Safety and efficacy of the Oxford-AstraZeneca vaccine against SARS-CoV-2 after 2nd and 3rd dose in adults: a cross-sectional study
Abstract
Background: The rapid global spread of the coronavirus SARS-CoV-2 and the lack of established therapeutic choices led to development of several vaccines. Our aim was to investigate the safety and effectiveness of the Oxford-AstraZeneca adenovirus vectored vaccine against SARS-CoV-2 breakthrough infections in people who were vaccinated with 2 or 3 doses of it.
Methods: This cross-sectional study was conducted from February 2021 to September 2021 on 997 people, who had received at least 2 doses of the Oxford-AstraZeneca vaccine. Demographic and clinical data were recorded using a questionnaire. The effectiveness of the vaccine was calculated based on the percentage of vaccinated people with confirmed and probable cases of SARS-CoV-2 infection. SPSS software was used for data analysis, and a significance level of p < 0.05 was chosen.
Results: After vaccination with the 2nd and 3rd doses, 355 (35.6%) and 26 (8.3%) participants contracted the SARS-CoV-2 infection, respectively. Breakthrough infection after the 2nd dose was significantly higher in females (p < 0.001), those with older age (p = 0.021), diabetes (p = 0.003) and hypertension (p < 0.001). Additionally, a significant correlation was found between SARS-CoV-2 infection after the 3rd dose and chronic kidney disease (p = 0.022) and a history of infection after the second dose (p < 0.001). The prevalence of vaccine side effects after the 2nd and 3rd doses was 51.7% and 13.4%, respectively.
Conclusions: The effectiveness of the Oxford-AstraZeneca vaccine in preventing SARS-CoV-2 increased from 64.4% after 2 doses to 91.7% after the 3rd dose, therefore it is recommended to administer a 3rd dose to ensure strong immunity against SARS-CoV-2. Based on our data the safety of this vaccine is acceptable.
Citation
Sarooni S S, Mohammadi M, Riahi S M. Safety and efficacy of the Oxford-AstraZeneca vaccine against SARS-CoV-2 after 2nd and 3rd dose in adults: a cross-sectional study. Eur J Transl Clin Med. 2024;7(2):26-39Introduction
As of November 3rd 2024 the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected 776.63 million people and caused 7.071 million deaths globally [1]. Effective long-term control of SARS-CoV-2 relies on achieving herd immunity within the population and vaccination is recommended as a safe and effective method to attain such immunity [2-5]. Both the US Food and Drug Administration (FDA) and the World Health Organization (WHO) guidelines state that they will approve a new vaccine for a pandemic virus if it demonstrates at least 50% efficacy [6-7]. In response, several anti-SARS-CoV-2 vaccines were developed in a shorter time [8]. Several studies have demonstrated that SARS-CoV-2 vaccines provide significant short-term protection, thus reducing the severity of the disease and lowering mortality rates [9-11].
As suggested by its name, the Oxford-AstraZeneca vaccine ChAdOx1 nCoV-19 (AZD1222) was developed by AstraZeneca and Oxford University. It is a chimpanzee adenoviral vector vaccine primarily distributed in India, Brazil and the United Kingdom [12-13]. This vaccine has demonstrated acceptable efficacy and safety in adults [14]. Due to its lower cost and simpler logistical requirements compared to mRNA vaccines, the Oxford-AstraZeneca (OAZ) vaccine has seen wider use particularly in the low- and middle-income countries of South America, Africa and Asia [9, 15]. Some studies have shown that 1 and 2 doses of the OAZ vaccine are moderately effective against mild coronavirus disease 2019 (COVID-19) and highly effective against severe cases [13, 16-17]. The OAZ vaccine’s effectiveness against symptomatic disease peaks at ~ 50%, while its effectiveness against hospitalization reaches 85-90% [16]. However, randomized controlled trials have shown that the vaccine effectiveness waning over time, as shown by decreased levels of neutralizing antibodies [18-19]. Breakthrough infections have also been reported in people who received 2 doses of vaccine [18-19]. Another study indicated that antibody levels following thePfizer–BioNTech (mRNA), Moderna (mRNA) and OAZ vaccines can persist for at least 6 months but tend to diminish over time [3]. Thus, an urgent 3rd dose of the SARS-CoV-2 vaccine is necessary to control the increasing number of infections [20]. For instance, some studies have demonstrated that a 3rd dose of the Pfizer-BioNTech vaccine offers enhanced protection against infection and reinforces the immunity achieved after the initial 2 doses [18, 21-22]. In the case of OAZ vaccine, most studies on its efficacy and safety focus on the 1st and 2nd doses, with limited research on the booster dose’s effectiveness [15, 20]. Therefore, the aim of our study was to investigate in a sample of Iranian people the efficacy of the OAZ vaccine in preventing probable and confirmed COVID-19 breakthrough infections within 6 months after the 2nd and 3rd doses, to evaluate this vaccine’s adverse effects and to explore the association between demographic and clinical variables to identify potential risk factors for COVID-19 breakthrough infections among those vaccinated with that vaccine.
Material and methods This cross-sectional study was conducted in collaboration with Birjand University of Medical Sciences and healthcare facilities in the South Khorasan province (Iran). We enrolled 997 participants who were > 18 years of age and received at least 2 doses of the OAZ vaccine from February 2021 to September 2021. Exclusion criteria were: < 18 years of age, vaccination with a different vaccine type, dementia, pregnancy and lacking of cooperation with the research staff. We performed random cluster sampling. Then, the effectiveness of the OAZ vaccine in preventing COVID-19 was evaluated in people who received 2 doses of it Based on clinical manifestations, the 2020 interim WHO guideline described patients with COVID-19 were classified into 3 main groups: suspected case, probable case and confirmed case [23]. The guideline also allowed countries to adapt these definitions based on their specific health and epidemiological conditions [23-24]. Based on this WHO guideline, the Ministry of Health, Treatment and Medical Education of the Islamic Republic of Iran issued its recommendations for the treatment and control of SARS-CoV-2 infections and in our study, patients were classified accordingly [25] (Table 1).
Table 1. Classification of SARS-CoV-2 infections used in this study and based on [25]
Outcomes
The main outcome we assessed in this study was the rate of SARS-CoV-2 breakthrough infection (defined as the percentage of vaccinated people who had confirmed or probable SARS-CoV-2 infection) after vaccination with the 3rd dose of the OAZ vaccine and we compared it with the results after the 2nd dose of the vaccine. In addition, the severity of infection and the rate of hospital admissions were studied after each vaccine dose. The safety outcome were the local and systemic reactions that occurred within 14 days after each dose (reported by participants using a questionnaire).
Data sources
Demographic details, contact numbers, addresses, the type of COVID-19 vaccine administered, the total number of doses received by each person and the intervals between vaccine doses were extracted from the Integrated Health System (Samaneh Yekparche-ye Behdashti) in collaboration with the Health and Treatment Deputy of Birjand University of Medical Sciences. In the next stage, 997 people who met the inclusion criteria were randomly selected. Next, we contacted them via phone call and explained the purpose of the study and how to participate. Those who gave their consent to participate in this study were given a questionnaire containing 3 parts: demographic and clinical information (e.g. age, sex, level of education, occupation, weight, height, blood type), risk factors and habits (e.g. comorbidities, smoking, drug use) and vaccination-related information (e.g. the number of vaccine doses, symptoms after vaccination). Additional questions were asked about history of SARS-CoV-2 infection before and after vaccination, method of diagnosing SARS-CoV-2 infection before and after vaccination.
Ethics and oversight
The study protocols and questionnaire were approved by the Local Ethics Committee at each center. Informed consent was obtained from all subjects involved in the study. This study was carried out in accordance with the Helsinki Declaration principles and approved by the University of Sistan and Baluchestan ethics committee (approval ID: IR.USB. REC.1400.105).
Statistical Analysis
After collecting and recording the raw data, we used the IBM SPSS software version 26.0 (Armonk, NY, United States) to calculate the Pearson’s chi-square test. The normality of the data was checked using the Kolmogorov-Smirnov test. Statistical differences between groups were determined using non-parametric tests (Mann-Whitney U test and Kruskal-Wallis H test) due to its non-normal distribution. A significance level of p < 0.05 was considered statistically significant. The results were presented as mean ± standard deviation (SD) and frequency (percentage).
Results
The demographic and clinical characteristics of the participants are described in Table 2. Among the 997 participants who were vaccinated with 2 doses of the OAZ vaccine, 536 (53.8%) were men, and 461 (46.2%) were women. The median age of the participants was 50.96 ± 15.03 years and the age range of subjects was between 20 and 96 years old. Among the participants who had received 2 doses of the OAZ vaccine, 380 (38.1%) had comorbidities, most frequently hypertension (20.8%) and diabetes mellitus (18.4%).
Table 2. Demographic and clinical characteristics of the participants

* Some of this data is missing.
† Other underlying diseases included cancer, arthritis, liver diseases, autoimmune diseases, hyperthyroidism, hepatitis B and allergies.
CKD – chronic kidney disease
SARS-CoV-2 infection before and after vaccination with the 2nd and 3rd
doses Among the people who received the 2nd doses of the vaccine, 29.6% had a history of SARS-CoV-2 infection before vaccination. After the 2nd dose, 355 (35.6%) participants were infected within an average duration of 110.2 ± 39.0 days. Only 3 (0.85%) participants needed hospitalization, whereas the rest of them (n = 352, 99.15%) were treated at home. Additionally, 313 (31.4%) participants were vaccinated with 3 doses of the OAZ vaccine. Among them, 26 people (8.3%) contracted the SARS-CoV-2 infection after an average of 41.7 ± 16.9 days following the 3rd dose of the vaccine and none required hospitalisation (Table 3).
Table 3. Demographic and clinical characteristics of the participants
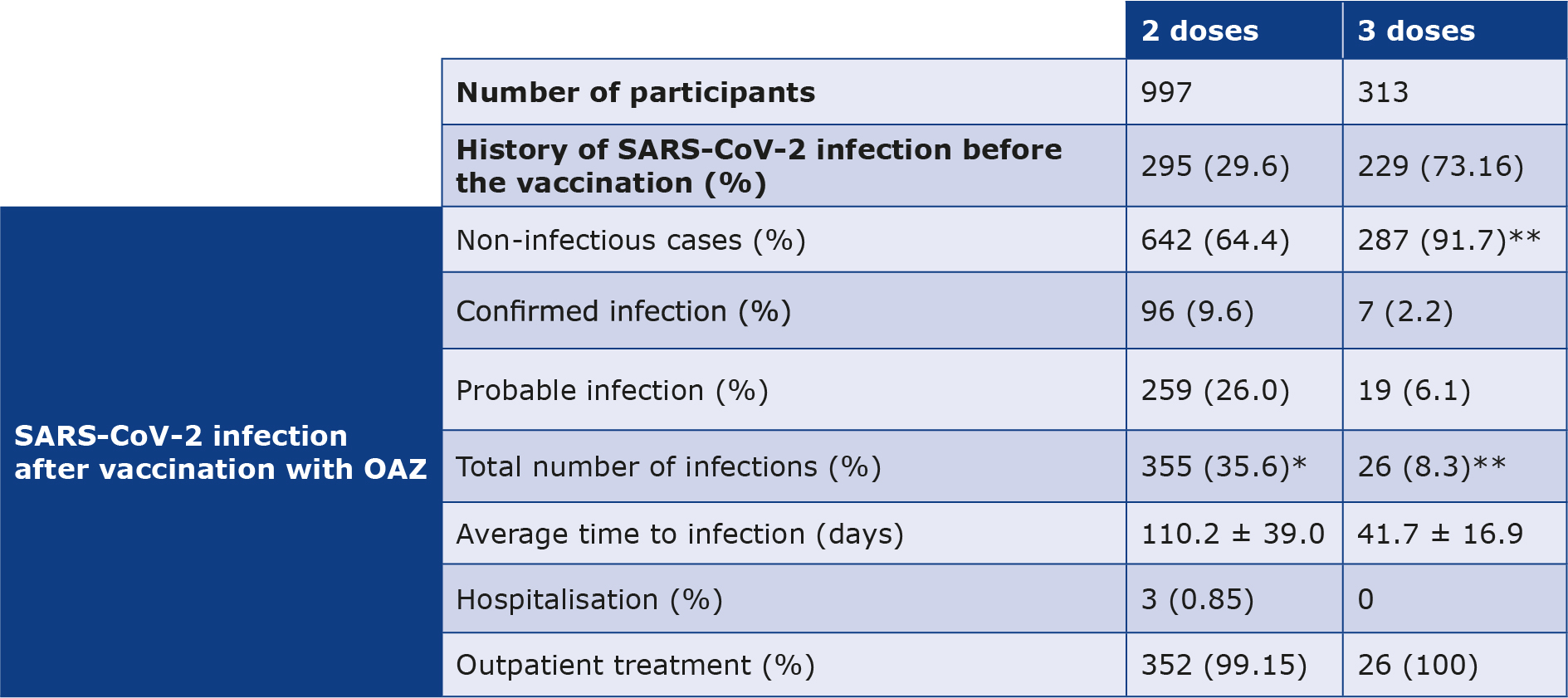
*Percentage of the total number (997 people).
**Percentage of the total number (313 people).
Effectiveness of the OAZ vaccine in preventing SARS-CoV-2 infection
The effectiveness of the OAZ vaccine was calculated based on the percentage of vaccinated people who were infected with confirmed and probable cases of SARS-CoV-2 infection after vaccination. Among the 355 participants who were diagnosed with SARS-CoV-2 after receiving the 2nd vaccine dose, 96 (9.6%) were diagnosed with confirmed SARS-CoV-2 infection and 259 (26.0%) were diagnosed with probable SARS-CoV-2 infection. Likewise, out of the 26 participants who were infected with SARS-CoV-2 after receiving the 3rd vaccine dose, 7 (2.2%) had confirmed SARS-CoV-2 infection, while 19 (6.1%) participants had probable SARS-CoV-2 infection. These results indicate that the OAZ vaccine efficacy in preventing COVID-19 has increased from 64.4% after the 2nd dose to 91.7% after the 3rd dose (Table 3).
Adverse effects
515 (51.7%) participants had ≥ 1 adverse effect after the 2nd dose of the OAZ vaccine, the most common of which included injection site pain (32.5%), fatigue (30.1%), cough (28.7%) and fever (28.1%). Whereas 42 (13.4%) participants had adverse effects after the 3rd dose of the vaccine, most commonly injection site pain (10.2%), swelling at the injection site (6.4%), local redness (4.2%) and cough (3.5%) (Table 4).
Table 4. OAZ vaccine adverse effects by dose
CT – computer tomography
Analytical results
In our study we found that SARS-CoV-2 infection rates after 2 doses of the OAZ vaccine were significantly higher in females (p < 0.001), participants with comorbidities (p = 0.012), particularly diabetes (p = 0.003) and hypertension (p < 0.001), as well as among those working outside the home (p = 0.021). Additionally, the average age of the infected participants was significantly higher than that of non-infected individuals (p = 0.021). After vaccination with the 3rd dose, the rate of COVID-19 infection was significantly higher in participants with chronic kidney disease (CKD) (p = 0.022) and those who had a history of COVID-19 infection after the second dose of the vaccine (p < 0.001) (Table 5).
Table 5. Comparison of independent variables in the case of contracting and not contracting SARS-CoV-2 after the 2nd and 3rd doses of the OAZ vaccine
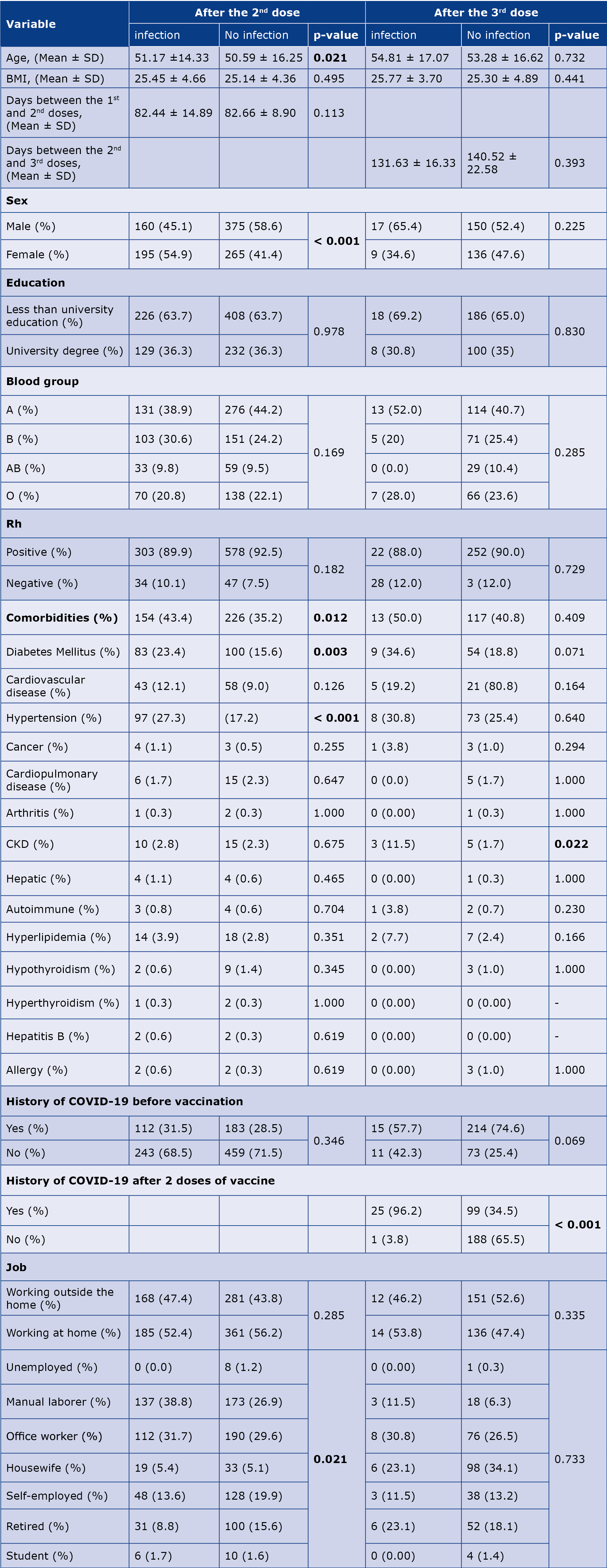
BMI – body mass index, CKD – chronic kidney disease, Rh – Rhesus (Rh) factor.
Statistically significant results are printed in bold.
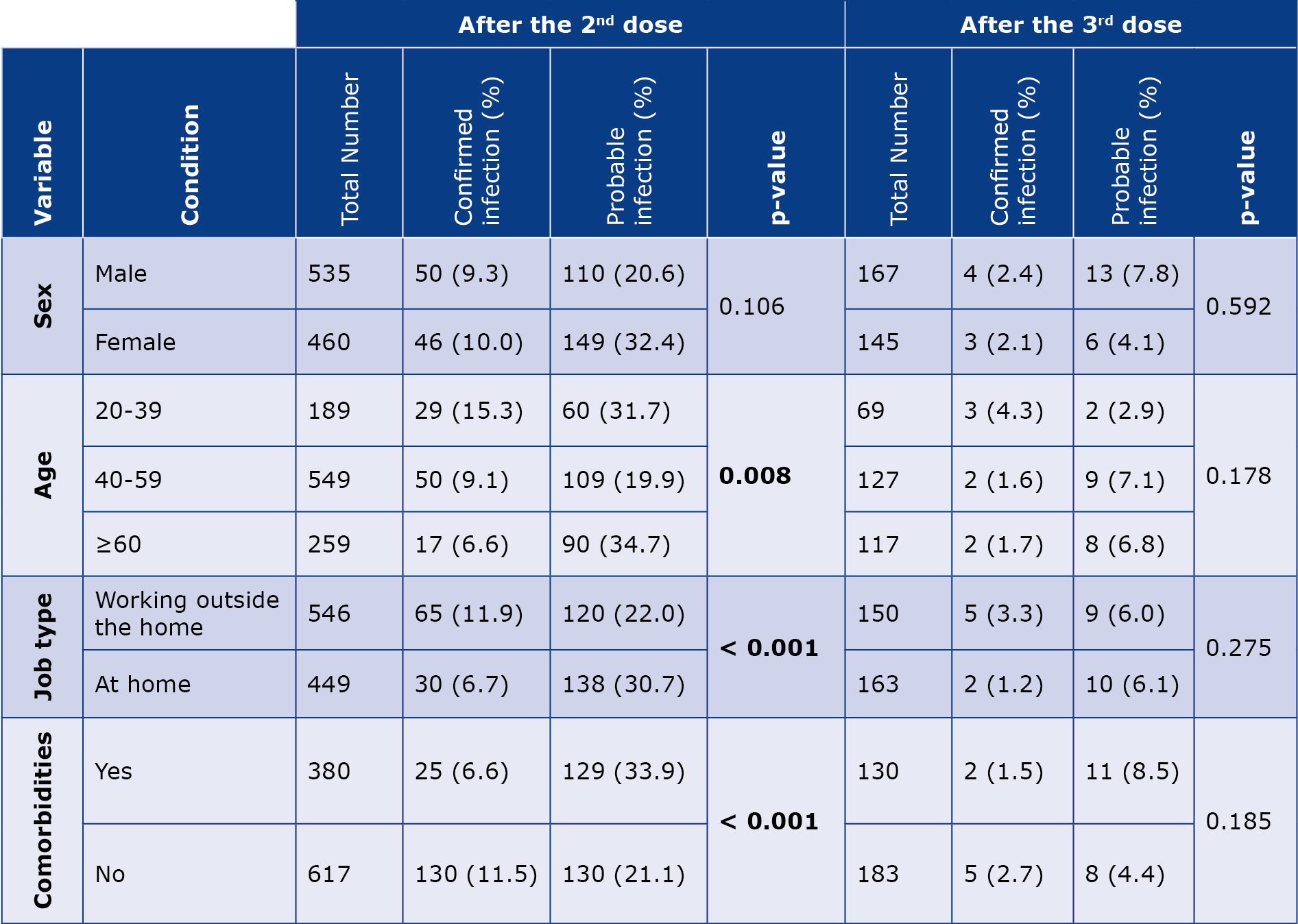
Our analysis revealed a significant correlation between confirmed and probable SARS-CoV-2 infection after 2 vaccinations with the age group (p = 0.008), occupation (p < 0.001) and comorbidities (p < 0.001). However, no significant correlation was observed among males and females (p = 0.106). The highest number of confirmed and probable SARS-CoV-2 cases was reported in the group < 40 years and > 60 years of age, respectively. Conversely, no significant difference was observed in the subgroups (sex, age, employment, underlying diseases) in terms of confirmed and probable infection after the 3rd dose (Table 6).
Table 6. Comparison of confirmed and probable coronavirus infection rates with various parameters after the 2nd and 3rd doses of the OAZ vaccine
Discussion
Public trust and confidence in SARS-CoV-2 vaccines based on assessments of their effectiveness are crucial for the longterm success of vaccination. Due the emergency use authorization, the clinical trials and adverse effect investigations for SARS-CoV-2 vaccines were significantly shorter than for other vaccines. Therefore, it is vital to conduct large-scale studies across populations of various countries to gain a better understanding of the SARS-CoV-2 vaccines‘ effectiveness [17]. Vaccine effectiveness is a measurement of disease incidence reduction in vaccinated individuals compared to those who are not vaccinated. This evaluation is typically performed under optimal conditions (e.g. in clinical trials) and the results can vary across populations [26]. Our results showed that the OAZ vaccine’s effectiveness against breakthrough infections and hospitalisation after the 2nd dose was 64.4% and 99.15%, respectively. In line with our results, in a study conducted in Brazil, South Africa and the UK it was found that the effectiveness of the OAZ vaccine against COVID-19 was 70.4% after 2 doses [16]. When a low dose was followed by a standard dose, the efficacy increased to 90.0% [16]. In an Iraqi study, 6.71% of participants contracted the SARS-CoV-2 infection, as determined by a positive PCR test, 14 days after vaccination with the 2nd dose of the OAZ vaccine, which is consistent with the 9.6% confirmed cases in our study. Mirroring our findings, none of the infected participants required hospitalisation and no deaths were reported [27]. In contrast to our results, in other studies, the overall efficacy against SARS-CoV-2 variants after the 2nd dose was higher: 73.73%, 74.0% in the United States, Chile, and Peru and 84.4% in Iran, whereas the overall efficacy against hospitalisation and death were lower than in our study [10, 17, 28]. Another study from Iran indicates that after vaccination with 2 doses of the OAZ vaccine, its maximum effectiveness in preventing regular hospitalisations and deaths is around 98% and 92%, respectively. These findings are consistent with ours [11]. Overall, the efficacy of the OAZ vaccine after the 2nd dose in our study was somewhat lower compared to most previous studies. However, its estimated efficacy in preventing hospitalisations was higher than in other studies. Factors such as study design, methodology, age distribution, demographic characteristics and the increasing prevalence of different SARS-CoV-2 variants all can influence the results regarding the effectiveness of vaccines in different studies [19].
In our study, the effectiveness of the OAZ booster dose against symptomatic disease and hospitalisation was 91.7% and 100%, respectively. It was demonstrated in a group of 177 participants from Bangkok, Thailand that administering either the OAZ or the Pfizer–BioNTech vaccine after 2 doses of the inactivated vaccine (Sinovac) can induce an immune response, including both IgA anti-spike and IFN-γ stimulation [20]. In the British population, the absolute effectiveness of the Pfizer-BioNTech or Moderna booster against symptomatic disease ranged from 94% to 97%, consistently across all age groups. For preventing hospitalisation or death, the absolute effectiveness of the Pfizer-BioNTech booster was approximately 97% to 99% across all age groups [21]. A study from England reported that the OAZ booster enhanced protection against symptomatic disease in both younger and older adults, counteracting the decline in immunity observed 25 weeks after the 2nd dose. Also, the booster doses of the OAZ and Pfizer-BioNTech vaccines significantly increased protection against symptomatic disease following infection with the Omicron variant of SARS-CoV-2, with effectiveness rates of 66.1% and 68.5% in older adults, respectively [15]. Additionally, the protection against hospitalisation peaked at 82.3% for OAZ and 90.9% for Pfizer-BioNTech [15]. Overall, previous studies indicate that a 3rd dose of an approved COVID-19 vaccine is effective in preventing COVID-19, including severe cases [15, 20-21, 29]. This finding is supported by data from the current study. However, there is limited information on the actual effectiveness of 3rd doses across different vaccines. The effectiveness of the third dose can vary depending on factors such as the specific vaccine used, the interval between the second and third doses, population characteristics, study design and the methods used to measure vaccine effectiveness. Additionally, adjustments for vaccine effectiveness may also influence the results [29].
Overall, participants in our study reported mild to moderate adverse reactions that did not require hospitalisation nor caused death. These findings align with those of other studies [13, 28, 30]. In a study from Iraq the adverse effects after the 2nd dose of the OAZ vaccine were reported in 12.45% of participants, which was lower than in our study [27]. Additionally, in the aforementioned study, the most common adverse effects after the 2nd dose were fever, pain at the injection site and myalgia, which is different than in our study [27]. In contrast to other authors, we did not report any uncommon symptoms e.g. excessive sweating, enlarged lymph nodes, loss of appetite, confusion thrombosis and thrombocytopenia [4, 9, 15]. On the other hand, we reported less frequent adverse effects e.g. conjunctivitis, lymphocytopenia, pulmonary involvement (diagnosed in CT scan images), loss of smell and hearing disorders, which have not been reported in other studies. In a study from Iran, the most common adverse effects after the 2nd dose of the OAZ vaccine were injection site pain (69.2%), headache (46.2%), fever (38.5%) and local stiffness at the injection site (23.1%). Additionally, 54.2% of the participants reported ≥ 1 adverse effect from the OAZ vaccine after the 2nd dose, which is consistent with the results of the present study [30]. In a prospective cohort study Zare et al. showed that after the second dose of the OAZ vaccine the local symptoms included pain (48.5%), tenderness (31.5%), firmness (6.3%) and swelling (4.4%), whereas the systemic symptoms were not life-threatening and included fever (43.3%), muscle pain (45.9%), joint pain (34.4%), chills (37.0%) and malaise (36.7%) [31]. The prevalence of fever in their study was higher than in ours (43.3% vs. 28.1%), but swelling was reported less than in our study (4.4% vs. 10.2% respectively). In another study, Sinaei et al. reported that the most common side effects after the 2nd dose of OAZ vaccine were injection site reaction (22.2%), fever and chills (21.5%), neurological symptoms (11.5%) and gastrointestinal symptoms (5%) [32]. Overall, adverse events reported after the second dose in the current study were relatively similar to those in most studies. Injection site pain and fever were the most commonly reported adverse events after the 2nd dose of OAZ vaccine in all reports.
Studies on adverse effects after the 3rd dose of vaccines are limited. Amer et al. reported that the prevalence of the adverse effects after 1st, 2nd and booster dose of the OAZ vaccine was respectively 23%, 22% and 3%, which was significantly different from our study [33]. Also, in contrast with our study, Yadegarynia et al. reported the prevalence of vaccine side effects increased after the 3rd dose compared to the second dose of the OAZ vaccine (57.8% vs. 48.2% respectively) and the most common side effect after the 3rd dose of the vaccine was myalgia (45.6%), followed by fever (39.9%), chills (37.3%) and headache (29.1%) [34]. The differences in the reported adverse effects can be attributed to the demographic and genetic characteristics of the studied population, the sample size and the study design [27].
According to our results, SARS-CoV-2 infection after vaccination with 2 doses of the OAZ vaccine was significantly associated with female sex, older age, and having diabetes and hypertension. Regarding the link between female sex and long COVID syndrome, Bai et al. reported that over half of COVID-19 patients experienced ongoing symptoms, with female sex being a significant factor [35]. In contrast to our findings, some studies have reported that the prevalence and severity of COVID-19 are higher in men [36-38]. On the other hand, Hossein et al. did not find significant relationship between sex and the rate of SARS-CoV-2 infection after the 2nd dose of OAZ vaccine [27]. However, the effects of COVID-19 can vary depending on factors such as socioeconomic status, ethnicity and geographic location [5]. Numerous studies have demonstrated a link between advanced age and comorbidities such as diabetes, hypertension, and CKD with the incidence and severity of COVID-19 [36-37, 39]. It is well-established that patients with diabetes have a heightened risk of infections, partly due to hyperglycemia-induced immune dysfunction [39]. Researchers in Wuhan (China) identified hypertension as the most prevalent comorbidity among COVID-19 patients, followed by diabetes and coronary artery disease. Additionally, older age was correlated with increased COVID-19 severity and mortality [36]. In another study, it was found that age ≥ 65, hypertension and diabetes were statistically more common in critical patients compared to non-critical ones [38]. However, Hussein et al. did not observe any significant correlation between age and post-vaccination infection rates [27]. Additionally, our results indicated that the incidence of contracting SARS-CoV-2 after the 2nd vaccine dose was higher among individuals working outside the home compared to those working at home. This is likely because they had a greater risk of exposure to viral particles due to their interactions with other people, however more studies are needed to confirm this conclusion.
In our sample, the rate of SARS-CoV-2 infection after vaccination with the OAZ booster dose was higher in individuals who had a history of infection following the 2nd dose, as well as in those with chronic kidney disease (CKD). In a study by Hall et al., 7.6% of participants experienced a primary infection and 0.6% experienced a secondary infection after vaccination with the Pfizer BioNTech and OAZ vaccines [40]. Research from Belgium indicated that vaccinated individuals with a history of SARS-CoV-2 infection had a significantly lower risk of new infections and symptoms, similarly to those who received an mRNA booster dose [41]. These findings contrast with our results and a possible explanation for this could be that individuals with a history of SARS-CoV-2 infection before receiving a booster vaccination were not very diligent in following health protocols (e.g. social distancing and wearing masks). Further studies are needed to confirm this hypothesis.
Previous studies have demonstrated that patients with CKD might experience elevated levels of pro-inflammatory cytokines, resulting in increased oxidative stress during a SARS-CoV-2 infection which can ultimately lead to pneumonia. Those studies have identified kidney diseases as a risk factor for the severe course of COVID-19 [37-39]. In our study, a significant relationship was observed between confirmed and probable cases of COVID-19 infection after the 2nd dose of the vaccine and factors such as age, type of work and comorbidities. The majority of confirmed cases were reported among individuals aged 20 to 39 years, those working outside the home, and those without comorbidities. This could be because people in the above-mentioned group are among the most active in the community and are more exposed to SARS-CoV-2. They are also more likely than older people with comorbidities to follow health protocols less carefully. However, more studies are needed to confirm this theory.
Limitations
Our study has some limitations. First, there is a lack of a control group or comparisons with other vaccines. Second, the weaknesses of the cross-sectional design of this study did not allow us to assess incidence, to make a causal inference, to follow up participants over time. In addition, our study is susceptible to sampling bias (e.g. non-response bias and recall bias). Third, we study included only participants > 18 years old and excluded children, adolescents and pregnant women, therefore our results may not be generalized to these populations. We did not investigate cellular and humoral immunity, which is another limitation in accurately assessing the effectiveness of the OAZ vaccine against SARS-CoV-2. Furthermore, we did not evaluate the actual effectiveness of vaccines against different strains of SARS-CoV-2. Self-reported data about adverse effects may also be prone to error, particularly because they were reported over a relatively short follow-up period.
Conclusions
In our study sample the OAZ vaccine was effective in preventing severe SARS-CoV-2 infection and hospitalisation after 2 doses. However, its effectiveness appears to decrease about 3-4 months after the 2nd dose, highlighting the need for a booster dose. The booster dose of the OAZ vaccine showed significant efficacy in preventing symptomatic infections and hospitalisation compared to the 2nd dose. Older age groups with comorbidities (particularly diabetes and hypertension), females and those at higher risk for SARS-CoV-2 exposure are more likely to have symptomatic infection after the 2nd dose. Additionally, a history of COVID-19 after the 2nd dose and CKD are associated with a higher likelihood of symptomatic infection after the 3rd dose. Investigations into the adverse effects of the vaccine after the 2nd and 3rd doses showed that the OAZ vaccine is safe. However, further studies involving larger populations and more accurate testing are needed to generalize these results.
Funding
This study was supported by the grant provided by the University of Sistan and Baluchestan (Zahedan, Iran).
Acknowledgments
The authors gratefully acknowledge the financial support for this work that was provided by the University of Sistan and Baluchestan. Also, we thank the participants, health workers, and the Health & Treatment Deputy of Birjand University of Medical Sciences for providing important collaboration to this study.
Conflict of interest
The authors declare that there is no conflict of interest.
-------
Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID; resource – flickr.com (CC BY 2.0)
References
| 1. |
COVID-19 Dashboard [Internet]. World Health Organization. [cited 2024 Nov 6]. Available from: https://covid19.who.int.
|
| 2. |
Rasmussen AL. Vaccination Is the Only Acceptable Path to Herd Immunity. Med [Internet]. 2020;1(1):21–3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666634020300325.
|
| 3. |
Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect [Internet]. 2022;28(2):202–21. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1198743X21006042.
|
| 4. |
Ghiasi N, Valizadeh R, Arabsorkhi M, Hoseyni TS, Esfandiari K, Sadighpour T, et al. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathol Persa [Internet]. 2021 Jun 5;7(2):e31–e31. Available from: http://immunopathol.com/Article/ipp-26260.
|
| 5. |
Delam H, Moghaddam S, Zare R. Efficacy and Safety of COVID-19 Vaccines in Different Variants and Doses: A Systematic Review. Epidemiol Heal Syst J [Internet]. 2022 Dec 7;9(4):184–95. Available from: https://ehsj.skums.ac.ir/Article/IJER-2205-1479.
|
| 6. |
Development and Licensure of Vaccines to Prevent COVID-19 [Internet]. FDA. U.S. Food & Drug Administration. Center for Biologics Evaluation and Research. [cited 2020 Nov 8]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19.
|
| 7. |
WHO target product profiles for COVID-19 vaccines. April 9, 2020 [Internet]. World Health Organization. [cited 2020 Nov 8]. Available from: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines.
|
| 8. |
Ball P. The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature [Internet]. 2021 Jan 7;589(7840):16–8. Available from: https://www.nature.com/articles/d41586-020-03626-1.
|
| 9. |
Katikireddi SV, Cerqueira-Silva T, Vasileiou E, Robertson C, Amele S, Pan J, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet [Internet]. 2022;399(10319):25–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673621027549.
|
| 10. |
Mirahmadizadeh A, Heiran A, Bagheri Lankarani K, Serati M, Habibi M, Eilami O, et al. Effectiveness of Coronavirus Disease 2019 Vaccines in Preventing Infection, Hospital Admission, and Death: A Historical Cohort Study Using Iranian Registration Data During Vaccination Program. Open Forum Infect Dis [Internet]. 2022;9(6). Available from: https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofac177/6582699.
|
| 11. |
Heidarzadeh A, Amini Moridani M, Khoshmanesh S, Kazemi S, Hajiaghabozorgi M, Karami M. Effectiveness of COVID-19 vaccines on hospitalization and death in Guilan, Iran: a test-negative case-control study. Int J Infect Dis [Internet]. 2023;128:212–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971222006646.
|
| 12. |
Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet [Internet]. 2021;397(10269):72–4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620326234.
|
| 13. |
Asano M, Okada H, Itoh Y, Hirata H, Ishikawa K, Yoshida E, et al. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: a double-blind, randomized controlled phase 1/2 trial. Int J Infect Dis [Internet]. 2022;114:165–74. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971221008183.
|
| 14. |
The Oxford/AstraZeneca (ChAdOx1-S [recombinant] vaccine) COVID-19 vaccine: what you need to know [Internet]. World Health Organization. [cited 2022 Jun 13]. Available from: https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know.
|
| 15. |
Kirsebom FCM, Andrews N, Sachdeva R, Stowe J, Ramsay M, Lopez Bernal J. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nat Commun [Internet]. 2022;13(1):7688. Available from: https://www.nature.com/articles/s41467-022-35168-7.
|
| 16. |
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet [Internet]. 2021;397(10269):99–111. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620326611.
|
| 17. |
Meo SA, Aftab S, Bayoumy NM, Meo AS. Efficacy of Oxford-AstraZeneca (ChAdOx1 CoV-19) vaccine against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) cases, hospital admissions, type of variants, and deaths. Eur Rev Med Pharmacol Sci [Internet]. 2023;27(20). Available from: https://www.europeanreview.org/wp/wp-content/uploads/10133-10143.pdf.
|
| 18. |
Hart JD, Chokephaibulkit K, Mayxay M, Ong-Lim ALT, Saketa ST, Russell FM. COVID-19 vaccine boosters in the Asia-Pacific region in the context of Omicron. Lancet Reg Heal - West Pacific [Internet]. 2022;20:100404. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666606522000190.
|
| 19. |
Wu N, Joyal-Desmarais K, Ribeiro PAB, Vieira AM, Stojanovic J, Sanuade C, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med [Internet]. 2023;11(5):439–52. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213260023000152.
|
| 20. |
Kanokudom S, Assawakosri S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines [Internet]. 2022;10(1):86. Available from: https://www.mdpi.com/2076-393X/10/1/86.
|
| 21. |
Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med [Internet]. 2022;28(4):831–7. Available from: https://www.nature.com/articles/s41591-022-01699-1.
|
| 22. |
Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet [Internet]. 2022;399(10332):1303–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673622004627.
|
| 23. |
Public health surveillance for COVID-19: interim guidance [Internet]. World Health Organization. [cited 2020 Dec 13]. Available from: https://apps.who.int/iris/handle/10665/333752.
|
| 24. |
Suthar AB, Schubert S, Garon J, Couture A, Brown AM, Charania S. Coronavirus Disease Case Definitions, Diagnostic Testing Criteria, and Surveillance in 25 Countries with Highest Reported Case Counts. Emerg Infect Dis [Internet]. 2022;28(1):148–56. Available from: https://wwwnc.cdc.gov/eid/article/28/1/21-1082_article.htm.
|
| 25. |
Flowchart of diagnosis and treatment of covid-19 disease at the levels of outpatient and inpatient services: 7th edition (July 2019) [Internet]. Ministry of Health. 2019 [cited 2019 Jul 1]. Available from: https://learn.irimc.org/article/flowchart-treatment-covid19-7th-gen.
|
| 26. |
Wilder-Smith A, Longini I, Zuber PL, Bärnighausen T, Edmunds WJ, Dean N, et al. The public health value of vaccines beyond efficacy: methods, measures and outcomes. BMC Med [Internet]. 2017;15(1):138. Available from: http://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-017-0911-8.
|
| 27. |
Hussein NR, Rasheed BN, Naqid IA, Dirbaz AM, Saleem ZSM, Ibrahim N, et al. A study of SARS-CoV-2 delta variant breakthrough infections and side effects of the Oxford-AstraZeneca vaccine. Public Heal Pract [Internet]. 2022;4:100303. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666535222000799.
|
| 28. |
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med [Internet]. 2021;385(25):2348–60. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2105290.
|
| 29. |
Baral P, Bhattarai N, Hossen ML, Stebliankin V, Gerstman BS, Narasimhan G, et al. Mutation-induced changes in the receptor-binding interface of the SARS-CoV-2 Delta variant B.1.617.2 and implications for immune evasion. Biochem Biophys Res Commun [Internet]. 2021;574:14–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X21012122.
|
| 30. |
Oghazian S, Tavanaei Tamanaei T, Haghighi R, Faregh M, Oghazian MB. Side effects of Sputnik V, Oxford–AstraZeneca, Sinopharm, and Covaxin and their associations with other variables among healthcare workers of a tertiary hospital in Iran. Int Immunopharmacol [Internet]. 2023;117:109784. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1567576923001078.
|
| 31. |
Zare Z, Assarroudi A, Armat MR, Laal Ahangar M, Estaji M, MoghaddamHosseini V, et al. Signs, Symptoms, and Side-Effects Presented by Different Types of COVID-19 Vaccines: A Prospective Cohort Study. Life [Internet]. 2022;12(12):2046. Available from: https://www.mdpi.com/2075-1729/12/12/2046.
|
| 32. |
Sinaei R, Najafzadeh MJ, Ghafari S, Hosseininasab A, Pardakhty A, Dalfardi B, et al. Short-term Safety of Covaxin, Sinopharm, Sputnik V, and AstraZeneca COVID-19 Vaccines Among Iranian Healthcare Workers: A Cross-Sectional Study. Arch Clin Infect Dis [Internet]. 2024;19(1). Available from: https://brieflands.com/articles/archcid-142157.
|
| 33. |
Amer SA, Al-Zahrani A, Imam EA, Ishteiwy EM, Djelleb IF, Abdullh LR, et al. Exploring the reported adverse effects of COVID-19 vaccines among vaccinated Arab populations: a multi-national survey study. Sci Rep [Internet]. 2024;14(1):4785. Available from: https://www.nature.com/articles/s41598-024-54886-0.
|
| 34. |
Yadegarynia D, Tehrani S, Hadavand F, Arshi S, Abtahian Z, Keyvanfar A, et al. Side effects after COVID-19 vaccination: a comparison between the most common available vaccines in Iran. Iran J Microbiol [Internet]. 2023; Available from: https://publish.kne-publishing.com/index.php/IJM/article/view/12467.
|
| 35. |
Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect [Internet]. 2022;28(4):611.e9-611.e16. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1198743X21006297.
|
| 36. |
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet]. 2020;395(10229):1054–62. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620305663.
|
| 37. |
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ [Internet]. 2020;m1966. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.m1966.
|
| 38. |
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect [Internet]. 2020;81(2):e16–25. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163445320302346.
|
| 39. |
Landstra CP, de Koning EJP. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front Endocrinol (Lausanne) [Internet]. 2021;12. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2021.649525/full.
|
| 40. |
Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med [Internet]. 2022;386(13):1207–20. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2118691.
|
| 41. |
Stouten V, Hubin P, Haarhuis F, van Loenhout J, Billuart M, Brondeel R, et al. Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. Viruses [Internet]. 2022;14(4):802. Available from: https://www.mdpi.com/1999-4915/14/4/802.
|













