Abstract
The ongoing outbreak of highly pathogenic avian influenza such as A/H5N1 virus, with its continued evolution and spread to various mammalian species, raises significant concerns about its potential to cause a human pandemic. This editorial examines the recent spillover events to mammals, the economic impact on the poultry industry and the importance of preparedness and preventive measures. The situation in the USA (widespread outbreaks in cows as well as in dairy farm workers) and in Europe (infections in cats and fur animals) highlights the urgency of implementing effective surveillance, biosecurity, vaccination and communication strategies. Particularly, we focus on the lessons learnt (and also those not learnt) from Poland and the rest of Europe in managing potentially being zoonotic outbreaks of unknown origin. Personal experience from these events, though potentially reflecting the subjective views of the authors, highlight the importance of regional preparedness and rapid response to mitigate the risks posed by avian influenza and other emerging infectious diseases. A One Health approach, integrating the animal, human and environmental health sectors with socioeconomic constraints, is crucial for mitigating the risks and preventing a potential global health crisis.
Citation
Jarynowski A, Maksymowicz S, Romanowska M, Skawina I. Avian influenza: the looming threat of Disease X and lessons from Poland and Europe. Eur J Transl Clin Med. 2024;7(2):5-21Introduction
Avian influenza, colloquially known as bird flu, is an infectious disease caused by type A influenza viruses (AIV) that occurs naturally in wild birds, mainly waterfowl. Classification of influenza viruses is based on the antigenic properties of their surface proteins; hemagglutinin (H) and neuraminidase (N). It is a disease with high mutation rates and the potential for zoonotic transmission [1]. Avian influenza challenges both the veterinary and medical communities. Recent outbreaks in various locations around the world revealed the urgent need for comprehensive mitigation strategies, particularly as concern rises over the emergence of a potential Disease X (a term reserved for an unknown pathogen that has the ability to cause a large-scale epidemic). AIV is shortlisted for high priority potential Pathogen X particularly for Europe [2]. Of particular concern are cases of virus spillover to mammals, i.e. its transfer from birds to other animal species, which increases the risk of mutation and adaptation of the virus to humans. An example of such a phenomenon in the last 2 years is an epizootic of H5N1 virus in cows and among other mammals in the United States of America (USA) in 2024 and cats in Poland or in fur animals in Finland in 2023 [3-5].
Poland, located in central Europe, has seen numerous outbreaks of avian influenza in years 2020-2024, providing crucial insights about disease prevention and control [6]. Poland’s geographical location and its extensive poultry industry make it an essential area for studying the spread and management of avian influenza across Europe [7].
In this editorial we aimed to discuss the various threats posed by avian influenza, particulary in the context of a potential Disease X [8]. By analyzing Poland’s experiences and the wider European perspective, we aim to identify strategies and policy recommendations that could be implemented on European scale, covering the biological, ecological and socioeconomic aspects of avian inflluenza. We highlight the importance of preparedness, quick response and international cooperation to prevent future pandemics of zoonotic origin [9]. Our discussion includes scientific findings in virology, epidemiology and veterinary science, as well as case studies from European countries, including Poland. We also want to signal what went wrong [10]. By synthesizing these insights, we hope to contribute to a more resilient and informed approach in managing the ever-imminent risk of avian influenza and other emerging infectious diseases.
History of avian influenza
The H1N1 influenza pandemic of 1918-1920 (incorrectly named “Spanish flu”) which caused the death of estimated 50-100 million people worldwide was an avian-like H1N1 virus [11]. The so-called Hongkong H3N2 pandemic virus was a reassortment virus (i.e. the exchange of genomes between different viruses living in the same host) of a seasonal human influenza virus and a low pathogenic avian influenza A virus [12-13]. It caused millions of infections in Europe between 1969-1971, which froze the economy and was one of the triggers of a reduced quality of life. In terms of registered cases, it was the largest epidemic in modern Polish history, far more so than the COVID-19 in 2020-2024. At the peak of the H3N2 epidemic (December 1970), almost half of the staff in Poland’s factories were either on sick leave or on strike. The avian strain H5N1, the lead topic of this editorial, killed at least 90 people in 2003/2004, mostly in South East Asia [14]. Although experience to date shows that human-to-human transmission is generally low, the mortality rate can be as high as 50% [15]. The 2009/2010 H1N1 pandemic was caused by a unique combination of swine, avian and human influenza. Other than HIV and coronaviruses, all major pandemics in modern history were caused by avian influenza [16]. It is worth noting that since 2022/2023 the seasonal human influenza rates have returned to pre-COVID-pandemic level in the European Union (EU) [17].
Virology and epidemiology
Due to the close contact and proximity of humans and animals throughout our shared history, many pathogenic microorganisms have evolved to effectively infect both types of hosts. These are zoonoses and reverse zoonoses (crossing from humans to the animal population) [18]. In the case of COVID-19, there is evidence that it might be a zoonotic disease from the start of the pandemic and later it evolved to a reverse zoonotic disease as well [19-20]. It led to disastrous implications for selected livestock populations. In particular, it caused the slaughtering of minks in 2020/2021 following the transmission of the SARS-CoV-2 virus from humans to minks, also in Poland [21]. In addition to the significant impairments caused by zoonotic diseases affecting livestock (e.g. the reduced access to food of animal origin), a region or country may experience a reduction in its ability to export or trade agricultural and food products during an outbreak/epizootic. Global livestock production and agriculture are heavily dependent on biosecurity measures along the production-consumption chain. We should also note the challenges to biosecurity requirements in the context of the current European Union’s Green Deal strategy of “from the farm to fork” [22]. Poultry producers are the first to experience the resulting restrictions (e.g. in antibiotic administration to livestock), while biosecurity (also against AIV) is promoted [23].
General virology of avian influenza
AIV, like all viruses (particularly RNA), are constantly mutating [24]. The farm environment allowed the reassortment of the H5 and H8 subtypes to occur. Thus, the viruses evolved from low pathogenic (LPAI) to lethal to domestic poultry (highly pathogenic, HPAI) [25]. If the virus mutates to a form that is easily transmitted from birds to humans (a so-called spillover), a serious public health crisis could emerge. In 2023 thousands of outbreaks have been reported in poultry, wild birds and, most alarmingly, in mammals [26]. The situation in the USA during the spring/summer of 2024 was unprecedented: an extremely large outbreak in mammals (mainly cows) and dozen confirmed cases in humans (with contact with animals) [27-28]. Upon submission (August 6th 2024), there were 1838 registered human cases of H5N1 since the beginning of this linkage around 1993 with 13 notifications from the current outbreak in the USA [29]. Probably more human cases are unreported. Besides, non-infectious AIV fragments appeared in dairy products or in sewage, also in the areas of USA without industrial dairy farms [30].
Genetic changes and adaptations of H5N1 in 2022-2024
The ongoing global outbreak of H5Nx virus, originating from the goose/Guangdong lineage, has persisted for over 25 years. It has diversified into 8 distinct groups (2.3.4.4a-2.3.4.4h) with 3 primary neuraminidase subtypes: N1, N8, and N6 [31]. Notably, the H5N1 subtype within this lineage (clade 2.3.4.4b) has been responsible for the last decade of global outbreaks. This strain has undergone rapid and significant genetic changes through reassortment of internal genes. In a short timeframe, it has outcompeted other avian influenza strains and is now prevalent in wild migratory bird populations across Asia (its primary source), Europe, Africa and the Americas [32]. Throughout this time, the virus has continuously evolved, resulting in the emergence of several distinct genetic clades of the H5 hemagglutinin gene. Variants of the H5N1 group have been monitored for several years and spread on all continents [31]. Mammals in Europe (2023) and the USA (2024) were infected with a reassortant virus and continuation of the lineage 2.3.4.4b [33]. There are several viral genes to be analyzed phylogenetically: polymerase-binding protein 1, 2 (PB1, PB2) matrix protein (MP), non-structural protein (NS), acidic protein (PA), nucleocapsid protein (NP). Changes in the NP part of AIV were linked with previous pandemics in humans, thus they are the most interesting in terms of zoonotic potential [34]. Mutations in PB2, specifically E627K, are genetic indicators of the virus ability to infect mammals [35]. All the viruses from cats and most other European mammals exhibit this mutation [36]. The virus found in the white stork in Poland in 2023 exhibited some of these characteristic mutations in NP and PB2 [35].
Thus, based on the available material, certain sets of SNPs (single nucleotide polymorphisms), previously marked as related to circulation in mammals, can persist in European bird populations, including both wild and domestic. However, these mutations do not tend to infect mammals unless they reassort with strains imported from Africa by the migrating bird population, which might explain the seasonal dynamics of the outbreaks. Phylodynamic of ongoing outbreak in cows in the USA suggest a different mechanism and Pan-American origin [37].
Social determinants of recent avian influenza outbreaks
Between April 1st (first confirmed human case in a dairy worker) and and April 21st 2024 (detection of non-infectious parts of AIV RNA in milk) the problem of avian influenza has caught the attention of the general public, which is usually not interested in non-human diseases (Figure 1) [38]. These dates started a new era in avian influenza. Although infections in cattle in March 2024 were of interest in the professional community only, the milk contamination and partially human cases among dairy farm workers gained greater recognition.
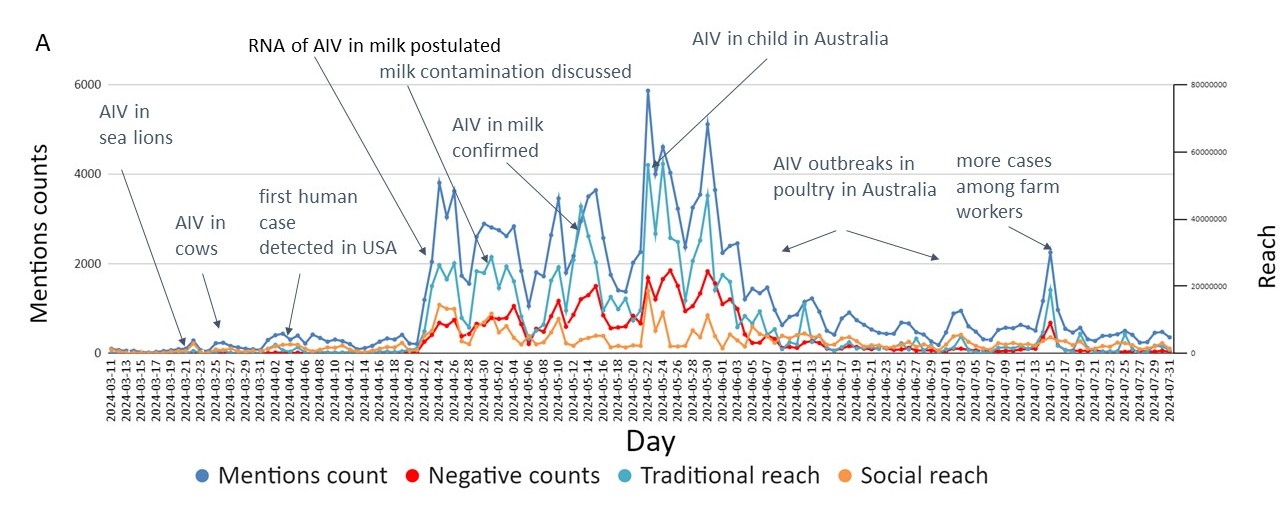
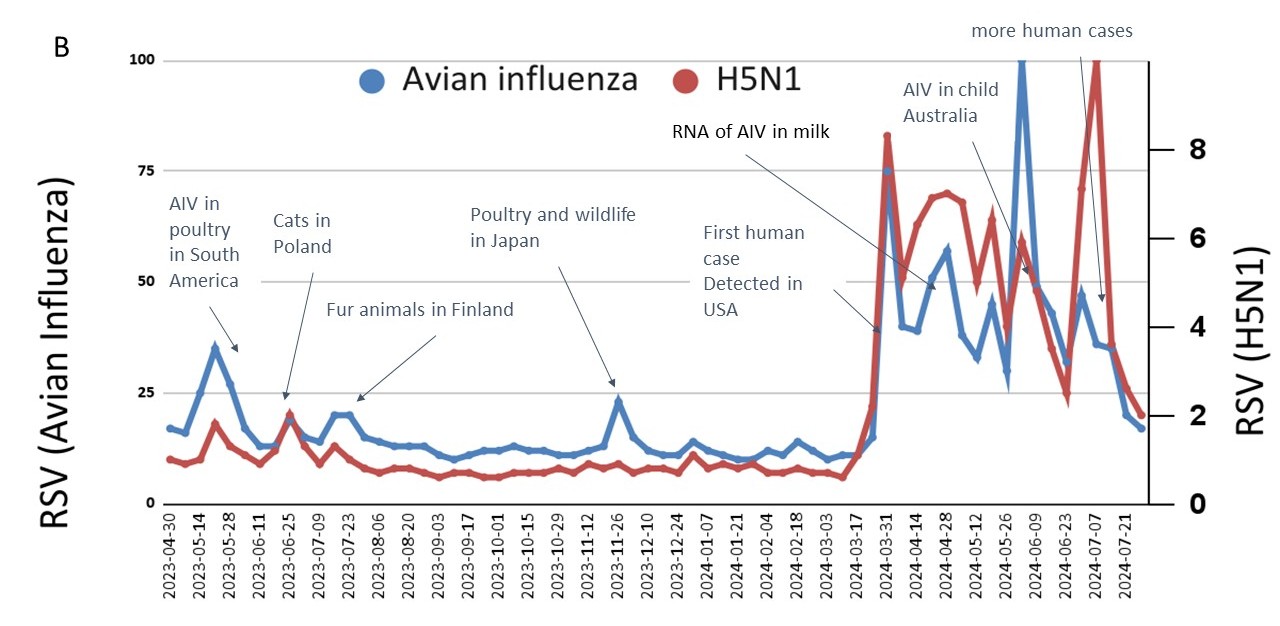
Figure 1. A– Discourse (information supply) volume with sentiment: the emotional attitude expressed in a text (left axis) and reach (right axis) for given keywords (avian influenza and bird flu) in English during March-July 2024 (collected with Brand 2024); B – Avian influenza (left axis) and A/H5N1 (right axis) in Google Trends (information demand) worldwide 5/2023-07/2024
The southern hemisphere’s influenza-like illness (ILI) season began in May 2024, with a significant impact on pediatric patients [39]. The case of H5N1 in a child in Australia attracted the most attention and generated considerable traditional media coverage and social media discourse (peak around May 22nd 2024, Figure 1) [40-41]. This case demonstrates that media and public interest depend not only on the epidemiological significance of an event, but also on its emotional and socioeconomic context [42].
Since peaking in 2021, cases in wild birds and poultry in the EU/European Economic Area were on decline until the beginning of the summer in 2024 [33]. However, viruses found new host species during the spillover in spring/summer 2023. Avian influenza used to have seasonal patterns (e.g. in Poland peaks around early spring, late summer), but these patterns are less and less visible since 2022 [26]. To cope with highly pathogenic avian influenza, the poultry industry and wild bird conservation programs must be transformed in line with an integrated One Health approach that recognizes the interconnectedness of human, animal and environmental health in addressing global health challenges [1].
Consequences of avian influenza
Avian influenza is not only a concern for epidemiologists, but also a major economic problem because if the virus is detected, all the birds in that particular flock have to be killed. The virus has infected wild birds in large areas of Europe (mainly in the West and the North), causing mass mortality. H5N1 is suspected of affecting biodiversity by causing mass mortality events in some mammals, such as the reported sudden deaths of over 20000 South American sea lions [43]. During 2020-2023 hundreds of millions of poultry were culled in Poland, France and Russia alone. It is also an economic diplomacy challenge to negotiate with countries that import European poultry meat due to concerns about AIV infection. It is noteworthy that Poland is the largest producer of poultry meat in the EU and the third largest exporter in the world [7].
Financial and social costs of avian influenza
In 2021 alone, the direct costs of combating avian influenza in Poland exceeded 100 million EUR (mainly due to the euthanasia of millions of birds) and if we add the indirect costs incurred by the farmers (problems with sales and biosecurity), it will easily exceed 250 million EUR [44]. The periods without any bird flu outbreaks in Poland or France are brief and in accordance with the regulations of many countries around the world, the occurrence of at least one case is associated with an embargo on the import of poultry [45].
Due to the fact that industrial farming is a breeding ground for new AIV variants, there are recommendations to limit the global poultry population, including reducing intensive production in countries such as Poland [46-47]. However, countries with less developed poultry production, e.g. most of the Global South, should increase production [48]. This is due to the fact that poultry and eggs are perceived as quality protein sources with good biomass conversion, relatively low CO2 emissions and lack of religious taboos. In contrast, countries with intensive poultry production, e.g. the USA, Poland or France, should drastically reduce production. On the other hand, climate change is making it more difficult to farm animals in subtropical climates (Africa, Central America, Asia and Southern Europe) due to insufficient access to water and heat waves, thus moving production to the colder zones. It is an opportunity for the development of the industry in Poland and the rest of Central/Northen Europe, but at the same time it poses threats [48]. Climate change also affects the migration corridors of birds, e.g. the southern coast of the Baltic Sea has become a new hot-spot, where contact between birds may occur and virus variants may spread over long distances [49-50].
High concentration of the poultry industry (e.g. in the Żuromin district, Central Poland) is a biosecurity problem (e.g. due to a multi-point outbreak in this area > 10 million birds were culled in a dozen days) [6]. Thus, deconcentrating these massive production centers in Poland and in France is worth considering. Biosecurity measures are very important, however there are also plenty of vaccines against HPAI H5 strains for poultry in use around the world [32, 51]. One of the reasons why vaccination is not so widespread is because of trade restrictions. The use of live vaccines causes vaccine strains to circulate in the population, which is a problem for trading with avian influenza-free countries (concerns about importing a vaccine strain). For example, France introduced mass vaccination of poultry in late 2023 because it had previously been blocked by trade restrictions [52]. Avian influenza vaccines (for H5N1) for humans are on the market, their production can probably be scaled up, but they have never been used in clinical practice until 2024 [53].
In 2023, there was a significant shortage of eggs in Russia, which led to price increases and widespread concern of availability among consumers and policymakers. The situation was also seen in the Kaliningrad Oblast (province) bordering Poland [54]. Although the export of eggs was officially forbidden, the demand provoked smuggling [55]. Among the constellation of factors related to ongoing Russia-Ukraine War (such as sanctions), avian outbreaks reduced the supply of eggs. In a single outbreak in Bashkiria Oblast in summer 2023, millions of laying hens were euthanized, and production in this area returned to normal conditions in 2024 [56]. The avian influenza-driven shortage of eggs occurred also in Australia in July 2024 (Figure 1) [57].
Case study: H5N1 in cats and fur animals in Europe
In Finland, the H5N1 virus outbreak at fur animal farms happened in the summer and autumn 2023. This time the virus most likely originated from wild birds, particularly gulls, and spread to farmed fur animals like foxes and minks [5]. Control measures included culling the infected animals, surveillance of the environment and wildlife, improving biosecurity, monitoring human exposure and securing vaccines for animal farmers. There were no problems with financing testing and monitoring, unlike in the case of the outbreak among cats in Poland. While no human infections were detected, the outbreak in Finland raises concerns about potential virus adaptation and transmission risks for future virus selection processes [48].
The H5N1 outbreak in cats in Poland did not have the same direct source as the Finnish outbreak and it resulted in circa 40 confirmed cases and hundreds of suspected dead cats. The exact case definition did not exist before, therefore the World Organization of Animal Health (WOAH) and other institutions (e.g. the Polish Veterinary Inspection) may provide slightly different numbers. The response of the authorities demonstrated effective regional control measures (primarily in Gdańsk), but revealed gaps in the national and international preparedness and communication [4]. Despite their limited financial capability, the local authorities quickly responded to contain the outbreak, while the lack of established protocols and coordination between agencies for human and animal health caused confusion and delays. The situation was further complicated by an infodemic from both the cat owners and the poultry industry, with conflicting information hindering effective control. The most important lesson learnt from the outbreak in Poland was the lack of effective collaboration between institutions. There were tensions between:
1) the regional and national inspections (compare with other One Health disasters, e.g. the 2022 massive fishkill in the Oder River caused by toxic algae blooms);
2) Departments of the Ministries responsible for health, agriculture and environmental protection (e.g. in Finland veterinary inspectors collected samples from wildlife, whereas in Poland this was not possible in case of certain species (e.g. white stork), due to the lack of permissions from environmental agencies);
3) citizens, governmental and scientific entities [4, 58].
In 2023/2024 more cases of AIV in Europe were detected among mammals such as dogs in Poland and Italy, multiple fur animals outside of Finland and cats outside of Poland among others [33, 59]. Course of the the avian influenza disease varies between species: it is similar to common cold in dairy cattle, while most often fatal in cats [36]. One and public health authorities both in Poland and the USA experienced similar challenges. For instance, a significant proportion of humans in contacts refused to stay under sanitary surveillance or get tested [60]. Similar mis/dis-information topics (e.g. conspiracy theories about “the next planned pandemic”) were circulating [4]. Although none of European mammal infections were proved to be transmitted from mammal to mammal, this was confirmed in the USA [37].
Preventive measures and preparedness
In the face of potential threats like avian influenza, we could implement the following key measures:
1) epidemiological monitoring;
2) counteracting processes that cause vulnerability;
3) developing new medical technology and promoting their use;
4) preparation of health infrastructure and coordination of service activities [61].
It is important to combine theoretical understanding with practical application as well as with the experience from COVID-19 and to utilize advanced technologies to create comprehensive plans for preventing and managing zoonotic diseases in our region (Baltic Sea) and across Europe [62-63] (Table 1). In humans, infections are typically related to direct contact with contaminated environments or animals that are either dead, sick or healthy-looking but infected (this is the case for dairy farm workers) [28]. Human infections of currently circulating H5N1 strains result in an asymptomatic or mild disease, causing symptoms ranging from fever, conjunctivitis and cough to ocular discharge. It can also cause atypical symptoms e.g. gastroenteritis, which makes it difficult to differentiate from another ILI [64]. In the late spring and summer between ILI seasons, an increase in the number of patients with respiratory symptoms (COVID-19 excluded) may need special attention. Moreover, a new spillover may result in a different symptom onset, thus close collaboration between family doctors and veterinary/sanitary inspection (mainly in rural areas) is needed to not overlook such events.
Table 1. Comprehensive preparedness approach to counteract potential avian influenza threats
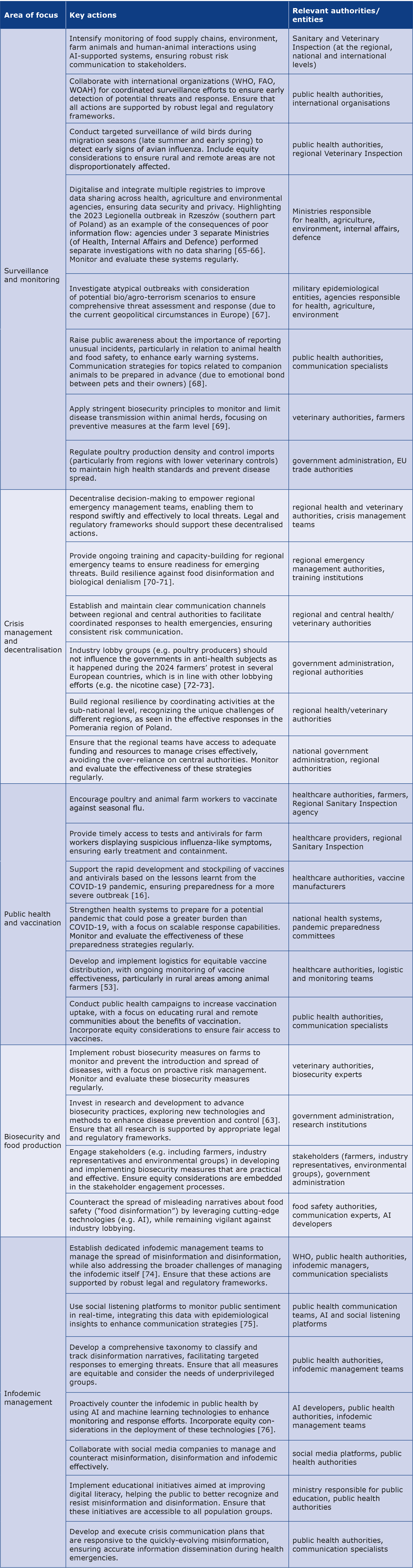
AI – artificial intelligence; FAO – Food and Agriculture Organization of the United Nations; WHO – World Health Organization; WOAH – World Organisation for Animal Health
Conclusions
The recent outbreaks of the AIV (mainly the H5N1 strain) have raised global concerns due to its spread to mammals and thus potential for human and possibly high mortality rates [77]. The economic impact has been substantial, with hundreds of millions of poultry culled yearly in Europe and trade restrictions imposed. The virus takes a much higher death toll in wildlife. Avian influenza is not just a biological condition of One Health [4, 78]. It is also a state of social condition and a state of global order. It is a vulnerability that we have created (e.g. due to intensive poultry farming) and might have to face again.
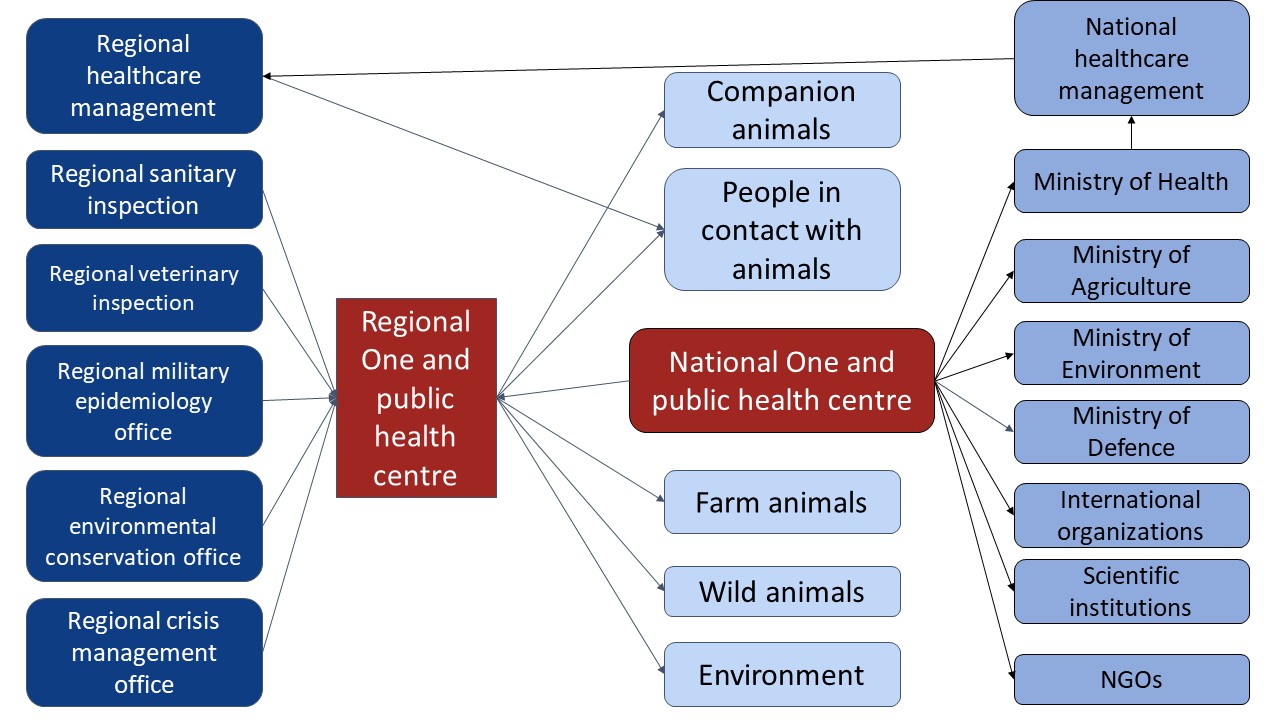
Figure 2. Structure of a surveillance/preparedness system (the initial phase of the EWRS – Early Warning and Response System [79]) which might be able to quickly react to Disease X threats such as avian influenza based on the experience with H5N1 in Europe in 2023 with major infectious agents; NGOs – non-governmental organisations
Expecting the unexpected
The main problem revealed in 2023 in Europe in a totally new event was the lack of intersectoral coordination and collaboration, which suggested rethinking the practical implementation of the One Health concept in the EWRS (Early Warning and Response System) [80] (Figure 2). First of all, we need to strengthen local One and public health inspection agencies, which will need to take responsibility to detect abnormality in the very first weeks of an outbreak and later the responsibility may be shifted to national and international bodies [81-82]. The just culture of quality model emphasizes learning from errors and failures to improve preparedness, thus actual (not on paper only) coordination between regional One and public health inspection agencies is needed [83]. According to the Polish law, this responsibility belongs to the voivodeship (provincial) governor authority, but this was not the case in any of the recent One health crises: AIV in cats in Pomerania (Northern Poland, 2023), the Legionella outbreak in Southern Poland (2023) or the massive fishkill in the Oder river in Southern Poland (2022) [4, 66, 84]. The modern concept of risk reduction required: localized strategies (which we emphasize in this editorial the most), breakthrough endeavors, collective actions and cross-disciplinary coordination [85].
In the Pomerania case of avian influenza in cats, the investigation started on about June 15th 2023, when excess mortality was observed by veterinarians with the support of local cat owners (lay people) [86]. The virus most likely originated almost a month earlier in Southeastern Poland, but was not detected there on time [86]. Local part of the investigation lasted until the last days of June when cooperative work of regional inspections excluded mammal to mammal transmission (including to humans) as the main route. Most literature on preparedness focuses on national and international collaboration, while regional efforts are overlooked, and we have seen consequences of that in 2023 [87]. Thus, the socalled Situation Definition Phase (assessing, specifying and interpreting the current situation) requires regional expertise and capacities [88]. Organizing One Health crisis management teams at the regional level seems to be the best solution as it combines competence and sub-regional knowledge. In Poland (Pomerania) and the USA (Texas), regional health/ veterinary authorities were able to identify the aetiology of unknown animal problems and manage them at an early stage with limited support from the national institutions. It is strongly recommended that cheaper new technologies, such as those based on artificial intelligence/machine learning, should be preferred to the traditional broad, expensive passive monitoring of different populations as advocated by some interest groups [63]. Most opinion articles and letters to the Editor simply emphasize the need for more AIV preparedness funding. We propose instead to use existing resources and skills and to use advances in technology and organisational knowledge to optimise the system.
The main problem in responding to unknown threats (e.g. AIV in cats in Pomerania) is the grey area between non- -standard risks and known threats [4, 84]. If the threat is well-defined (e.g. rabies and dirofilaria in pets of the war refugees from Ukraine or the typical HPAI in poultry), the infection control strategy can be planned in advance [2]. If rabies is suspected in a human following animal exposure, the medical doctor and the veterinarian have detailed procedures on what to do. Hazmat and CBRNE (Chemical, Biological, Radiological, Nuclear and Explosives) teams, emergency and infectious disease hospital staff and other inspectors regularly train in response (i.e. simulated outbreaks of the most likely agents) [67]. In the case of Disease X (a hypothetical case), rural doctors from the Żuławy region (Vistula agricultural zone) or epidemiologists from either one of the provincial hospitals in the Gdańsk Bay area (excluding the Tricity) may be the first to link the increase in ILI cases among their patients to the mass mortality of seagulls. Informal interdisciplinary ties between local community leaders will be crucial to defining the threat and responding smoothly [83]. Therefore, social reactions must be taken into account in mitigating epidemic threats [89].
Disease X
The current situation related to bird flu raises concerns about the possibility of another pandemic among humans, particularly in the context of the so-called Disease X. It is a term reserved for a hypothetical pathogen (most likely zoonotic virus), much more lethal than COVID-19 and capable of causing a global health crisis on an unknown scale.
Geopolitical challenges and climate change destabilize human flow systems, creating conditions for the migration of species and the development of new pathogens, pose new categories of risks to humans, animals and the environment [90]. Gdańsk (where this Journal is published) and the Tricity metropolitan area (Gdańsk, Sopot and Gdynia) in Poland, seems to be susceptible, particularly during the tourist season with an additional > 10 million visitors each summer and new problems not related to typical foodborne outbreaks [91-92]. In terms of possible spillovers and reassortments of avian influenza, the Poland’s Baltic coastline (particularly the Gdańsk Bay) was identified as one of the most important hotspots in the entire Europe due to crossing of main bird migration paths [93]. Increased mobility of goods (e.g. the Gdańsk and Gdynia ports together are 4th in Europe in terms of cargo tonnage handled) and people (planned construction of a mega airport hub in Central Poland), growing numbers of migrations (e.g. war refugees from Ukraine or flows from the Global South) and further urbanization of metropolises (increased population density) with simultaneous depopulation of the provinces create new challenges and threats. These factors have enabled the rapid spread of new diseases and may change the most likely entry point of new pathogens from Western to Eastern Europe.
The situation described above highlights the particular vulnerability of Gdańsk and the Tricity area on a micro scale (as well as the entire world on a macro scale) and aptly illustrates broader trends related to civilizational development. These trends have long been discussed in sociological literature, notably through the concept of the “risk society” detailed by the German sociologist Ulrich Beck in his 1986 book “Risk Society: Towards a New Modernity” [94]. According to Beck, a “risk society” is a modern society dominated by global threats and risks arising from technological advancement, industrial economy and modernization processes [95]. A classic example of such global risk is undoubtedly Disease X, which poses both a tangible threat and one immersed in uncertainty. It is unknown what epidemiological problem will lead to a crisis or when it will happen.
In the recently popular futurological BANI theory by Jamais Cascio, the post-pandemic world is described using four words: brittle, anxious, non-linear and incomprehensible [96]. We can view the major public health challenges through a similar lens. The COVID-19 crisis showed that the healthcare systems we considered stable and predictable, turned out to be fragile: there was a shortage of masks and disinfectants, the preventive measures failed and hospitals were closed to some extent [97]. The response to this was adaptation: the launch of telehealth services, lockdowns and restrictions. After the pandemic, there was a sense of regaining control although it is immersed in the uncertainty of anticipation of Disease X. The world of health is also non-linear. As some fields develop, others may be neglected. We are using cutting-edge science to develop gene therapies, while not conducting enough research into new groups of antibiotics. Medicine is also an increasingly complex system, susceptible to geopolitical and climate changes.
All this means that in a chaotic world, it is necessary to be as flexible as possible in taking action. Monitoring definable threats (while not closing our eyes to new ones) and preparing strategies for mitigating the risks posed by an unknown disease. This is exemplified by the case of avian influenza as the most likely agent of the upcoming crisis [98].
The potential pandemic of Disease X – there is not a question of “if” but “when” it will happen [99]. To mitigate this risk we need to enhance surveillance, strengthen biosecurity, build strong vaccination strategies and improve the communication between animal and human health agencies. Responding to new avian influenza outbreaks through a One Health approach particularly involves monitoring exposed individuals for potential infections, conducting syndromic and laboratory surveillance to identify and track cases, planning and preparing for in-depth epidemiologic investigations and evaluating the effectiveness of existing medical countermeasures (e.g. diagnostic tests: antigen, antibody and PCR), vaccines (e.g. the 100 days concept) and therapeutics (e.g. oseltamivir) all together [100].
A collaborative One Health approach is essential not only in theory. This is the lesson that we need to learn from the situation in Europe in 2023. And it has to be implemented in practice to address the complex challenges posed by avian influenza and other diseases, ensuring the protection of human health, animal welfare, and economic stability [62]. In conclusion, further discussion on the reorganization of preparedness efforts in Europe appears warranted, particularly regarding the enhancement of EWS, interdisciplinary collaboration and resource allocation for effective outbreak response.
The situation escalated in November 2024, as the H5N1 virus not only continued its spread among known mammalian hosts in the USA (including humans and companion animals) but also exhibited a concerning leap to pigs, marking the first confirmed cases within this species [101]. This development has alarmed the virology community, as recombinants of swine and avian influenza viruses pose a significant pandemic threat. The emergence of AH5N1 in pigs suggests we are edging closer to a potential pandemic scenario.
Conflict of interest
The first author was involved personally in the 2023 investigation of the epizootic outbreak among cats in the Gdańsk area, with access to internal data. However, the information provided in this article is based on publicly available sources integrated with non-classified personal communications, his own unpublished analysis and with the personal opinions of the authors.
Funding
None.
---------
Image – Avian Influenza A Virus (H5N1/Bird Flu)
Colorized transmission electron micrograph of avian influenza A H5N1 virus particles (blue), grown in Madin-Darby Canine Kidney (MDCK) epithelial cells. Microscopy by CDC; repositioned and recolored by NIAID. Credit: CDC and NIAID; resource – flickr.com (CC BY 2.0)
References
| 1. |
Aarestrup FM, Bonten M, Koopmans M. Pandemics – One Health preparedness for the next. Lancet Reg Heal - Eur [Internet]. 2021;9:100210. Available from: https://doi.org/10.1016/j.lanepe.2021.100210.
|
| 2. |
Pathogens prioritization: a scientific framework for epidemic and pandemic research preparedness [Internet]. WHO. 2024. Available from: https://cdn.who.int/media/docs/default-source/consultation-rdb/prioritization-pathogens-v6final.pdf?sfvrsn=c98effa7_7&download=true.
|
| 3. |
Lewis N, Beer M. Stop H5N1 influenza in US cattle now. Science (80- ) [Internet]. 2024;385(6705):123. Available from: https://doi.org/10.1126/science.adr5866.
|
| 4. |
Jarynowski A, Romanowska M, Maksymowicz S, Belik V. One health multimodal surveillance in time of change: lessons not learnt from case study of a/h5n1 spillover to mammals in Gdańsk metropolitan area. One Heal J [Internet]. 2024;2(III):45–61. Available from: https://onehealthjournal.org.ua/index.php/main/article/view/2024-III-06.
|
| 5. |
Lindh E, Lounela H, Ikonen N, Kantala T, Savolainen-Kopra C, Kauppinen A, et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Eurosurveillance [Internet]. 2023;28(31). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.31.2300400.
|
| 6. |
Dziadek K, Świętoń E, Kozak E, Wyrostek K, Tarasiuk K, Styś-Fijoł N, et al. Phylogenetic and Molecular Characteristics of Wild Bird-Origin Avian Influenza Viruses Circulating in Poland in 2018−2022: Reassortment, Multiple Introductions, and Wild Bird–Poultry Epidemiological Links. Ozawa M, editor. Transbound Emerg Dis [Internet]. 2024;2024:1–15. Available from: https://www.hindawi.com/journals/tbed/2024/6661672/.
|
| 7. |
Vorotnikov V. Poland remains largest poultry producer in the EU [Internet]. Poultry World. 2023 [cited 2024 Oct 3]. Available from: https://www.poultryworld.net/the-industrymarkets/market-trends-analysis-the-industrymarkets-2/poland-remains-largest-poultry-producer-in-the-eu/.
|
| 8. |
Possas C, Marques ETA, Kuchipudi S V., Kumar P, Kim JH, Homma A. Disease X in the Tropics, preventing the next pandemic: how to accelerate spillover prevention and vaccine preparedness? Front Trop Dis [Internet]. 2024;5. Available from: https://www.frontiersin.org/articles/10.3389/fitd.2024.1417065/full.
|
| 9. |
Fiegler-Rudol J, Lau D n. med. K, Kasperczyk D. hab. n. med. J. Public health threat of novel zoonotic diseases: literature review. Przegl Epidemiol [Internet]. 2024;78(1):69–80. Available from: https://www.przeglepidemiol.pzh.gov.pl/Public-health-threat-of-novel-zoonotic-diseases-literature-review,188161,0,2.html.
|
| 10. |
Wiśniewska MZ. Whistleblowing in Health Care Organizations: a comprehensive literature review. Probl Zarządzania [Internet]. 2021;19(4 (94)):131–65. Available from: https://repozytorium.bg.ug.edu.pl/info/article/UOGb56e91cc814445b5922cbe140cdc5778/.
|
| 11. |
Swayne DE, editor. Animal Influenza [Internet]. Wiley; 2016. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118924341.
|
| 12. |
Bedford T, Suchard MA, Lemey P, Dudas G, Gregory V, Hay AJ, et al. Integrating influenza antigenic dynamics with molecular evolution. Elife [Internet]. 2014;3. Available from: https://elifesciences.org/articles/01914.
|
| 13. |
Kostrzewski J, Magdzik W, Wiśniewski M. Epidemia grypy w Polsce w 1971 r. Przegl Epidemiol [Internet]. 1972;27(1–2):1–13. Available from: https://epibaza.pzh.gov.pl/sites/default/files/Przegląd Epidemiologiczny 1973 nr 1-2.pdf.
|
| 14. |
Guan Y, Poon LLM, Cheung CY, Ellis TM, Lim W, Lipatov AS, et al. H5N1 influenza: A protean pandemic threat. Proc Natl Acad Sci [Internet]. 2004;101(21):8156–61. Available from: https://pnas.org/doi/full/10.1073/pnas.0402443101.
|
| 15. |
Cumulative number of confirmed human cases for avian influenza A (H5N1) reported to WHO, 2003-2011 [Internet]. WHO. 2011 [cited 2024 Aug 12]. Available from: http://www.who.int/entity/influenza/human_animal_interface/EN_GIP_20111215CumulativeNumberH5N1cases.pdf.
|
| 16. |
Al-Tawfiq JA, Tirupathi R, Temsah M-H. Feathered fears: Could avian H5N1 influenza be the next pandemic threat of disease X? New Microbes New Infect [Internet]. 2024;59:101416. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2052297524002002.
|
| 17. |
European Centre for Disease Prevention and Control. Seasonal influenza 2022−2023. In: ECDC Annual Epidemiological Report for 2023 [Internet]. Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-annual-epidemiological-report-20222023.
|
| 18. |
Sing A. Zoonoses: Infections Affecting Humans and Animals [Internet]. Springer; 2023. Available from: https://link.springer.com/referencework/10.1007/978-3-031-27164-9.
|
| 19. |
Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, et al. The origins of SARS-CoV-2: A critical review. Cell [Internet]. 2021;184(19):4848–56. Available from: https://doi.org/10.1016/j.cell.2021.08.017.
|
| 20. |
Goraichuk I V., Arefiev V, Stegniy BT, Gerilovych AP. Zoonotic and Reverse Zoonotic Transmissibility of SARS-CoV-2. Virus Res [Internet]. 2021;302:198473. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168170221001805.
|
| 21. |
Rabalski L, Kosinski M, Mazur-Panasiuk N, Szewczyk B, Bienkowska-Szewczyk K, Kant R, et al. Zoonotic spill-over of SARS-CoV-2: mink-adapted virus in humans. Clin Microbiol Infect [Internet]. 2022;28(3):451.e1-451.e4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1198743X21006984.
|
| 22. |
Barreiro Hurle J, Bogonos M, Himics M, Hristov J, Perez Dominguez I, Sahoo A, et al. Modelling environmental and climate ambition in the agricultural sector with the CAPRI model [Internet]. Luxembourg: Joint Research Centre (Seville site); 2021. Available from: https://econpapers.repec.org/paper/iptiptwpa/jrc121368.htm.
|
| 23. |
Antimicrobial consumption and resistance in bacteria from humans and food‐producing animals. EFSA J [Internet]. 2024;22(2). Available from: http://doi.wiley.com/10.2903/j.efsa.2024.8589.
|
| 24. |
Flint SJ, Racaniello VR, Rall GF, Hatziioannou T, Skalka AM. Principles of virology, Volume 2: pathogenesis and control [Internet]. John Wiley & Sons; 2020. Available from: https://www.google.com/books?hl=pl&lr=&id=LFwBEAAAQBAJ&oi=fnd&pg=PR17&dq=Flint+SJ,+Racaniello+VR,+Rall+GF,+Hatziioannou+T,+Skalka+AM.+Principles+of+virology.+John+Wiley+%26+Sons%3B+2020.&ots=OYVagwTbJn&sig=u-4v3Fy_byM98_dqZcbxtIOiH-A.
|
| 25. |
Pohlmann A, King J, Fusaro A, Zecchin B, Banyard AC, Brown IH, et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe, 2021. Peiris JSM, editor. MBio [Internet]. 2022;13(4). Available from: https://journals.asm.org/doi/10.1128/mbio.00609-22.
|
| 26. |
Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Mirinavičiūtė G, Niqueux É, et al. Avian influenza overview June–September 2023. EFSA J [Internet]. 2023;21(10). Available from: http://doi.wiley.com/10.2903/j.efsa.2023.8328.
|
| 27. |
Nguyen T-Q, Hutter C, Markin A, Thomas M, Lantz K, Killian ML, et al. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle. bioRxiv [Internet]. 2024;2024.05.01.591751. Available from: http://biorxiv.org/content/early/2024/05/01/2024.05.01.591751.abstract.
|
| 28. |
Garg S, Reed C, Davis CT, Uyeki TM, Behravesh CB, Kniss K, et al. Outbreak of Highly Pathogenic Avian Influenza A(H5N1) Viruses in U.S. Dairy Cattle and Detection of Two Human Cases — United States, 2024. MMWR Morb Mortal Wkly Rep [Internet]. 2024;73(21):501–5. Available from: http://www.cdc.gov/mmwr/volumes/73/wr/mm7321e1.htm?s_cid=mm7321e1_w.
|
| 29. |
Past Reported Global Human Cases with Highly Pathogenic Avian Influenza A(H5N1) (HPAI H5N1) by Country, 1997-2024 [Internet]. CDC, Avian Influenza (Bird Flu). 2024 [cited 2024 Oct 4]. Available from: https://www.cdc.gov/bird-flu/php/avian-flu-summary/chart-epi-curve-ah5n1.html.
|
| 30. |
Singh G, Trujillo JD, McDowell CD, Matias-Ferreyra F, Kafle S, Kwon T, et al. Detection and characterization of H5N1 HPAIV in environmental samples from a dairy farm. Virus Genes [Internet]. 2024;60(5):517–27. Available from: https://link.springer.com/10.1007/s11262-024-02085-4.
|
| 31. |
Abdelkawi A, Zinoune Z, Slim A, Pathak Y V. Avian Influenza Outbreaks over the Last Decade. In: Rising Contagious Diseases [Internet]. Wiley; 2024. p. 42–9. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9781394188741.ch5.
|
| 32. |
Graziosi G, Lupini C, Catelli E, Carnaccini S. Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals. Animals [Internet]. 2024;14(9):1372. Available from: https://www.mdpi.com/2076-2615/14/9/1372.
|
| 33. |
Alexakis L, Fusaro A, Kuiken T, Mirinavičiūtė G, Ståhl K, Staubach C, et al. Avian influenza overview March–June 2024. EFSA J [Internet]. 2024;22(7). Available from: http://doi.wiley.com/10.2903/j.efsa.2024.8930.
|
| 34. |
Chernyshova AI, Zhirnov OP. Two Phylogenetic Cohorts of the Nucleocapsid Protein NP and Their Correlation with the Host Range of Influenza A Viruses. Dokl Biochem Biophys [Internet]. 2024;516(1):93–7. Available from: https://link.springer.com/10.1134/S1607672924700789.
|
| 35. |
Rabalski L, Milewska A, Pohlmann A, Gackowska K, Lepionka T, Szczepaniak K, et al. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Eurosurveillance [Internet]. 2023;28(31). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.31.2300390.
|
| 36. |
Domańska-Blicharz K, Świętoń E, Świątalska A, Monne I, Fusaro A, Tarasiuk K, et al. Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Eurosurveillance [Internet]. 2023;28(31). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.31.2300366.
|
| 37. |
Caserta LC, Frye EA, Butt SL, Laverack M, Nooruzzaman M, Covaleda LM, et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature [Internet]. 2024; Available from: https://www.nature.com/articles/s41586-024-07849-4.
|
| 38. |
Jarynowski A, Semenov A, Belik V. Perception of infectious diseases with animal and human hosts on the Polish internet. In: Proceedings of the 20th Congress of the International Society for Animal Hygiene, Berlin, Germany [Internet]. 2022. p. 5–7. Available from: http://interdisciplinary-research.eu/wp-content/uploads/2022/08/Abstract-form-ISAH_jarynowski_corr.pdf.
|
| 39. |
Integrated inflenza and other respiratory viruses surveillance outputs [Internet]. WHO. 2024 [cited 2024 Oct 4]. Available from: https://app.powerbi.com/view?r=eyJrIjoiNzdjZTVmY2YtNzY2NC00NTM0LTkzY2QtMWM0MzY0Mjg0YTZjIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9.
|
| 40. |
Sołtysiak A, Żminda J, Makarova O, Jarynowski A, Belik V. Perception of antibiotics in Polish Internet during the infection season 2023/2024. E-Methodology. 2023;10(10):54–82.
|
| 41. |
Kumar P, Sharma A, Apostolopoulos V, Gaidhane AM, Satapathy P. Australia’s first human case of H5N1 and the current H7 poultry outbreaks: implications for public health and biosecurity measures. Lancet Reg Heal – West Pacific [Internet]. 2024;48. Available from: https://doi.org/10.1016/j.lanwpc.2024.101141.
|
| 42. |
SteelFisher GK, Blendon RJ, Kang M, Ward JRM, Kahn EB, Maddox KEW, et al. Adoption of preventive behaviors in response to the 2009 H1N1 influenza pandemic: a multiethnic perspective. Influenza Other Respi Viruses [Internet]. 2015;9(3):131–42. Available from: https://onlinelibrary.wiley.com/doi/10.1111/irv.12306.
|
| 43. |
Flavia O, Sascha K, Carola S, Christoph S, Valerie A, Alina A, et al. The role of mammals in Avian Influenza: a review. EFSA Support Publ [Internet]. 2024;21(3). Available from: http://doi.wiley.com/10.2903/sp.efsa.2024.EN-8692.
|
| 44. |
Comparission in world farming. Bird flu: only major farm reforms can end it [Internet]. 2024. Available from: https://www.ciwf.pl/media/7454836/ptasia-grypa-raport-2023-compassion-polska-ang.pdf.
|
| 45. |
Lipińska I. Zwalczanie chorób zakaźnych zwierząt gospodarskich–wybrane aspekty prawne. Stud Iurid Agrar [Internet]. 2017;157. Available from: https://www.google.com/books?hl=pl&lr=&id=3YqXDwAAQBAJ&oi=fnd&pg=PA157&dq=Lipińska+I.+Zwalczanie+chorób+zakaźnych+zwierząt+gospodarskich–wybrane+aspekty+prawne.+Stud+Iurid+Agrar.+2017%3B157.&ots=s144lId-Zj&sig=KSHVlMWKf5u9xa6AR4Hd3aNVSuY.
|
| 46. |
Gržinić G, Piotrowicz-Cieślak A, Klimkowicz-Pawlas A, Górny RL, Ławniczek-Wałczyk A, Piechowicz L, et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci Total Environ [Internet]. 2023;858:160014. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048969722071145.
|
| 47. |
Regan T. Empty cages: Facing the challenge of animal rights [Internet]. Rowman & Littlefield; 2004. Available from: https://www.google.com/books?hl=pl&lr=&id=PUDXwO22eqgC&oi=fnd&pg=PR9&dq=Regan+T.+Empty+cages:+Facing+the+challenge+of+animal+rights.+Rowman+%26+Littlefield%3B+2004.&ots=SyH3jSco_Q&sig=Bmbs2qf0CcEDY5ecLNV2Jr-xwks.
|
| 48. |
Kuiken T. The global expansion of H5N1 highly pathogenic avian influenza. Sydney Infectious Diseases Institute; 2024.
|
| 49. |
Fusaro A, Gonzales JL, Kuiken T, Mirinavičiūtė G, Niqueux É, Ståhl K, et al. Avian influenza overview December 2023–March 2024. EFSA J [Internet]. 2024;22(3). Available from: http://doi.wiley.com/10.2903/j.efsa.2024.8754.
|
| 50. |
Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Mirinavičiūtė G, Niqueux É, et al. Avian influenza overview September–December 2023. EFSA J [Internet]. 2023;21(12). Available from: http://doi.wiley.com/10.2903/j.efsa.2023.8539.
|
| 51. |
Gierak A, Śmietanka K. The impact of selected risk factors on the occurrence of highly pathogenic avian influenza in commercial poultry flocks in Poland. J Vet Res [Internet]. 2021;65(1):45–52. Available from: https://www.sciendo.com/article/10.2478/jvetres-2021-0013.
|
| 52. |
Vaccination contre l’influenza aviaire: pourquoi cela n’est pas un obstacle à la sécurité des échanges commerciaux [Internet]. Organisation mondiale de la sante animale; 2023. Available from: https://www.woah.org/app/uploads/2023/12/fr-woah-policybrief-vaccinationinfluenzaaviaireetcommerce.pdf.
|
| 53. |
Nohynek H, Helve OM. One health, many interpretations: vaccinating risk groups against H5 avian influenza in Finland. Eurosurveillance [Internet]. 2024;29(25). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2024.29.25.2400383.
|
| 54. |
Kamenev P, Kalugina E. ‘Impossible Grief’: A Frenzy for Second-Class Eggs Emerged in Kaliningrad Oblast [in Russian] [Internet]. New Kaliningrad. 2023 [cited 2024 Oct 9]. Available from: https://www.newkaliningrad.ru/news/community/24072584-skorb-nevozmozhnaya-v-kaliningradskoy-oblasti-voznik-azhiotazh-na-yaytsa-vtoroy-kategorii.html.
|
| 55. |
Otwarte klatki [in Polish] [Internet]. Instagram profile. 2023 [cited 2010 Oct 9]. Available from: https://www.instagram.com/otwarteklatki/reel/CyL0fY6s8DV/.
|
| 56. |
Bolee milliona kur unichtozhat iz za ptichego grippa v bashkirii {in Russian] [Internet]. https://mir24.tv. 2024 [cited 2024 Aug 1]. Available from: https://mir24.tv/news/16561920/bolee-milliona-kur-unichtozhat-iz-za-ptichego-grippa-v-bashkirii.
|
| 57. |
Dahlstrom M. Warning about ‘risky’ farming change amid shortages at Coles, Woolworths, Aldi [Internet]. Yahoo! News. 2024 [cited 2024 Oct 9]. Available from: https://au.news.yahoo.com/warning-about-risky-farming-change-amid-shortages-at-coles-woolworths-aldi-040151828.html?guccounter=1.
|
| 58. |
Grelowska M, Hibner M, Deneka W, Jarynowski A. Information flow during culmination of online public discourse based on the Oder river environmental disaster (accepted in E-methodology). 2024.
|
| 59. |
Szaluś-Jordanow O, Golke A, Dzieciątkowski T, Czopowicz M, Kardas M, Mickiewicz M, et al. Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms [Internet]. 2024;12(4):689. Available from: https://www.mdpi.com/2076-2607/12/4/689.
|
| 60. |
Branswell H. CDC’s top flu scientist says the risk to the public from H5N1 is low, but she isn’t sleeping well. Here’s why [Internet]. STATnews.com. 2024 [cited 2024 Oct 9]. Available from: https://www.statnews.com/2024/05/03/bird-flu-why-h5n1-keeping-awake-cdc-top-flu-scientist/.
|
| 61. |
Maksymowicz S. Choroba X może wywołać kryzys większy niż COVID-19. Podatność już wytworzyliśmy [in Polish] [Internet]. Nowa Konfederacja. 2024 [cited 2024 Oct 8]. Available from: https://nowakonfederacja.pl/choroba-x-moze-wywolac-kryzys-wiekszy-niz-covid-19-podatnosc-juz-wytworzylismy/.
|
| 62. |
Meletis E, Jarynowski A, Maksymowicz S, Kostoulas P, Belik V. Animal Health Discourse during Ecological Crises in the Media — Lessons Learnt from the Flood in Thessaly from the One Health Perspective. Vet Sci [Internet]. 2024;11(4):140. Available from: https://www.mdpi.com/2306-7381/11/4/140.
|
| 63. |
Zhang L, Guo W, Lv C. Modern technologies and solutions to enhance surveillance and response systems for emerging zoonotic diseases. Sci One Heal [Internet]. 2024;3:100061. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2949704323000550.
|
| 64. |
Zoonotic influenza – Annual Epidemiological Report for 2022 [Internet]. European Centre for Disease Prevention and Control. 2023 [cited 2024 Oct 8]. Available from: https://www.ecdc.europa.eu/en/publications-data/zoonotic-influenza-annual-epidemiological-report-2022.
|
| 65. |
Sidor A, Ślączka J, Rylska-Malita D, Rajzer K, Sierakowska A, Mikulska U. Outbreak of Legionnaires’ disease in Rzeszów in 2023. Przegl Epidemiol [Internet]. 2024;78(1):44–55. Available from: https://www.przeglepidemiol.pzh.gov.pl/Outbreak-of-Legionnaires-disease-in-Rzeszow-in-2023,188372,0,2.html.
|
| 66. |
Krzowski L, Ostrowska A. Legionnaires’ disease Poland. Warsaw; 2023.
|
| 67. |
Jarynowski A. Agroterrorism involving biological agents and related threats in Poland and Europe in the context of the COVID-19 pandemic and the war in Ukraine. Terror Stud Anal prewencja [Internet]. 2023;(4 (4)):405–44. Available from: https://www.ejournals.eu/pliki/art/24138/.
|
| 68. |
Ly H. Highly pathogenic avian influenza H5N1 virus infection of companion animals. Virulence [Internet]. 2024;15(1). Available from: https://www.tandfonline.com/doi/full/10.1080/21505594.2023.2289780.
|
| 69. |
Delpont M, Salazar LG, Dewulf J, Zbikowski A, Szeleszczuk P, Dufay-Lefort A-C, et al. Monitoring biosecurity in poultry production: an overview of databases reporting biosecurity compliance from seven European countries. Front Vet Sci [Internet]. 2023;10. Available from: https://www.frontiersin.org/articles/10.3389/fvets.2023.1231377/full.
|
| 70. |
Chowdhury A, Kabir KH, Abdulai A-R, Alam MF. Systematic Review of Misinformation in Social and Online Media for the Development of an Analytical Framework for Agri-Food Sector. Sustainability [Internet]. 2023;15(6):4753. Available from: https://www.mdpi.com/2071-1050/15/6/4753.
|
| 71. |
Jarynowski A, Krzowski Ł, Maksymowicz S. Biological mis (dis)-information in the Internet as a possible Kremlin warfare [Internet]. IBI; 2023. Available from: https://www.researchgate.net/profile/Andrzej-Jarynowski/publication/372523777_Biological_misdis-information_in_the_Internet_as_a_possible_Kremlin_warfare/links/64bc1aebc41fb852dd91619b/Biological-misdis-information-in-the-Internet-as-a-possible-Kremlin-wa.
|
| 72. |
Jarynowski A, Platek D, Belik V. Digital traces of protests by animal breeders and animal right defenders in the European Union. Wiesbaden; 2024.
|
| 73. |
Rotaub R. Prof. GUMed Łukasz Balwicki: To smutne, że politycy dają się rozgrywać koncernom tytoniowym [in Polish] [Internet]. RynekZdrowia.pl. 2024 [cited 2024 Oct 9]. Available from: https://www.rynekzdrowia.pl/Polityka-zdrowotna/Prof-GUMed-Lukasz-Balwicki-To-smutne-ze-politycy-daja-sie-rozgrywac-koncernom-tytoniowym,260630,14.html.
|
| 74. |
White B, Cabalero I, Nguyen T, Briand S, Pastorino A, Purnat TD. An Adaptive Digital Intelligence System to Support Infodemic Management: The WHO EARS Platform. In 2023. Available from: https://ebooks.iospress.nl/doi/10.3233/SHTI230296.
|
| 75. |
Boender TS, Schneider PH, Houareau C, Wehrli S, Purnat TD, Ishizumi A, et al. Establishing Infodemic Management in Germany: A Framework for Social Listening and Integrated Analysis to Report Infodemic Insights at the National Public Health Institute. JMIR Infodemiology [Internet]. 2023;3:e43646. Available from: https://infodemiology.jmir.org/2023/1/e43646.
|
| 76. |
Gradoń KT, Hołyst JA, Moy WR, Sienkiewicz J, Suchecki K. Countering misinformation: A multidisciplinary approach. Big Data Soc [Internet]. 2021;8(1). Available from: https://journals.sagepub.com/doi/10.1177/20539517211013848.
|
| 77. |
Granata G, Simonsen L, Petrosillo N, Petersen E. Mortality of H5N1 human infections might be due to H5N1 virus pneumonia and could decrease by switching receptor. Lancet Infect Dis [Internet]. 2024;24(9):e544–5. Available from: https://doi.org/10.1016/S1473-3099(24)00460-2.
|
| 78. |
Koopmans MPG, Barton Behravesh C, Cunningham AA, Adisasmito WB, Almuhairi S, Bilivogui P, et al. The panzootic spread of highly pathogenic avian influenza H5N1 sublineage 2.3.4.4b: a critical appraisal of One Health preparedness and prevention. Lancet Infect Dis [Internet]. 2024 Aug 8; Available from: https://doi.org/10.1016/S1473-3099(24)00438-9.
|
| 79. |
Ricks PM, Njie GJ, Dawood FS, Blain AE, Winstead A, Popoola A, et al. Lessons Learned from CDC’s Global COVID-19 Early Warning and Response Surveillance System. Emerg Infect Dis [Internet]. 2022;28(13). Available from: https://wwwnc.cdc.gov/eid/article/28/13/21-2544_article.htm.
|
| 80. |
Taleb NN. Black Swans and the Domains of Statistics. Am Stat [Internet]. 2007;61(3):198–200. Available from: http://www.tandfonline.com/doi/abs/10.1198/000313007X219996.
|
| 81. |
Jephcott FL. Stuck in ‘the field’: why applied epidemiology needs to go home. BMJ Glob Heal [Internet]. 2024;9(4):e015692. Available from: https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2024-015692.
|
| 82. |
Dzau V, Swaminathan S, Baker C, Bright RA, Castillo J, Chuan TC, et al. The 100 Days Mission: how a new medical-countermeasures network can deliver equity and innovation. Lancet [Internet]. 2023;402(10412):1507–10. Available from: https://doi.org/10.1016/S0140-6736(23)01775-0.
|
| 83. |
Wiśniewska M. Just culture and the reporting of food safety incidents. Br Food J [Internet]. 2023;125(1):302–17. Available from: https://www.researchgate.net/profile/Malgorzata-Wisniewska-3/publication/366556173_Just_culture_and_the_reporting_of_food_safety_incidents/links/63c55b74d9fb5967c2e01753/Just-culture-and-the-reporting-of-food-safety-incidents.pdf.
|
| 84. |
Jarynowski A, Maksymowicz S. Die Tragödie an der Oder. Wie Polen und Deutsche aneinander vorbeireden [in German] [Internet]. Dialog Forum. 2024 [cited 2024 Oct 10]. Available from: https://forumdialog.eu/2024/05/02/die-tragoedie-des-allgemeinguts-an-der-oder-wie-polen-und-deutsche-aneinander-vorbeireden/.
|
| 85. |
McLennan M. The global risks report 2024. 19th Edition. Insight Report [Internet]. 2024. Available from: https://www3.weforum.org/docs/WEF_The_Global_Risks_Report_2024.pdf.
|
| 86. |
Jarynowski A, Belik V. Triangulated epidynamic-spatio-temporal analysis of A/H5N1 spillover to cats in Poland in June/July 2023 – letter to Eurosurveillance [Internet]. 2023. Available from: https://zenodo.org/records/10845907.
|
| 87. |
Plaza PI, Gamarra-Toledo V, Euguí JR, Lambertucci SA. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg Infect Dis [Internet]. 2024;30(3). Available from: https://wwwnc.cdc.gov/eid/article/30/3/23-1098_article.
|
| 88. |
Voss M, Dittmer C, Schulze K, Rüger A, Bock N. Katastrophenbewältigung alssozialer Prozess: Vom Ideal-zumRealverständnis von Risiko-, Krisen und Katastrophenmanagement. Notfallvorsorge [Internet]. 2024;53(1):22–32. Available from: https://www.nomos-elibrary.de/10.5771/2941-0371-2022-1/notfallvorsorge-volume-53-2022-issue-1.
|
| 89. |
Golinowska S, Czepiel J, Domagała A, Duplaga M, Grodzicki T, Hałuszka J, et al. Public Health: The Social and Ecological Dimension [Internet]. Wydawnictwo Naukowe Scholar; 2024. Available from: https://books.google.pl/books?id=2e0OEQAAQBAJ.
|
| 90. |
Komunikat 04/2024 Komitetu Problemowego ds. Kryzysu Klimatycznego przy Prezydium PAN na temat wpływu zmiany klimatu na zdrowie [in Polish]. PAN. 2024.
|
| 91. |
Szkudlarek Ł. Diagnoza adaptacji i mitygacji do zmian klimatu Obszaru Metropolitalnego Gdańsk-Gdynia-Sopot [in Polish] [Internet]. Wrocław; 2021. Available from: https://www.metropoliagdansk.pl/upload/files/DIAGNOZA_ADAPTACJI_I_MITYGACJI_DO_ZMIAN_KLIMATU_OMGGS_PROJEKT(1).pdf.
|
| 92. |
Milczarek M, Czarkowski MP, Sadkowska-Todys M. Salmonellosis in Poland in 2021. Epidemiol Rev Epidemiol [Internet]. 2023;77(4). Available from: https://scholar.archive.org/work/bwks6vxlinguzppmt3wre6vg2i/access/wayback/https://www.przeglepidemiol.pzh.gov.pl/pdf-188471-110141?filename=Salmonellosis in Poland.pdf.
|
| 93. |
Waldenström J, van Toor M, Lewis N, Lopes S, Javakhishvili Z, Muzika D, et al. Active wild bird surveillance of avian influenza viruses, a report. EFSA Support Publ [Internet]. 2022;19(12). Available from: http://doi.wiley.com/10.2903/sp.efsa.2022.EN-7791.
|
| 94. |
Beck U. Risikogesellschaft: Auf dem Weg in eine andere Moderne. Suhrkamp Verlag; 2016.
|
| 95. |
Milliken A. Thank God it’s just COVID! Eur J Transl Clin Med [Internet]. 2020;3(2):7–10. Available from: https://ejtcm.gumed.edu.pl/articles/73.
|
| 96. |
Szczypiorski K. Cybersecurity and Data Science. Electronics [Internet]. 2022;11(15):2309. Available from: https://www.mdpi.com/2079-9292/11/15/2309.
|
| 97. |
Smiatacz T. It didn’t have to happen this way – what COVID-19 tells us about translational medicine. Eur J Transl Clin Med [Internet]. 2020;3(1):7–10. Available from: https://ejtcm.gumed.edu.pl/articles/60.
|
| 98. |
Al Asfoor D, Tabche C, Al-Zadjali M, Mataria A, Saikat S, Rawaf S. Concept analysis of health system resilience. Heal Res Policy Syst [Internet]. 2024;22(1):43. Available from: https://health-policy-systems.biomedcentral.com/articles/10.1186/s12961-024-01114-w.
|
| 99. |
Klamser PP, D’Andrea V, Di Lauro F, Zachariae A, Bontorin S, Di Nardo A, et al. Enhancing global preparedness during an ongoing pandemic from partial and noisy data. Yortsos Y, editor. PNAS Nexus [Internet]. 2023;2(6). Available from: https://academic.oup.com/pnasnexus/article/doi/10.1093/pnasnexus/pgad192/7191545.
|
| 100. |
Gouglas D, Christodoulou M, Hatchett R. The 100 Days Mission—2022 Global Pandemic Preparedness Summit. Emerg Infect Dis [Internet]. 2023;29(3). Available from: https://wwwnc.cdc.gov/eid/article/29/3/22-1142_article.
|
| 101. |
United States of America - Influenza A viruses of high pathogenicity (Inf. with) (non-poultry including wild birds) (2017-) – Follow up report 75 [Internet]. 2024. Available from: https://wahis.woah.org/#/in-review/4451.
|











