Abstract
Rhabdomyosarcoma (RMS) is a pediatric soft tissue cancer with poor prognosis in cases of metastasis. Different signal transduction pathways have been studied in RMS cells as to shed some light into the tumorigenesis and metastasis mechanisms of this cancer in the search for new diagnostic and therapeutic strategies. The Notch pathway, which regulates cell survival, is widely studied in different cancers, including sarcomas and its activation has been known to induce cell motility. To this end, the present review explores the role of the Notch signaling pathway in the progression of RMS and in potential therapeutic strategies.
Citation
Kalali D, Milošević E, Anestakis D. The role of the Notch signaling pathway in rhabdomyosarcomas. Eur J Transl Clin Med. 2025;8(1):97-102Introduction
Rhabdomyosarcoma (RMS) is the most frequent type of pediatric soft tissue cancer in the global population, accounting for approximately 7% of malignancies in patients under 15 years of age [1]. Although the survival rates of patients have improved considerably over the past years due to advances in therapeutic strategies, RMS is an aggressive tumor with poor prognosis in case of metastasis [2-3]. RMS is histologically divided into two main subtypes: alveolar and embryonal. Although these subtypes differ anatomically and in terms of the clinical picture, they share many common molecular characteristics [4].
Different signaling pathways have been studied in all types of RMS cells, with the hope of shedding some light on the process of oncogenesis and finding potential novel therapeutic targets [5-6]. The socalled ‘pathways of embryonic development’ (e.g. the Notch, Hippo, Wnt and Hedgehog pathways) are widely studied in malignant neoplasms and are viewed as potential targets in anticancer therapy [7]. The Notch pathway can result in both cell survival and death, thus it is one of the key pathways in tumorigenesis and tumor progression and is interlinked with various other tumorigenic signal transduction pathways [8-9]. It is triggered by the activation of the Notch transmembrane receptors, namely the Notch1, Notch2, Notch3 and Notch4 proteins encoded by four distinct genes. The binding of Notch proteins with transmembrane Delta and Jagged proteins expressed on adjacent cells, activates the signal transduction pathway [10]. The pathway results in the cleavage and activation of the Notch intracellular domain (NotchIC) with the assistance of gamma-secretase. Subsequently, the NotchIC domain enters the nucleus and induces the expression of different pro-proliferative and anti-apoptotic genes through the RBP-J (recombination signal binding protein for immunoglobulin kappa J region) [10-11].
The evidence on the role of the Notch pathway in RMS cells is scattered in different sources. In this narrative review we aimed to explore in depth the role of this pathway in the tumorigenesis and metastasis of RMS and insights into targeting its proteins as possible therapeutic strategies.
Material and methods
A systematic search was performed in the online databases of PubMed, Scopus, and EMBASE to retrieve all available literature on the role of the Notch pathway in RMS, published from inception till July 2024. A combination of the keywords “Notch”, “Embryonic signaling pathways”, “Rhabdomyosarcoma” and “RMS” were used in combination with the Boolean operators “OR” and “AND.” Due to the narrative nature of the review, there were no specific inclusion and exclusion criteria or screening protocol. Overall, all relevant original research and review articles, written in English were included in our review (total of 11 articles).
Results and discussion
The Notch pathway and oncogenesis of RMS
Recently, various studies have found that the Notch pathway plays a significant role in the tumorigenesis of RMS. Specifically, the Notch pathway has been found to induce overexpression of the transcription factor HES1 (hairy and enhancer of split-1) in RMS cell lines and consequently, promote cell proliferation and inhibit apoptotic pathways [12-13]. Simultaneously, studies have shown that RMS cells tend to express the Notch1 and Notch3 receptors at higher levels compared to healthy muscle cells and that the induction of their respective pathways amplifies the proliferation ability of these cells [14]. Moreover, RMS cells have been found to overexpress RBP-J and therefore activate the p38 MAPKS and MMK3 genes that support malignant proliferation through the Notch pathway [15]. The Notch pathway is also known to activate muscle satellite cells (SMCs) and thus amplify myoblast formation in infants [16]. In this manner, the Notch pathway activation can induce myoblast-like properties in RMS cells. It is also worth mentioning that SMCs are known to trigger the upregulation of MMPs and at the same time, MMPs are necessary for the regulation and proliferation of SMCs [17].
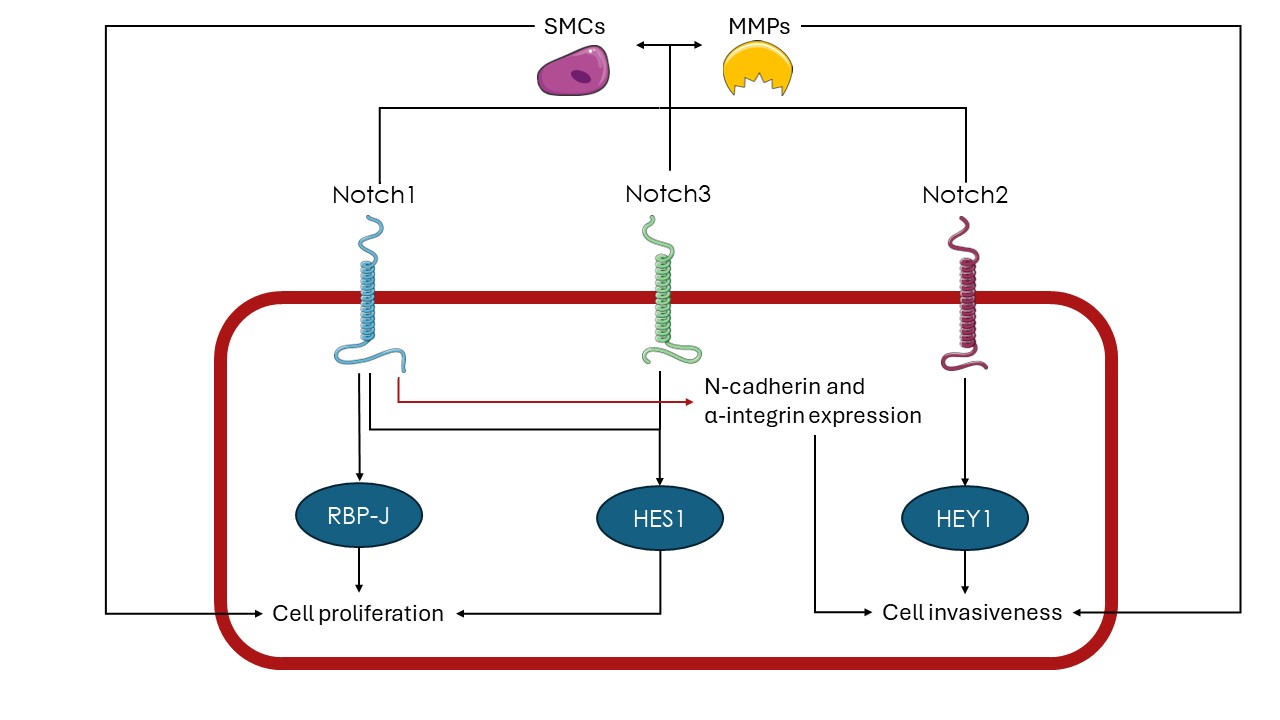
Figure 1. Notch signaling in RMS
HES1 – hairy and enhancer of split-1, HEY1 – hairy/enhancer-of-split related with YRPW motif protein 1, MMPs – matrix metalloproteinases, RBP-J – recombination signal binding protein for immunoglobulin kappa J region, SMCs – satellite muscle cells
The Notch pathway and metastatic potential of RMS
The activation of the Notch pathway has been found to enhance the metastatic potential of RMS cells. More specifically, Roma et al. showed that the Notch receptor and the transcription factor HEY 1 (hairy/enhancer-of-split related with YRPW motif protein 1) are overexpressed in RMS patients and that the Notch pathway inhibition decreases invasiveness and motility of RMS cells [18]. Furthermore, the induction of the Notch pathway, triggered by the Notch1 receptor has been found to increase the invasiveness of RMS cells through the induction of the N-cadherin and alpha-integrin proteins [19]. It is also worth noting that the Notch pathway is generally known to induce the expression of matrix metalloproteinases in the microenvironment of different tumors including sarcomas. The aforementioned enzymes can assist the process of metastasis by degrading components of the extracellular matrix [20-22]. Overall, by regulating the expression of different proteins and enzymes, the Notch pathway assists RMS cells to overcome barriers of their progression and in turn migrate to other sites. Figure 1 summarizes the pathways through which Notch signaling can contribute to the progression and metastasis of RMS.
The Notch pathway as a potential therapeutic target in RMS
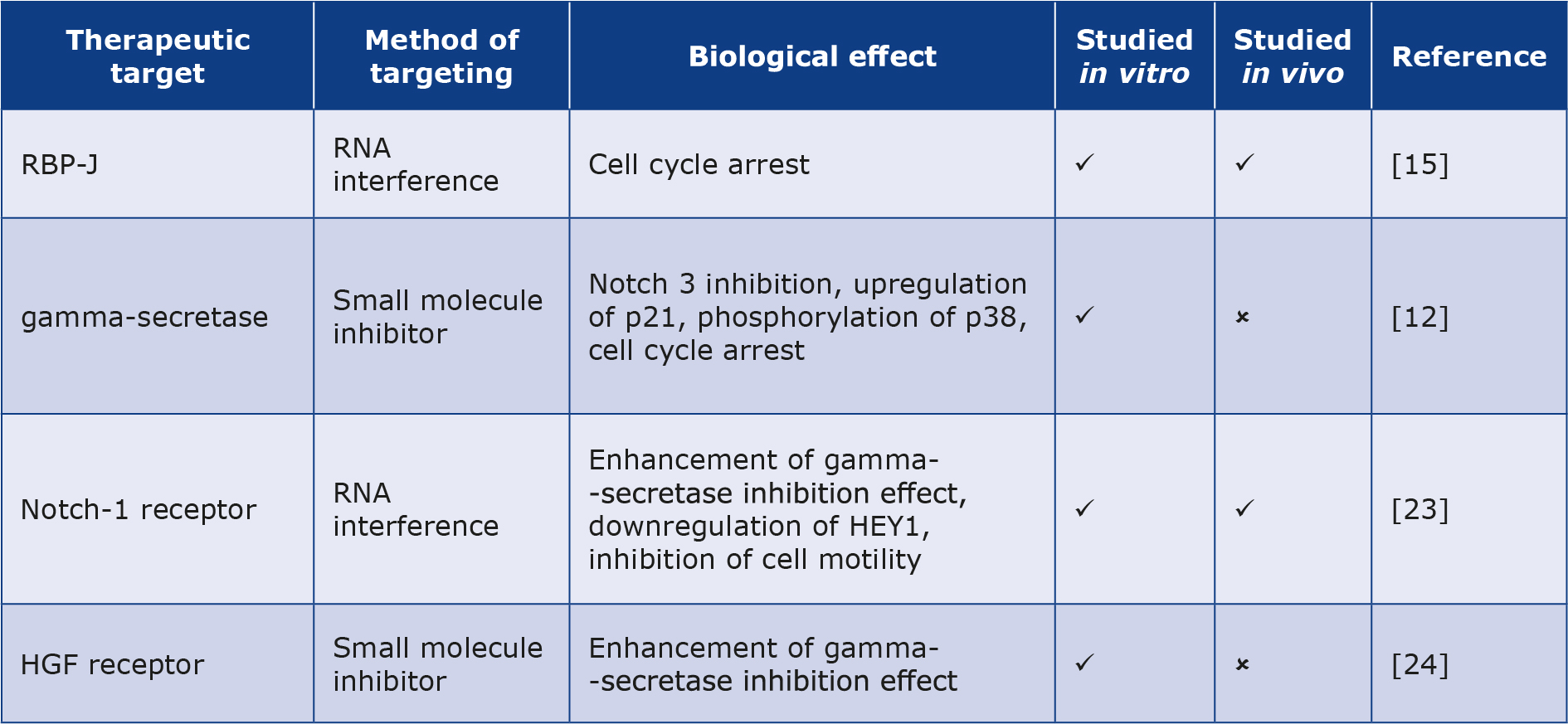
Due to the role of Notch signaling in the proliferation and motility of RMS cells, different studies have been conducted to investigate the effect of inhibiting the aforementioned pathway as a way of controlling the disease. Nagao et al. showed that the knockdown of RBP-J by RNA interference significantly reduced the growth of RMS cells and induced cell cycle arrest, indicating that the inhibition of the Notch pathway through silencing RBP-J can be a potential therapeutic strategy for RMS [15]. Moreover, inhibition of Notch3 signaling using an inhibitor of gamma-secretase has been shown to amplify p21 expression and induce p38 phosphorylation, hence inhibiting cell proliferation and activating apoptotic pathways in RMS cells [12]. Likewise, the use of gamma-secretase inhibitors alongside Notch1 silencing through RNA interference has been shown to exert antiproliferative effects on RMS cells and at the same time, downregulate HEY1 expression, hence decreasing invasiveness in vitro and in vivo [23]. It has been shown that inhibiting the hepatocyte growth factor receptor (HGF receptor), can make RMS cells respond more sensitively to gamma-secretase inhibitors [24].
Furthermore, it is worth mentioning that many clinical trials have been undertaken for potential therapeutic drugs that inhibit the Notch pathway in other types of malignancies [25]. Specifically, phase I clinical trials on anti-Notch1 monoclonal antibody brontictuzumab have shown promising results in patients with lymphomas and refractory solid tumors, with the main toxicity being the induction of diarrhea [26-27]. Moreover, a phase I clinical trial on the monoclonal antibody tarextumab which targets Notch2 and Notch3 simultaneously, presented encouraging results in patients with solid tumors [28]. Gamma-secretase inhibitors were not shown to be effective in the treatment of other advanced solid tumors, although they presented few adverse effects (diarrhea, nausea and vomiting) [29-30]. Table 1 summarizes the potential targets for the Notch pathway inhibition and thus control of RMS cells.
Table 1. Potential therapeutic targets inhibiting Notch in RMS
Conclusions
RMS research has been focused on discovering the mechanisms through which the neoplasm progresses, and the ways of inhibiting them [31]. Also, in the last three years, several promising therapeutics have been investigated, but the exact mechanisms through which they act are still unknown [32-33]. As seen in the present review, in vitro and in vivo research hasConflicts of interest demonstrated a significant role of the Notch signaling pathway in the development of RMS. Hence, it can be viewed as a potential target for developing new targeted therapy drugs for this type of cancer. Combining potential monoclonal antibodies or small molecule inhibitors targeting the Notch pathway with other existing drugs may help reduce the chances of developing drug resistance. Nonetheless, the exact mechanisms through which the Notch pathway promotes proliferation and migration in RMS cells remain unknown. Therefore, more research should be conducted to assess the different biological effects of the Notch pathway activity in RMS.
None.
Funding
None.
References
| 1. |
Martin-Giacalone BA, Weinstein PA, Plon SE, Lupo PJ. Pediatric Rhabdomyosarcoma: Epidemiology and Genetic Susceptibility. J Clin Med. 2021;10(9):2028. Available from: https://www.mdpi.com/2077-0383/10/9/2028.
|
| 2. |
Haduong JH, Heske CM, Allen‐Rhoades W, Xue W, Teot LA, Rodeberg DA, et al. An update on rhabdomyosarcoma risk stratification and the rationale for current and future Children’s Oncology Group clinical trials. Pediatr Blood Cancer 2022;69(4). Available from: https://onlinelibrary.wiley.com/doi/10.1002/pbc.29511.
|
| 3. |
Kim JR, Yoon HM, Koh K-N, Jung AY, Cho YA, Lee JS. Rhabdomyosarcoma in Children and Adolescents: Patterns and Risk Factors of Distant Metastasis. Am J Roentgenol. 2017;209(2):409–16. Available from: https://www.ajronline.org/doi/10.2214/AJR.16.17466.
|
| 4. |
Agaram NP. Evolving classification of rhabdomyosarcoma. Histopathology 2022;80(1):98–108. Available from: https://onlinelibrary.wiley.com/doi/10.1111/his.14449.
|
| 5. |
Sun X, Guo W, Shen JK, Mankin HJ, Hornicek FJ, Duan Z. Rhabdomyosarcoma: Advances in Molecular and Cellular Biology. Sarcoma 2015;2015:1–14. Available from: http://www.hindawi.com/journals/sarcoma/2015/232010/.
|
| 6. |
Stewart E, McEvoy J, Wang H, Chen X, Honnell V, Ocarz M, et al. Identification of Therapeutic Targets in Rhabdomyosarcoma through Integrated Genomic, Epigenomic, and Proteomic Analyses. Cancer Cell. 2018;34(3):411-426.e19. Available from: https://doi.org/10.1016/j.ccell.2018.07.012.
|
| 7. |
Dreesen O, Brivanlou AH. Signaling Pathways in Cancer and Embryonic Stem Cells. Stem Cell Rev. 2007;3(1):7–17. Available from: https://link.springer.com/10.1007/s12015-007-0004-8.
|
| 8. |
Anusewicz D, Orzechowska M, Bednarek AK. Notch Signaling Pathway in Cancer—Review with Bioinformatic Analysis. Cancers (Basel) 2021;13(4):768. Available from: https://www.mdpi.com/2072-6694/13/4/768.
|
| 9. |
Previs RA, Coleman RL, Harris AL, Sood AK. Molecular Pathways: Translational and Therapeutic Implications of the Notch Signaling Pathway in Cancer. Clin Cancer Res. 2015;21(5):955–61. Available from: https://aacrjournals.org/clincancerres/article/21/5/955/247574/Molecular-Pathways-Translational-and-Therapeutic.
|
| 10. |
Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95. Available from: https://www.nature.com/articles/s41392-022-00934-y.
|
| 11. |
Lake RJ, Tsai P-F, Choi I, Won K-J, Fan H-Y. RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking. Fortini M, editor. PLoS Genet. 2014;10(3):e1004204. Available from: https://dx.plos.org/10.1371/journal.pgen.1004204.
|
| 12. |
Raimondi L, Ciarapica R, De Salvo M, Verginelli F, Gueguen M, Martini C, et al. Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21Cip1 expression and hampers tumour cell growth in vitro and in vivo. Cell Death Differ. 2012;19(5):871–81. Available from: https://www.nature.com/articles/cdd2011171.
|
| 13. |
Sang L, Coller HA, Roberts JM. Control of the Reversibility of Cellular Quiescence by the Transcriptional Repressor HES1. Science (80- ) 2008;321(5892):1095–100. Available from: https://www.science.org/doi/10.1126/science.1155998.
|
| 14. |
De Salvo M, Raimondi L, Vella S, Adesso L, Ciarapica R, Verginelli F, et al. Hyper-Activation of Notch3 Amplifies the Proliferative Potential of Rhabdomyosarcoma Cells. Wang Q, editor. PLoS One 2014;9(5):e96238. Available from: https://dx.plos.org/10.1371/journal.pone.0096238.
|
| 15. |
Nagao H, Setoguchi T, Kitamoto S, Ishidou Y, Nagano S, Yokouchi M, et al. RBPJ Is a Novel Target for Rhabdomyosarcoma Therapy. Wang Q, editor. PLoS One. 2012;7(7):e39268. Available from: https://dx.plos.org/10.1371/journal.pone.0039268.
|
| 16. |
Conboy IM, Rando TA. The Regulation of Notch Signaling Controls Satellite Cell Activation and Cell Fate Determination in Postnatal Myogenesis. Dev Cell. 2002;3(3):397–409. Available from: https://linkinghub.elsevier.com/retrieve/pii/S153458070200254X.
|
| 17. |
Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle. Cell Adh Migr. 2009;3(4):337–41. Available from: http://www.tandfonline.com/doi/abs/10.4161/cam.3.4.9338.
|
| 18. |
Roma J, Masià A, Reventós J, Sánchez de Toledo J, Gallego S. Notch Pathway Inhibition Significantly Reduces Rhabdomyosarcoma Invasiveness and Mobility In Vitro. Clin Cancer Res. 2011;17(3):505–13. Available from: https://aacrjournals.org/clincancerres/article/17/3/505/76773/Notch-Pathway-Inhibition-Significantly-Reduces.
|
| 19. |
Masià A, Almazán-Moga A, Velasco P, Reventós J, Torán N, Sánchez de Toledo J, et al. Notch-mediated induction of N-cadherin and α9-integrin confers higher invasive phenotype on rhabdomyosarcoma cells. Br J Cancer 2012;107(8):1374–83. Available from: https://www.nature.com/articles/bjc2012411.
|
| 20. |
Hernole J, Narayanappa R. Interactions between Notch and matrix metalloproteinases: the role in cancer. Biul Pol Tow Onkol Nowotw. 2023;8(6):436–43. Available from: https://journals.viamedica.pl/biuletyn_pto/article/view/98685.
|
| 21. |
Jawad MU, Garamszegi N, Garamszegi SP, Correa-Medina M, Diez JA, Wen R, et al. Matrix Metalloproteinase 1: Role in Sarcoma Biology. Deb S, editor. PLoS One 2010;5(12):e14250. Available from: https://dx.plos.org/10.1371/journal.pone.0014250.
|
| 22. |
Kalali D. The Role of the Matrix Metalloproteinase-9 Gene in Tumor Development and Metastasis: A Narrative Review. Glob Med Genet. 2023 Jun;10(02):048–53. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0043-1768166.
|
| 23. |
Belyea BC, Naini S, Bentley RC, Linardic CM. Inhibition of the Notch-Hey1 Axis Blocks Embryonal Rhabdomyosarcoma Tumorigenesis. Clin Cancer Res. 2011;17(23):7324–36. Available from: https://aacrjournals.org/clincancerres/article/17/23/7324/76712/Inhibition-of-the-Notch-Hey1-Axis-Blocks-Embryonal.
|
| 24. |
Perrone C, Pomella S, Cassandri M, Pezzella M, Milano GM, Colletti M, et al. MET Inhibition Sensitizes Rhabdomyosarcoma Cells to NOTCH Signaling Suppression. Front Oncol. 2022;12. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2022.835642/full.
|
| 25. |
Li X, Yan X, Wang Y, Kaur B, Han H, Yu J. The Notch signaling pathway: a potential target for cancer immunotherapy. J Hematol Oncol. 2023;16(1):45. Available from: https://jhoonline.biomedcentral.com/articles/10.1186/s13045-023-01439-z.
|
| 26. |
Ferrarotto R, Eckhardt G, Patnaik A, LoRusso P, Faoro L, Heymach J V, et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. 2018;29(7):1561–8. Available from: https://www.sciencedirect.com/science/article/pii/S092375341932109X.
|
| 27. |
Casulo C, Ruan J, Dang NH, Gore L, Diefenbach C, Beaven AW, et al. Safety and Preliminary Efficacy Results of a Phase I First-in-Human Study of the Novel Notch-1 Targeting Antibody Brontictuzumab (OMP-52M51) Administered Intravenously to Patients with Hematologic Malignancies. Blood 2016;128(22):5108. Available from: https://www.sciencedirect.com/science/article/pii/S0006497119351092.
|
| 28. |
Smith DC, Chugh R, Patnaik A, Papadopoulos KP, Wang M, Kapoun AM, et al. A phase 1 dose escalation and expansion study of Tarextumab (OMP-59R5) in patients with solid tumors. Invest New Drugs. 2019;37(4):722–30. Available from: https://link.springer.com/10.1007/s10637-018-0714-6.
|
| 29. |
Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I Pharmacologic and Pharmacodynamic Study of the Gamma Secretase (Notch) Inhibitor MK-0752 in Adult Patients With Advanced Solid Tumors. J Clin Oncol. 2012;30(19):2307–13. Available from: https://ascopubs.org/doi/10.1200/JCO.2011.39.1540.
|
| 30. |
Gounder MM, Rosenbaum E, Wu N, Dickson MA, Sheikh TN, D’Angelo SP, et al. A Phase Ib/II Randomized Study of RO4929097, a Gamma-Secretase or Notch Inhibitor with or without Vismodegib, a Hedgehog Inhibitor, in Advanced Sarcoma. Clin Cancer Res. 2022;28(8):1586–94. Available from: https://aacrjournals.org/clincancerres/article/28/8/1586/694158/A-Phase-Ib-II-Randomized-Study-of-RO4929097-a.
|
| 31. |
Zarrabi A, Perrin D, Kavoosi M, Sommer M, Sezen S, Mehrbod P, et al. Rhabdomyosarcoma: Current Therapy, Challenges, and Future Approaches to Treatment Strategies. Cancers (Basel) 2023;15(21):5269. Available from: https://www.mdpi.com/2072-6694/15/21/5269.
|
| 32. |
Milosevic E, Stanisavljevic N, Boskovic S, Stamenkovic N, Novkovic M, Bavelloni A, et al. Antitumor activity of natural pigment violacein against osteosarcoma and rhabdomyosarcoma cell lines. J Cancer Res Clin Oncol. 2023;149(13):10975–87. Available from: https://link.springer.com/10.1007/s00432-023-04930-9.
|
| 33. |
Morel VJ, Rössler J, Bernasconi M. Targeted immunotherapy and nanomedicine for rhabdomyosarcoma: The way of the future. Med Res Rev. 2024;44(6):2730-73. Available from: https://onlinelibrary.wiley.com/doi/10.1002/med.22059.
|









