ahmad R, Shaju R, Atfi A, Razzaque MS. Zinc and Diabetes: A Connection between Micronutrient and Metabolism. Cells 2024;13(16):1359. Available from: https://doi.org/10.3390/cells13161359.
|
| 13. |
Piao M, Liu Y, Yu T, Lu Y. Dietary zinc reduces endoplasmic reticulum stress and autophagy to protect against diabetic renal damage in streptozotocin-induced diabetic rats. Int J Diabetes Dev Ctries. 2019;39:340-345. Available from: https://link.springer.com/article/10.1007/s13410-018-0681-7.
|
| 14. |
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Cai L. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 2010;21(3):237-246. Available from: https://doi.org/10.1016/j.jnutbio.2008.12.010.
|
| 15. |
Zhang X, Liang D, Lian X, Chi ZH, Wang X, Zhao Y, Ping Z. Effect of zinc deficiency on mouse renal interstitial fibrosis in diabetic nephropathy. Mol Med Rep. 2016;14(6):5245-5252. Available from: https://www.spandidos-publications.com/10.3892/mmr.2016.5870?text=fulltext.
|
| 16. |
Fidan F, Alkan BM, Tosun A. Çağın pandemisi: D vitamini eksikliği ve yetersizliği. Turk J Osteoporos. 2014;20(2):71-74. Available from: https://turkosteoporozdergisi.org/articles/doi/tod.94830.
|
| 17. |
Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo Jr CA, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115-1125. Available from: https://journals.lww.com/jasn/abstract/2005/04000/activated_injectable_vitamin_d_and_hemodialysis.39.aspx.
|
| 18. |
Vasdeki D, Tsamos G, Dimakakos E, Patriarcheas V, Koufakis T, Kotsa K, Cholewka A, Stanek A. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients 2024;16(21):3651. Available from: https://doi.org/10.3390/nu16213651.
|
| 19. |
Ali A, Kumar M, Srivastava N, Khan MM, Khan MA. Free radicals and diabetes mellitus. Int J Pharm Sci Med. 2023; 8;1-19. Available from: http://dx.doi.org/10.47760/ijpsm.2023.v08i03.001.
|
| 20. |
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US); 2011. Available from: https://doi.org/10.17226/12910.
|
| 21. |
Hamadjida A, Tchiengang FT, Metechie LC, Otto GN, Eteme ON, Mingoas JK. Hypoglycemic and hypolipidemic effects of hydroethanolic stem bark extract of Boswellia dalzielii in alloxan-induced diabetic rats. World J Pharm Pharmaceut Sci. 2023;12:1910-1929. Available from: http://dx.doi.org/10.20959/wjpps20237-25388.
|
| 22. |
Bolkent S, Yanardag R, Bolkent S, Mutlu O, Yildirim S, Kangawa K, Suzuki H. The effect of zinc supplementation on ghrelin-immunoreactive cells and lipid parameters in gastrointestinal tissue of streptozotocin-induced female diabetic rats. Mol Cell Biochem. 2006;286:77-85. Available from: https://link.springer.com/article/10.1007/s11010-005-9095-1.
|
| 23. |
Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29(3):650-656. Available from: https://doi.org/10.2337/diacare.29.03.06.dc05-1961.
|
| 24. |
Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017;53(6):365-374. Available from: https://doi.org/10.1016/j.medici.2018.02.001.
|
| 25. |
Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res. 2002;46(3):251-255. Available from: https://doi.org/10.1016/S1043-6618(02)00131-7.
|
| 26. |
Hamadjida A, Metechie LC, Tchiengang FDT, Otto GLN, Eteme ON, Njintang NY, Mingoas JPK. Antidiabetic potential of Hibiscus sabdariffa extract in alloxan-induced diabetic rats. GSC Biol Pharm Sci. 2023;23(1):193-203. Available from: https://doi.org/10.30574/gscbps.2023.23.1.0158.
|
| 27. |
Levison DA. Book Reviews: Theory and practice of histological techniques. 4th Edition. J Pathol. 1997;183:243-244.
|
| 28. |
Madhan Kumar SJ, Malarvani T, Sathiyanarayamurthy S. Nephroprotective effects of Cynodon dactylon aqueous extract in STZ induced diabetic male rats – histological study. Int J Pharmacogn Phytochem Res. 2016;8:1812-1817. Available from: https://impactfactor.org/PDF/IJPPR/8/IJPPR,Vol8,Issue11,Article10.pdf.
|
| 29. |
Gupta P, Sharma P, Shanno K, Jain V, Pareek A, Agarwal P, Sharma V. Nephroprotective role of alcoholic extract of Pterocarpus marsupium heartwood against experimentally induced diabetic nephropathy. J Pharm Pharmacogn Res. 2016;4(5):174-186. Available from: http://www.redalyc.org/articulo.oa?id=496053937002.
|
| 30. |
Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia‐induced free radical generation leading to oxidative stress. J Cell Physiol. 2019;234(2):1300-1312. Available from: https://doi.org/10.1002/jcp.27164.
|
| 31. |
Das K, Ghosh M. Structured DAG oil ameliorates renal injury in streptozotocin-induced diabetic rats through inhibition of NF-κB and activation of Nrf2 pathway. Food Chem Toxicol. 2017;100:225-238. Available from: https://doi.org/10.1016/j.fct.2016.12.033.
|
| 32. |
Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10(9):493-503. Available from: https://www.nature.com/articles/nrneph.2014.114.
|
| 33. |
Zanchi C, Macconi D, Trionfini P, Tomasoni S, Rottoli D, Locatelli M, Rudnicki M, Vandesompele J, Mestdagh P, Remuzzi G, Benigni A, Zoja C. MicroRNA-184 is a downstream effector of albuminuria driving renal fibrosis in rats with diabetic nephropathy, Diabetologia 2017;60:1114-1125. Available from: https://link.springer.com/article/10.1007/s00125-017-4248-9.
|
| 34. |
Ashrafi M, Nazifi S, Namazi F, Kazemipour N, Karimi B, Goudarzi T, Talebanzadeh S. Renal protective effect of saffron aqueous extract in streptozotocin induced diabetic rats. Int J Med Sci Public Health 2017;6:151-161. Available from: https://www.ijmrhs.com/medical-research/renal-protective-effect-of-saffron-aqueous-extract-in-streptozotocin-induced-diabetic-rats.pdf.
|
| 35. |
Jamshidi HR, Zeinabady Z, Zamani E, Shokrzadeh M. Attenuation of diabetic nephropathy by carvacrol through anti-oxidative effects in alloxan-induced diabetic rats. Res J Pharmacogn. 2018;5(2):57-64. Available from: https://doi.org/10.22127/rjp.2018.58508.
|
| 36. |
Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44(9):1763-1771. Available from: https://doi.org/10.1016/j.jacc.2004.06.075.
|
| 37. |
Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 1998;41:1241-1248. Available from: https://link.springer.com/article/10.1007/s001250051058.
|
| 38. |
Sun L, Kanwar YS. Relevance of TNF-α in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015;88(4):662-665. Available from: https://doi.org/10.1038/ki.2015.250.
|
| 39. |
Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134(2):419. Available from: https://pubmed.ncbi.nlm.nih.gov/2916653/.
|
| 40. |
Boyle JJ, Weissberg PL, Bennett MR. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler Thromb Vasc Biol. 2002; 22(10):1624-1630. Available from: https://doi.org/10.1161/01.ATV.0000033517.48444.1A.
|
| 41. |
Carey RM, Siragy HM. The intrarenal renin–angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14(6):274-281. Available from: https://www.cell.com/ajhg/abstract/S1043-2760(03)00111-5#.
|
| 42. |
Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. J Am Soc Nephrol. 2004;15(1_suppl):S55-S57. Available from: https://doi.org/10.1097/01.asn.0000093460.24823.5b.
|
| 43. |
Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58(2):684-690. Available from: https://doi.org/10.1046/j.1523-1755.2000.00214.x.
|
| 44. |
Price DA, Porter LE, Gordon M, Fisher ND, De’ Oliveira JMF, Laffel LM, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10(11):2382-2391. Available from: https://doi.org/10.1681/asn.v10112382.
|
| 45. |
Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 2002;39(1):129-134. Available from: https://doi.org/10.1161/hy0102.100536.
|
| 46. |
Gilbert RE, Krum H, Wilkinson‐Berka J, Kelly DJ. The renin–angiotensin system and the long‐term complications of diabetes: pathophysiological and therapeutic considerations. Diabet Med. 2003;20(8):607-621. Available from: https://doi.org/10.1046/j.1464-5491.2003.00979.x.
|
| 47. |
King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130(5):1360S-1366S. Available from: https://doi.org/10.1093/jn/130.5.1360S.
|
| 48. |
Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341-363. Available from: https://doi.org/10.1146/annurev.nu.05.070185.002013.
|
| 49. |
Prasad AS, Bao B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants. 2019;8(6):164. Available from: https://doi.org/10.3390/antiox8060164.
|
| 50. |
Parham M, Amini M, Aminorroaya A, Heidarian E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud. 2008;5(2):102-9. Available from: https://doi.org/10.1900/rds.2008.5.102.
|
| 51. |
Matzke GR, Aronoff GR, Atkinson AJ Jr, Bennett WM, Decker BS, Eckardt KU, Golper T, Grabe DW, Kasiske B, Keller F, Kielstein JT, Mehta R, Mueller BA, Pasko DA, Schaefer F, Sica DA, Inker LA, Umans JG, Murray P. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80(11):1122-37. Available from: https://doi.org/10.1038/ki.2011.322.
|
| 52. |
Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, Poursafa P, Rouzbahani A. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. 2010;8(6):505-10. Available from: https://doi.org/10.1089/met.2010.0020.
|
| 53. |
Vardatsikos G, Pandey NR, Srivastava AK. Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem. 2013;120:8-17. Available from: https://doi.org/10.1016/j.jinorgbio.2012.11.006.
|
| 54. |
Chimienti F. Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev. 2013;26(1):1-11. Available from: https://doi.org/10.1017/s0954422412000212.
|
| 55. |
Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine 2014;45(2):178-89. Available from: https://doi.org/10.1007/s12020-013-0032-x.
|
| 56. |
Myers SA. Zinc transporters and zinc signaling: new insights into their role in type 2 diabetes. Int J Endocrinol. 2015;2015:167503. Available from: https://doi.org/10.1155/2015/167503.
|
| 57. |
Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71(17):3281-95. Available from: https://doi.org/10.1007/s00018-014-1617-0.
|
| 58. |
Cai Y, Kirschke CP, Huang L. SLC30A family expression in the pancreatic islets of humans and mice: cellular localization in the β-cells. J Mol Histol. 2018;49(2):133-145. Available from: https://doi.org/10.1007/s10735-017-9753-0.
|
| 59. |
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010;53(8):1656-68. Available from: https://doi.org/10.1007/s00125-010-1733-9.
|
| 60. |
Davidson HW, Wenzlau JM, O’Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014;25(8):415-24. Available from: https://doi.org/10.1016/j.tem.2014.03.008.
|
| 61. |
Foster M, Chu A, Petocz P, Samman S. Zinc transporter gene expression and glycemic control in post-menopausal women with Type 2 diabetes mellitus. J Trace Elem Med Biol. 2014;28(4):448-52. Available from: https://doi.org/10.1016/j.jtemb.2014.07.012.
|
| 62. |
Lefebvre B, Vandewalle B, Balavoine AS, Queniat G, Moerman E, Vantyghem MC, Le Bacquer O, Gmyr V, Pawlowski V, Kerr-Conte J, Pattou F. Regulation and functional effects of ZNT8 in human pancreatic islets. J Endocrinol. 2012;214(2):225-32. Available from: https://doi.org/10.1530/joe-12-0071.
|
| 63. |
Cruz KJC, de Oliveira ARS, Morais JBS, Severo JS, Mendes PMV, de Sousa Melo SR, de Sousa GS, Marreiro DDN. Zinc and Insulin Resistance: Biochemical and Molecular Aspects. Biol Trace Elem Res. 2018;186(2):407-412. Available from: https://doi.org/10.1007/s12011-018-1308-z.
|
| 64. |
Huang L, Yan M, Kirschke CP. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp Cell Res. 2010;316(16):2630-43. Available from: https://doi.org/10.1016/j.yexcr.2010.06.017.
|
| 65. |
Özcelik D, Nazıroglu M, Tunçdemir M, Çelik Ö, Öztürk M, Flores-Arce MF. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2012;150:342-349. Available from: https://link.springer.com/article/10.1007/s12011-012-9508-4.
|
| 66. |
Zhou Z, Sun X, Kang YJ. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227(3):214-222. Available from: https://doi.org/10.1177/153537020222700310.
|
| 67. |
Cai L. Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol Toxicol Med. 2004;2(2):15401420490464367. Available from: https://doi.org/10.1080/15401420490464367.
|
| 68. |
Zhang X, Zhao Y, Chu Q, Wang ZY, Li H, Chi ZH. Zinc modulates high glucose-induced apoptosis by suppressing oxidative stress in renal tubular epithelial cells. Biol Trace Elem Res. 2014;158:259-267. Available from: https://doi.org/10.1007/s12011-014-9922-x.
|
| 69. |
Derakhshanian H, Shab-Bidar S, Speakman JR, Nadimi H, Djafarian K. Vitamin D and diabetic nephropathy: a systematic review and meta-analysis. Nutrition 2015;31(10):1189-1194. Available from: https://doi.org/10.1016/j.nut.2015.04.009.
|
| 70. |
Flores M. A role of vitamin D in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus? Nutr Res Rev. 2005;18(2):175-82. Available from: https://doi.org/10.1079/nrr2005104.
|
| 71. |
Karnchanasorn R, Ou HY, Chiu KC. Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas 2012;41(6):863-8. Available from: https://doi.org/10.1097/mpa.0b013e31823c947c.
|
| 72. |
Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, Nishi S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014;27(4):586-595. Available from: https://doi.org/10.1093/ajh/hpt160.
|
| 73. |
Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients 2014;6(6):2206-2216. Available from: https://doi.org/10.3390/nu6062206.
|
| 74. |
Wang Y, Deb DK, Zhang Z, Sun T, Liu W, Yoon D, Li YC. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012;23(12):1977-1986. Available from: https://doi.org/10.1681/asn.2012040383.
|
| 75. |
Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58(4):1389-99. Available from: https://doi.org/10.1046/j.1523-1755.2000.00301.x.
|
| 76. |
Goldschmeding R, Aten J, Ito Y, Blom I, Rabelink T, Weening JJ. Connective tissue growth factor: just another factor in renal fibrosis? Nephrol Dial Transplant. 2000;15(3):296-9. Available from: https://doi.org/10.1093/ndt/15.3.296.
|
| 77. |
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229-38. Available from: https://doi.org/10.1172/jci15219.
|
| 78. |
Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14(6):274-81. Available from: https://doi.org/10.1016/s1043-2760(03)00111-5.
|
| 79. |
Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993;265(4 Pt 2):F477-86. Available from: https://doi.org/10.1152/ajprenal.1993.265.4.f477.
|
| 80. |
Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93(6):2431-7. Available from: https://doi.org/10.1172/jci117251.
|
| 81. |
Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57(2):601-6. Available from: https://doi.org/10.1046/j.1523-1755.2000.00880.x.
|
| 82. |
Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128-35. Available from: https://doi.org/10.1172/jci21398.
|

























