Abstract
Vascular access is an essential component of coronary angiography and percutaneous coronary intervention. The choice of access site significantly impacts on procedural outcome. Distal transradial access (dTRA) via the anatomical snuffbox has emerged as an alternative to the conventional transradial approach. This article presents an analysis of dTRA, examining its anatomical considerations, procedural aspects, and clinical outcomes. While dTRA offers potential benefits such as improved patient comfort and a reduced risk of radial artery injury, challenges remain, including a steeper learning curve for operators and limitations in specific patient populations. This review aims to provide a comprehensive understanding of dTRA, enabling informed decisions regarding its adoption and advancement in contemporary cardiac catheterization.
Citation
Koziński Ł, Dąbrowska-Kugacka A, Orzałkiewicz Z. Distal transradial access via snuffbox for cardiac catheterization: a review. Eur J Transl Clin Med. 2024;7(2):59-77Introduction
Transradial access (TRA) has become the preferred approach for cardiac catheterization, surpassing femoral access due to its reduced local complications and improved patient comfort [1-8]. However, a novel technique known as distal transradial access (dTRA) has emerged, offering potential advantages over the conventional TRA (cTRA) [9-10]. The dTRA involves cannulating the radial artery (RA) in the anatomical snuffbox (also known as the radial fossa or fovea radialis), a triangular depression located at the base of the thumb.
This distal puncture site, compared to the traditional proximal RA access, provides distinct advantages [11-12].
The shift towards dTRA is motivated by its potential to reduce vascular complications, improve patient comfort and preserve the RA for future access [9-10, 13]. This preservation is critical, as the RA access may be required for subsequent procedures, including coronary or non-coronary interventions, arteriovenous fistula creation for hemodialysis or use as a graft in coronary surgery. These benefits stem from the anatomical characteristics of the RA in the snuffbox region [11]. This review aims to present the literature on dTRA through the snuffbox, focusing on its benefits, limitations and future applications beyond coronary procedures.
Material and methods
A literature search was conducted using PubMed and Google Scholar databases to identify relevant articles published in English about the use of dTRA. The search strategy incorporated a combination of keywords and their synonyms, including but not limited to: distal transradial access, dTRA, anatomical snuffbox access, distal radial artery access, cardiac catheterization, coronary angiography, percutaneous coronary intervention, PCI, transradial intervention.
Results
The search yielded 239 abstracts. After screening and a comprehensive assessment, 49 full-text articles (21 randomized controlled trials, 24 prospective and retrospective observational trials, 2 large registries and 2 meta-analyses) were deemed eligible for inclusion in this review.
Discussion
Historical aspects
The first mention of TRA in the literature dates back to 1948, when surgical cut-down provided access for aortic catheterization [14-15]. TRA for cardiac catheterization was pioneered by Campeau in 1989 and later developed by Kiemeneij and Larman in 1993 [16-17]. This was a paradigm shift in interventional cardiology because the TRA offers significant advantages over the traditional femoral approach, particularly in reducing access site complications [3-4, 8]. This has driven the widespread adoption of the TRA and led to continuous refinement of techniques and exploration of alternative access points along the RA.
The dTRA (also known as snuffbox access) emerged as a further advancement of the TRA. Kiemeneij is credited with pioneering this approach in 2017, recognizing the potential benefits of accessing the RA in the anatomical snuffbox [13]. Although the first mentions of accessing the dorsal RA for blood pressure monitoring in children and adults occurred nearly 50 years ago [18-19], and its usefulness for coronary and non-coronary procedures was reported by other researchers a few years before Kiemeneij, these early reports did not generate much attention or enthusiasm [20]. In 2011, Babunashvili and Dundua introduced a procedure utilizing dTRA access for retrograde recanalization of forearm RA within several days of an initial cTRA procedure complicated by occlusion [21].
Anatomy of the distal radial artery
This region, characterized by its superficial location and unique anatomical features, offered the possibility of minimizing vascular injury, enhancing patient comfort and preserving the proximal RA for future access. The key landmark for dTRA is the anatomical snuffbox, a triangular depression on the dorsolateral wrist which is best visualized when the thumb is extended. It is bordered by the tendons of the extensor pollicis longus (medially), extensor pollicis brevis and abductor pollicis longus (laterally) and the radial styloid process (proximally) [11, 22-23]. Its floor consists of the scaphoid and trapezium bones. The distal RA, the target for dTRA, passes through this region, located superficially beneath the skin (see Figure 1). This location facilitates easier access and potentially reduces the risk of deep punctures (see Figure 2).
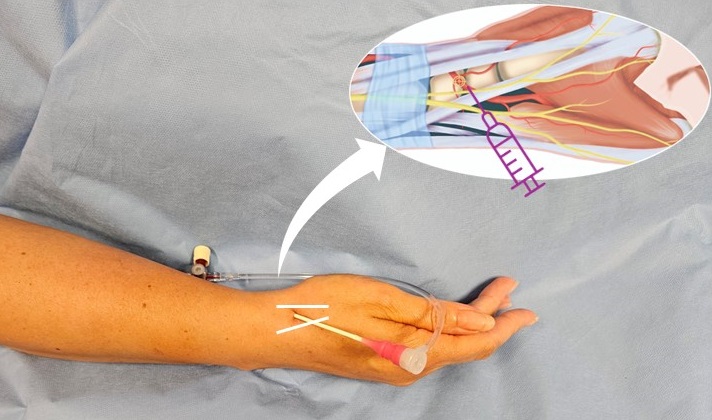
Figure 1. Distal transradial access via the anatomical snuffbox (fovea radialis)
White lines next to the arterial sheath indicate the boundaries of the snuffbox area: the tendon of the extensor pollicis longus (medially) and the tendons of the extensor pollicis brevis and abductor pollicis longus (laterally).
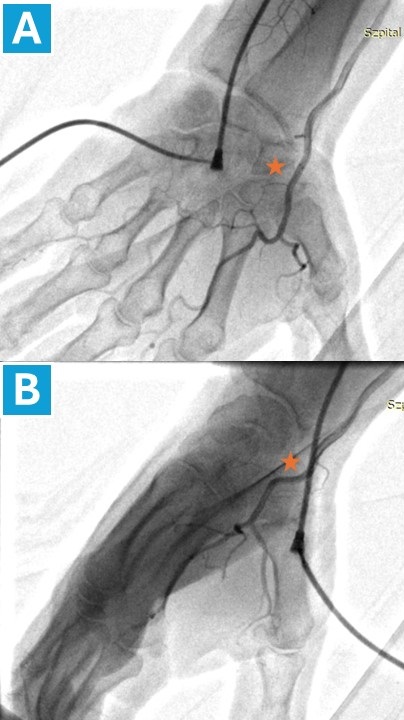
Figure 2. Angiography of the distal radial artery at the snuffbox
This angiogram was performed by administering contrast media through a sheath placed in the ulnar artery. The * symbol marks the site of distal radial artery puncture at the level of the snuffbox, visualized with the hand in pronation (A) and in a neutral position (B).
The RA’s distal anatomy contributes significantly to the safety of TRA. Near the anatomical snuffbox, the RA branches to form both the superficial palmar arch (anastomosing with the ulnar artery) and distally the deep palmar arch (anastomosing with the deep palmar branch of the ulnar artery). Interconnected by extensive collateral vessels, this robust dual-arch system ensures continued antegrade flow to the hand even if the distal RA is occluded, minimizing the risk of retrograde thrombosis [11, 22-25]. This anatomical characteristic is the key to its potential benefits in reducing radial artery occlusion (RAO):
- Preserved Blood Flow. Because the access site is located after the palmar arch branches off, therefore even if a thrombus were to form at the access site, blood flow to the hand can often continue through the palmar arch. This collateral circulation helps maintain perfusion and reduces the risk of hand ischemia.
- Lower Occlusion Risk. By preserving blood flow through the palmar arch, dTRA minimizes the duration of complete RAO during and after the procedure. This reduced occlusion time is considered significant factor in lowering the overall risk of RAO.
The snuffbox also houses the cephalic vein and branches of the radial nerve. The initial segment of the cephalic vein, is often prominent within the snuffbox. Branches of the radial nerve, responsible for sensation in the dorsal hand, are typically located deeper and lateral to the RA [11, 22-23]. While the superficial location of the RA in the snuffbox is advantageous, the smaller arterial caliber and proximity of tendons necessitate a thorough understanding of the anatomy for safe and successful cannulation without complications. It is important to distinguish dTRA within the snuffbox from the “very distal TRA,” which involves puncture distal to the snuffbox and carries additional anatomical considerations [26]. This “very dTRA” modification was not assessed in this review because of insufficient published evidence regarding its efficacy and safety.
RA size
The success of dTRA is significantly influenced by the diameter of the distal RA [27-30]. Studies show that the distal RA is significantly smaller than the proximal RA, with average diameters ranging from 1.70 to 2.99 mm [30-43]. Reported distal RA diameter measurements vary due to inconsistent methods, including measurement techniques, anatomical landmark identification, vessel diameter definition (inner vs. outer) and study conditions. Studies conducted in European populations have report an average distal RA diameter of approximately 2.30 mm [30, 37, 43]. A strong positive correlation (r = 0.66) exists between the distal RA size and forearm RA size, with the distal RA measuring 80-89% of the forearm segment’s dimension [30-32, 40]. Studies highlight a correlation between specific anthropometric and clinical factors and distal RA size. For instance, males have RA diameters that are on average 14% larger compared to females [30]. Furthermore, height, body weight, body mass index and body surface are consistently correlated with RA diameter [30, 32, 36, 40]. Certain comorbidities (e.g. diabetes and hypertension) are associated with smaller distal RA dimensions [40].
The reduced size of the distal RA, particularly in women and individuals with smaller body surface area, is correlated with technical difficulties for operators, potentially elevating the risk of complications (e.g. vasospasm or occlusion) [30]. Using larger vascular sheaths can exacerbate this challenge, as the size discrepancy between the sheath and the artery increases the likelihood of damage to the arterial wall and occlusion. Ultrasound-guided assessment of RA size, particularly comparing the distal and proximal segments and considering gender differences, is essential for appropriate patient selection, guiding procedural technique and minimizing complications [27]. In the absence of ultrasonography, a well-palpable distal RA pulse and a proximal RA pulse may indicate a satisfactory distal RA size, as there is a positive correlation between those two parameters (r = 0.5) [30].
The dTRA technique
Numerous studies were focused on refining the technique for dTRA, with a particular emphasis on optimizing patient comfort during the procedure [12-13]. Ultrasound plays a crucial role in assessing the suitability of dTRA by measuring the distal RA diameter and identifying potential anatomical challenges [27]. This pre-procedural assessment allows for informed decision-making and selection of the most appropriate access site.
Patient positioning is another important element of successful dTRA. For right-sided access, the patient’s arm should be placed neutrally alongside their body, with the lateral side facing upwards. Left-sided access can be achieved by either flexing the left hand medially towards the patient’s groin or abducting the left arm on a supportive board for easier access. The patient’s arm should be maintained in a neutral position throughout the procedure, which may be particularly beneficial for patients who are obese and those with limited range of motion in the arm. Both the left- and right-sided dTRA may improve the ergonomics of the procedure for both the patient (comfortable forearm position) and the operator (not leaning over the patient).
Once the patient is appropriately positioned, the operator should identify the optimal access site based on anatomical landmarks and the course of the distal RA [12-13]. A diminished RA pulse can be observed following the subcutaneous administration of an anesthetic (lidocaine or bipuvacaine), thus obscuring the ideal puncture site and necessitating additional access attempts. This may be attributable to anesthetic infiltration within the confined anatomical space of the snuffbox, potentially inducing vasospasm. Therefore, using smaller volumes of anesthetic (0.5-2.0 ml) are be recommended. The preferred technique for RA puncture is the modified Seldinger technique [12-13]. This approach involves directly accessing the arterial lumen with the needle and without traversing the posterior wall, as opposed to the through-and-through technique. Periosteal puncture at the base of the anatomical snuffbox can cause discomfort and potentially contribute to vasospasm and therefore should be avoided. Repeated punctures carry an inherent risk of iatrogenic arterial spasm, further complicating another puncture attempt. Therefore, maximizing first-attempt success is crucial. Due to the RA’s curvature, the needle’s angle relative to the skin will influence its entry angle into the vessel. To minimize the risk of arterial dissection, the needle should be slightly angled downwards (30o-45o) before advancing the guidewire. Due to their lubricity and flexibility, hydrophilic guidewires are recommended when navigating tortuous anatomy or encountering arterial spasm. If distal RA tortuosity impedes guidewire advancement, maneuvers such as pronating/supinating the hand or ulnar deviation can mitigate vessel curvature and facilitate passage. A combination of 2 or 3 spasmolytic drugs (typically nitroglycerin, verapamil or papaverine) effectively minimizes the risk of RA spasm.
Due to the anatomy of the distal RA, smaller arterial sheaths are generally preferred. Specifically, hydrophilic-coated slender sheaths are advantageous due to their smoother insertion. Thanks to their thin-walled design, the outer diameter is reduced by one French (Fr) size while preserving the inner diameter. Slender sheaths are commercially available in sizes 5 Fr, 6 Fr and 7 Fr.
Fluoroscopy or ultrasound guidance can be invaluable during guidewire insertion, particularly if there is any uncertainty about its positioning [27]. Visualizing the guidewire’s path helps ensure proper placement within the artery and minimizes the risk of complications. Additionally, the pressure waveform from the introducer sheath can provide further confirmation of successful access. Catheter selection for dTRA is determined by the same factors as for cTRA: patient anatomy and the particular procedure. In all patients undergoing dTRA, an additional 3-5 cm of catheter length should be factored in to accommodate the longer course of the artery, particularly in taller patients (> 185 cm in height). Therefore, diagnostic and guiding catheters exceeding the standard length of 100 cm are recommended. In clinical scenarios necessitating larger catheter sizes, a sheathless approach may be a viable alternative. Resistance during guidewire or catheter advancement at the level of the antecubital fossa may occasionally occur when using the left-sided access. This is attributable to arterial angulation secondary to joint flexion and transient forearm extension during passage typically mitigates this issue.
Learning curve of the dTRA
The adoption of dTRA has a significant learning curve [44-45]. Achieving and sustaining a high success rate (> 94%) required approximately 200 procedures [44]. As operator experience grew, both procedural duration and the number of access attempts decreased [44-45]. Furthermore, female sex (odds ratio (OR) 1.84, 95% confidence interval (CI) 1.01- -3.39, p = 0.049) and systolic blood pressure below 120 mmHg (OR 1.87, 95% CI 1.04-3.36, p = 0.036) were identified as independent predictors of unsuccessful dTRA cannulation [44]. These findings underscore the critical role of operator experience and careful patient selection in optimizing dTRA success.
Radial artery occlusion
Table 1 presents collected data (including RAO rates) from two large registries and the available prospective randomized clinical trials (RCTs) on dTRA [46-70]. RAO is the main complication of TRA, precluding future access to the RA. Reported rates of RAO after cTRA vary significantly in the literature, ranging from < 1% to as high as 33% [71]. This variability partly stems from differences in the timing and methods used to assess RA patency. A meta-analysis of RCTs revealed an overall incidence of early (within 24 hours) RAO of 7.7% and 5.5% after 1 week follow-up, highlighting the need for standardized reporting and assessment of this complication [72]. Over the years, a decline in RAO rates down to 3.7% has been observed, reflecting a growing awareness and implementation of best practices aimed at preventing this complication [72].
Table 1. Chronological list of large registries and randomized clinical trials comparing distal vs. conventional transradial access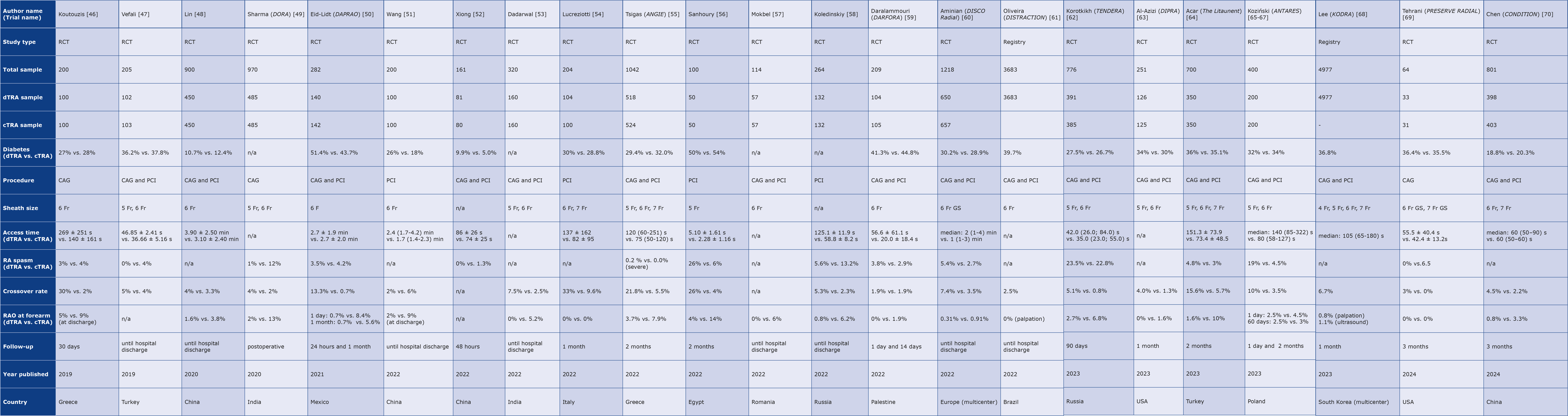
CAG – coronary angiography; cTRA – conventional transradial access; dTRA – distal transradial access; Fr – French (size); GS – glidesheath slender; n/a – not available; min – minute; PCI – percutaneous intervention; RCT – randomized controlled trial; s – second
The development of RAO is a complex process involving multiple contributing factors. Acute RAO occurs shortly after TRA, is primarily driven by arterial thrombosis due to a confluence of factors, including vessel wall injury induced by sheath and catheter manipulation, a localized hypercoagulable state and reduced blood flow due to hemostasis via compression [73]. In contrast, chronic RAO is characterized by a gradual thickening of the arterial wall, specifically the intimal and medial layers. This thickening results from the hyperplasia of vascular smooth muscle cells, representing a response to the initial injury [74]. Numerous randomized trials have established a clear understanding of the risk factors associated with RAO. These factors can be categorized as either modifiable or non-modifiable. Non-modifiable factors include female sex, low body mass index, diabetes and previous RA cannulation [72]. Modifiable risk factors for RAO include suboptimal sheath-to-artery ratio (> 1), inadequate anticoagulation, multiple unsuccessful puncture attempts, occlusive and/ or prolonged hemostasis and RA spasm [72, 75].
The PROPHET study demonstrated that implementing the so-called patent hemostasis strategy (hemostasis with the preservation of blood flow) during cTRA significantly reduced the incidence of both early and late RAO. This approach resulted in a 59% reduction in early RAO and a 75% reduction in late RAO [75]. Furthermore, the PROPHET-II trial revealed that prophylactic ipsilateral ulnar compression during RA hemostasis, in conjunction with a patent hemostasis protocol, led to a significant decrease in RAO rates at 30 days post-procedure [76]. This combined approach resulted in an occlusion rate of 0.9%, compared to 3.0% without ulnar artery compression. Discrepancies in reported RAO incidence rates are partly due to inconsistencies in assessment methods and the limitations of pulse palpation. Even in the presence of significant occlusion collateral circulation can often maintain a palpable pulse, leading to an underestimation of true RAO rates [77]. Accurate determination of RAO incidence necessitates more objective evaluation methods, such as doppler ultrasound.
It was precisely the need for the reduction of RAO after TRA that was the main driver of the switch to the dTRA access. Initial observational studies on small groups estimate the rate of in-hospital forearm RAO after dTRA at 0% to 7% [13, 45-46, 78-82]. The majority of RCTs have demonstrated a lower incidence of RAO following dTRA compared to cTRA. This difference is often statistically significant, with reported RAO rates ranging from 0% to 5% for dTRA versus 0% to 13% for cTRA [46-60, 62-65, 69-70]. Importantly, none of the trials have shown inferior RAO outcomes for dTRA compared to cTRA. The ANGIE study, a larger trial in a Greek population, demonstrated a significant reduction in RAO incidence at 60 days post-procedure with dTRA (3.7%) compared to cTRA (7.9%; p = 0.014) [55]. The multicenter DISCO RADIAL trial included 1218 participants in Europe and demonstrated remarkably low RAO rates in both the dTRA (0.31%) and cTRA (0.91%) groups when measured until hospital discharge [60]. Although the difference in RAO rates wasn’t statistically significant (p = 0.29), it is worth noting that both groups benefited from a strict hemostasis protocol adhering to current best practice recommendations, which likely contributed to the low overall RAO occurrence [60]. In the Brazilian DISTRACTION registry (3683 patients), there are no cases of RAO at discharge upon assessment by palpation [61]. A zero incidence rate, while impressive, might be influenced by the sensitivity of the detection method used. The South Korean KODRA registry is the largest (4977 patients) and reported a 0.8% RAO rate after 1 month detected upon palpation and a 1.1% rate when assessed by ultrasonography [68]. A recent meta-analysis of 15 randomized studies (7,196 patients, dTRA = 3,475, cTRA = 3,721), found a significantly lower risk of RAO in the dTRA group (1.8%) compared to the cTRA group (6.6%). This translates to a risk ratio of 0.31 (95% CI: 0.21-0.46, p < 0.001) favoring dTRA [9].
The anatomical advantages of dTRA likely contribute to its lower RAO rates. Even with distal RA occlusion during hemostasis, antegrade flow through the superficial palmar arch helps maintain perfusion and reduce retrograde thrombus propagation into the RA [13]. An experimental study simulating RAO in healthy subjects demonstrated a key difference between distal and proximal occlusion sites. While simulated occlusion at the wrist (proximal RA) led to significant flow reduction in the forearm RA, simulated occlusion in the anatomical snuffbox (distal RA) did not significantly impact flow [83]. Furthermore, the location of the superficial palmar branch within the anatomical snuffbox allows for efficient compression and faster hemostasis, further minimizing the risk of RAO development. Using thin-walled, hydrophilic sheaths during dTRA procedures can potentially lower the occurrence of RAO in both the forearm and the anatomical snuffbox [84].
The recently published prospective, multicenter ‘Open Radial Artery Study,’ might challenge the pursuit of novel dTRA techniques as its authors reported a reduction in RAO incidence after cTRA using a ‘patent hemostasis’ technique [85]. After 2 weeks, none of the 2181 patients who underwent the procedure experienced RAO. These impressive results were achieved by meticulously adhering to a protocol designed to minimize the risk of RAO using appropriate sheath sizes, anticoagulation, minimizing hemostasis time (60 ± 6 min) and verifying patency during hemostasis with plethysmography [85]. This highlights how that attention to such procedural details might be more crucial to reducing RAO occurrence than the choice of access site.
Non-RAO complications, access failure and access time
The use of dTRA has a high safety profile for non-RAO access-site complications, particularly bleeding. Real-world data collected after implementing best practices revealed a 3.3% incidence of dTRA-related bleeding. This breakdown included mild 1.1% BARC (Bleeding Academic Research Consortium) type 1 and 2.2% BARC type 2 bleeding [9, 68]. Importantly, there were no instances of severe or life-threatening bleeding events (BARC type 3 or 5) [68, 86]. Furthermore, mild hematoma (EASY grade I (Early Discharge After Transradial Stenting of Coronary Arteries Study)) occurred in only 3.1% of patients, while serious hematoma was exceptionally rare in a study population of approximately 5000 (68, 87). Other potential complications (e.g. pseudoaneurysm or arteriovenous fistula) were infrequent and often detected via ultrasound. When recognized early, they can typically be managed with extended compression at the radial fossa. [88-89]. Transient, mild neuropathy, presenting as thumb numbness within a few hours post-procedure, are observed in up to 29% of individuals, likely due to radial nerve irritation [65]. Interestingly, data from two studies incorporated in the meta-analysis showed significantly greater post-procedural pain in dTRA compared with cTRA, with no differences in pain while accessing the RA [9].
Undeniably, the dTRA is more technically demanding than cTRA, as evidenced by higher rates of access failure and crossover, typically to the ipsilateral proximal RA. Early reports cited crossover rates as high as 30% [46]. A recent meta-analysis of 18 RCTs showed a significantly greater relative risk of crossover with dTRA compared to cTRA (10.3% vs. 3.7%, p < 0.001) [9]. The KODRA registry and a largest randomized trial (DISCO RADIAL), reported crossover rates of 6.7% and 7.4%, respectively [60, 68]. Factors potentially contributing to access failure include unsuccessful arterial puncture, limited operator experience, weak RA pulse, smaller RA diameter, lower body mass index, vasospasm and possibly female sex [60, 65, 68].
While meta-analyses did not demonstrate a difference in RA spasm rates between dTRA and cTRA, some authors report a higher incidence with dTRA (up to 19% vs. 4.5% for cTRA). This discrepancy may stem from differences in RA vasospasm definition and diagnostic methods (ideally it should be confirmed through angiography, see Figure 3). Regardless of the definition used, RA vasospasm can contribute to access failure. A recent study found that applying a transdermal nitroglycerin patch on the puncture site before dTRA significantly increased first-attempt success rates for those using palpation-guided techniques [90]. This improvement, attributed to a likely reduction in RA spasm, was accompanied by a noticeable increase in the average diameter of the distal RA [90].
Figure 3. Severe distal radial artery spasm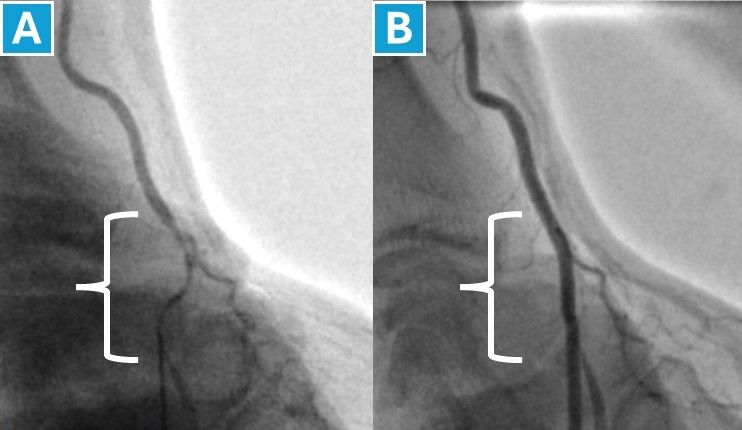
This angiogram demonstrates severe spasm of the distal radial artery (A), during an attempt to advance a standard 0.018” J-shaped miniguidewire following puncture with a 22-gauge needle. Contrast media was administered through the needle to visualize the spasm. Image (B) shows the same radial artery after the spasm resolved.
Although dTRA requires a significantly longer mean access time when compared with cTRA (2.6 ± 1.8 vs. 1.8 ± 1.3 min; p < 0.001), the overall procedure time, contrast volume use and fluoroscopy time are comparable between the two techniques [9]. The dTRA does not influence the motor or sensory function of the hand, both in early and long-term follow-up [91-92].
Hemostasis
Following sheath removal, hemostasis is typically achieved more rapidly with dTRA compared to cTRA [9, 12]. Traditional radial hemostasis relies on distal wrist immobility, which may not be sufficient for the more mobile dorsal hand [12]. A more secure method involves tamponade with a gauze plug and elastic bandage at the access site. Alternatively, a dedicated dTRA hemostatic device can be employed. Its advantages are secure positioning and precise compression, achieving results comparable to the traditional gauze and bandage technique. Currently, only one such dedicated dTRA hemostatic device is commercially available (Preclude SYNC DISTAL radial compression device by Merit Medical Systems) [28]. Although many operators report successful use of hemostasis devices designed for cTRA.
Despite general agreement that dTRA requires shorter hemostasis times to balance RAO risk without increasing access-site bleeding, no standardized protocol has been accepted in clinical practice. Published data on optimal hemostasis time varies widely, ranging from 135 ± 62 minutes in a recent meta-analysis to 180 minutes proposed by some operators [9, 93]. In the largest studies, including the KODRA registry and DISCO RADIAL, the hemostasis time was 153 minutes [60, 68]. Preliminary unpublished data from our group suggests even shorter durations are feasible, with 90, 60 and 30 minutes routinely applied after 7 Fr, 6 Fr, and 5 Fr thin-wall sheaths, respectively. This inconsistency highlights the need for further research to establish a standardized, evidence-based protocol for dTRA hemostasis.
Coronary and noncoronary procedures via dTRA
Experienced operators can successfully use the dTRA for a range of coronary interventions, from straightforward procedures to complex scenarios e.g. high-risk interventions, chronic total occlusion and even ST-elevation myocardial infarction [81, 94-97]. With appropriate patient selection, comparable access times and overall procedural success can be achieved in myocardial infarction cases via dTRA as with cTRA [81, 95-96].
Beyond its established role in coronary interventions, dTRA has shown promise in carotid interventions, limb ischemia treatment, pelvic organ procedures, neuroradiology and even perioperative blood pressure monitoring [52, 96-99]. Preliminary reports indicate both the safety and high success rates of these procedures. It is very interesting to see reports of dTRA use in structural heart disease procedures. Achim et al. successfully utilized bilateral dTRA with an 8 Fr sheath in 32 high-risk patients with severe symptomatic aortic stenosis undergoing balloon aortic valvuloplasty [100]. They achieved a 100% technical success rate and most patients were mobilized within 24 hours.
Conclusion
Distal transradial access can be a versatile technique for percutaneous interventions. The data suggests that in the hands of experienced operators and after implementation of best practices, the dTRA offers efficacy comparable to cTRA, with potentially lower rates of RA occlusion, shorter hemostasis time and reduced bleeding complications.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
Not applicable.
References
| 1. |
Lindner SM, McNeely CA, Amin AP. The value of transradial. Interv Cardiol Clin [Internet]. 2020;9(1):107–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211745819300598.
|
| 2. |
Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J [Internet]. 1999;138(3):430–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002870399701432.
|
| 3. |
Doll JA, Beaver K, Naranjo D, Waldo SW, Maynard C, Helfrich CD, et al. Trends in Arterial Access Site Selection and Bleeding Outcomes Following Coronary Procedures, 2011–2018. Circ Cardiovasc Qual Outcomes [Internet]. 2022;15(5). Available from: https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.121.008359.
|
| 4. |
Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, et al. Trends in U.S. Cardiovascular Care. J Am Coll Cardiol [Internet]. 2017;69(11):1427–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109716373387.
|
| 5. |
Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, et al. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv [Internet]. 2018;11(9). Available from: https://www.ahajournals.org/doi/10.1161/HCV.0000000000000035.
|
| 6. |
Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J [Internet]. 2019;40(2):87–165. Available from: https://academic.oup.com/eurheartj/article/40/2/87/5079120.
|
| 7. |
Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic Review and Cost–Benefit Analysis of Radial Artery Access for Coronary Angiography and Intervention. Circ Cardiovasc Qual Outcomes [Internet]. 2012;5(4):454–62. Available from: https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.112.965269.
|
| 8. |
Ferrante G, Rao S V., Jüni P, Da Costa BR, Reimers B, Condorelli G, et al. Radial Versus Femoral Access for Coronary Interventions Across the Entire Spectrum of Patients With Coronary Artery Disease. JACC Cardiovasc Interv [Internet]. 2016;9(14):1419–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879816304289.
|
| 9. |
Mufarrih SH, Haider S, Qureshi NQ, Khan MS, Kazimuddin M, Akbar MS, et al. Distal Versus Proximal Radial Arterial Access for Percutaneous Coronary Angiography and Intervention: Updated Meta-Analysis of Randomized Controlled Trials. Am J Cardiol [Internet]. 2024;218:34–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914924001565.
|
| 10. |
Bittar V, Trevisan T, Clemente MRC, Pontes G, Felix N, Gomes WF. Distal versus traditional radial access in patients undergoing emergency coronary angiography or percutaneous coronary intervention: a systematic review and meta-analysis. Coron Artery Dis [Internet]. 2024; Available from: https://journals.lww.com/10.1097/MCA.0000000000001411.
|
| 11. |
Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv [Internet]. 2018;11(20):2113–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879818310331.
|
| 12. |
Corcos T. Distal radial access for coronary angiography and percutaneous coronary intervention: A state‐of‐the‐art review. Catheter Cardiovasc Interv [Internet]. 2019;93(4):639–44. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.28016.
|
| 13. |
Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention [Internet]. 2017;13(7):851–7. Available from: http://www.pcronline.com/eurointervention/122nd_issue/124.
|
| 14. |
Radner S. Thoracal Aortography by Catheterization from the Radlal Artery. Acta radiol [Internet]. 1948;29(2):178–80. Available from: http://www.tandfonline.com/doi/full/10.3109/00016924809132437.
|
| 15. |
Rao S V., Stone GW. Arterial access and arteriotomy site closure devices. Nat Rev Cardiol [Internet]. 2016;13(11):641–50. Available from: https://www.nature.com/articles/nrcardio.2016.133.
|
| 16. |
Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn [Internet]. 1989;16(1):3–7. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.1810160103.
|
| 17. |
Kiemeneij F, Jan Laarman G. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn [Internet]. 1993;30(2):173–8. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.1810300220.
|
| 18. |
Amato JJ, Solod E, Cleveland RJ. A “second” radial artery for monitoring the perioperative pediatric cardiac patient. J Pediatr Surg [Internet]. 1977;12(5):715–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/0022346877903992.
|
| 19. |
Pyles ST, Scher KS, Vega ET, Harrah JD, Rubis LJ. Cannulation of the Dorsal Radial Artery: A New Technique. Anesth Analg [Internet]. 1982;61(10):876–8. Available from: https://journals.lww.com/anesthesia-analgesia/fulltext/1982/10000/cannulation_of_the_dorsal_radial_artery__a_new.15.aspx.
|
| 20. |
Kaledin A, Kochanov I, Podmetin P, Seletsky S, Ardeev V. Distal radial artery in endovascular interventions. 2017.
|
| 21. |
Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Catheter Cardiovasc Interv [Internet]. 2011;77(4):530–6. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.22846.
|
| 22. |
Nguyen JD, Black AC, Duong H. Anatomy, Shoulder and Upper Limb, Hand Arteries. In: StatPearls [Internet]. Florida Atlantic University: StatPearls Publishing, Treasure Island (FL); 2023. Available from: http://europepmc.org/abstract/MED/31536192.
|
| 23. |
Marchese RM, Black AC, Geiger Z. Anatomy, Shoulder and Upper Limb, Forearm Radial Artery. In: StatPearls [Internet]. Albany Medical Center: StatPearls Publishing, Treasure Island (FL); 2023. Available from: http://europepmc.org/abstract/MED/31536233.
|
| 24. |
Cai G, Huang H, Li F, Shi G, Yu X, Yu L. Distal transradial access: a review of the feasibility and safety in cardiovascular angiography and intervention. BMC Cardiovasc Disord [Internet]. 2020;20(1):356. Available from: https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-020-01625-8.
|
| 25. |
Liontou C, Kontopodis E, Oikonomidis N, Maniotis C, Tassopoulos A, Tsiafoutis I, et al. Distal Radial Access: A Review Article. Cardiovasc Revascularization Med [Internet]. 2020;21(3):412–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1553838919303367.
|
| 26. |
Wretowski D, Krakowian M, Łabyk A, Pruszczyk P, Roik M. Very distal transradial approach (VITRO) for coronary interventions. Adv Interv Cardiol [Internet]. 2019;15(1):42–5. Available from: https://www.termedia.pl/doi/10.5114/aic.2019.83771.
|
| 27. |
Achim A, Péter OÁ, Kákonyi K, Sasi V, Nemes A, Homorodean C, et al. The Role of Ultrasound in Accessing the Distal Radial Artery at the Anatomical Snuffbox for Cardiovascular Interventions. Life [Internet]. 2022;13(1):25. Available from: https://www.mdpi.com/2075-1729/13/1/25.
|
| 28. |
Kawamura Y, Yoshimachi F, Nakamura N, Yamamoto Y, Kudo T, Ikari Y. Impact of dedicated hemostasis device for distal radial arterial access with an adequate hemostasis protocol on radial arterial observation by ultrasound. Cardiovasc Interv Ther [Internet]. 2021;36(1):104–10. Available from: http://link.springer.com/10.1007/s12928-020-00656-4.
|
| 29. |
Goldman DT, Bageac D, Mills A, Yim B, Yaeger K, Majidi S, et al. Transradial Approach for Neuroendovascular Procedures: A Single-Center Review of Safety and Feasibility. Am J Neuroradiol [Internet]. 2021;42(2):313–8. Available from: http://www.ajnr.org/lookup/doi/10.3174/ajnr.A6971.
|
| 30. |
Koziński Ł, Orzałkiewicz Z, Zagożdżon P, Dąbrowska-Kugacka A. Distal Transradial Access Optimization: A Prospective Trial of Ultrasound-Guided Radial Artery Characterization for the Anatomical Snuffbox. Diagnostics [Internet]. 2024;14(18):2081. Available from: https://www.mdpi.com/2075-4418/14/18/2081.
|
| 31. |
Lee J-W, Son J-W, Go T-H, Kang DR, Lee SJ, Kim SE, et al. Reference diameter and characteristics of the distal radial artery based on ultrasonographic assessment. Korean J Intern Med [Internet]. 2022;37(1):109–18. Available from: http://kjim.org/journal/view.php?doi=10.3904/kjim.2020.685.
|
| 32. |
Norimatsu K, Kusumoto T, Yoshimoto K, Tsukamoto M, Kuwano T, Nishikawa H, et al. Importance of measurement of the diameter of the distal radial artery in a distal radial approach from the anatomical snuffbox before coronary catheterization. Heart Vessels [Internet]. 2019;34(10):1615–20. Available from: http://link.springer.com/10.1007/s00380-019-01404-2.
|
| 33. |
Mizuguchi Y, Izumikawa T, Hashimoto S, Yamada T, Taniguchi N, Nakajima S, et al. Efficacy and safety of the distal transradial approach in coronary angiography and percutaneous coronary intervention: a Japanese multicenter experience. Cardiovasc Interv Ther [Internet]. 2020;35(2):162–7. Available from: http://link.springer.com/10.1007/s12928-019-00590-0.
|
| 34. |
Yu W, Hu P, Wang S, Yao L, Wang H, Dou L, et al. Distal radial artery access in the anatomical snuffbox for coronary angiography and intervention. Medicine (Baltimore) [Internet]. 2020;99(3):e18330. Available from: https://journals.lww.com/10.1097/MD.0000000000018330.
|
| 35. |
Wang H, Peng W-J, Liu Y-H, Ma G-Q, Wang D, Su B, et al. A comparison of the clinical effects and safety between the distal radial artery and the classic radial artery approaches in percutaneous coronary intervention. Ann Palliat Med [Internet]. 2020;9(5):2568–74. Available from: http://apm.amegroups.com/article/view/47682/html.
|
| 36. |
Meo D, Falsaperla D, Modica A, Calcagno MC, Libra F, Desiderio C, et al. Proximal and distal radial artery approaches for endovascular percutaneous procedures: anatomical suitability by ultrasound evaluation. Radiol Med [Internet]. 2021;126(4):630–5. Available from: http://link.springer.com/10.1007/s11547-020-01299-4.
|
| 37. |
Achim A, Kákonyi K, Jambrik Z, Nagy F, Tóth J, Sasi V, et al. Distal Radial Artery Access for Coronary and Peripheral Procedures: A Multicenter Experience. J Clin Med [Internet]. 2021;10(24):5974. Available from: https://www.mdpi.com/2077-0383/10/24/5974.
|
| 38. |
Deora S, Sharma SK, Choudhary R, Kaushik A, Garg PK, Khera PS, et al. Assessment and comparison of distal radial artery diameter in anatomical snuff box with conventional radial artery before coronary catheterization. Indian Heart J [Internet]. 2022;74(4):322–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0019483222001031.
|
| 39. |
Kim Y, Ahn Y, Kim MC, Sim DS, Hong YJ, Kim JH, et al. Gender differences in the distal radial artery diameter for the snuffbox approach. Cardiol J [Internet]. 2018;25(5):639–41. Available from: https://journals.viamedica.pl/cardiology_journal/article/view/59709.
|
| 40. |
Chen T, Yu X, Song R, Li L, Cai G. Application of ultrasound in cardiovascular intervention via the distal radial artery approach: New wine in old bottles? Front Cardiovasc Med [Internet]. 2022;9. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1019053/full.
|
| 41. |
Hadjivassiliou A, Cardarelli-Leite L, Jalal S, Chung J, Liu D, Ho S, et al. Left Distal Transradial Access (ldTRA): A Comparative Assessment of Conventional and Distal Radial Artery Size. Cardiovasc Intervent Radiol [Internet]. 2020;43(6):850–7. Available from: https://link.springer.com/10.1007/s00270-020-02485-7.
|
| 42. |
Tehrani BN, Sherwood MW, Damluji AA, Epps KC, Bakhshi H, Cilia L, et al. A Randomized Comparison of Radial Artery Intimal Hyperplasia Following Distal Versus Proximal Transradial Access for Coronary Angiography: PRESERVE RADIAL. J Am Heart Assoc [Internet]. 2024;13(4):e031504. Available from: https://doi.org/10.1161/JAHA.123.031504.
|
| 43. |
Horák D, Bernat I, Jirouš Š, Slezák D, Rokyta R. Distal radial access and postprocedural ultrasound evaluation of proximal and distal radial artery. Cardiovasc Interv Ther [Internet]. 2022;37(4):710–6. Available from: https://link.springer.com/10.1007/s12928-022-00857-z.
|
| 44. |
Roh JW, Kim Y, Lee O-H, Im E, Cho D-K, Choi D, et al. The learning curve of the distal radial access for coronary intervention. Sci Rep [Internet]. 2021;11(1):13217. Available from: https://www.nature.com/articles/s41598-021-92742-7.
|
| 45. |
Lee J-W, Park SW, Son J-W, Ahn S-G, Lee S-H. Real-world experience of the left distal transradial approach for coronary angiography and percutaneous coronary intervention: a prospective observational study (LeDRA). EuroIntervention [Internet]. 2018 Oct;14(9):e995–1003. Available from: http://www.pcronline.com/eurointervention/142nd_issue/179.
|
| 46. |
Koutouzis M, Kontopodis E, Tassopoulos A, Tsiafoutis I, Katsanou K, Rigatou A, et al. Distal Versus Traditional Radial Approach for Coronary Angiography. Cardiovasc Revascularization Med [Internet]. 2019;20(8):678–80. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1553838918304391.
|
| 47. |
Vefalı V, Sarıçam E. The Comparison of Traditional Radial Access and Novel Distal Radial Access for Cardiac Catheterization. Cardiovasc Revascularization Med [Internet]. 2020;21(4):496–500. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1553838919303781.
|
| 48. |
Lin Y, Sun X, Chen R, Liu H, Pang X, Chen J, et al. Feasibility and Safety of the Distal Transradial Artery for Coronary Diagnostic or Interventional Catheterization. Musialek P, editor. J Interv Cardiol [Internet]. 2020 Dec;2020:1–6. Available from: https://www.hindawi.com/journals/jitc/2020/4794838/.
|
| 49. |
Sharma AK, Razi MM, Prakash N, Sharma A, Sarraf S, Sinha S, et al. A comparative assessment of Dorsal radial artery access versus classical radial artery access for percutaneous coronary angiography-a randomized control trial (DORA trial). Indian Heart J [Internet]. 2020;72(5):435–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0019483220301292.
|
| 50. |
Eid-Lidt G, Rivera Rodríguez A, Jimenez Castellanos J, Farjat Pasos JI, Estrada López KE, Gaspar J. Distal Radial Artery Approach to Prevent Radial Artery Occlusion Trial. JACC Cardiovasc Interv [Internet]. 2021;14(4):378–85. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879820320963.
|
| 51. |
Wang Y, Liu Z, Wu Y, Li Z, Wang Y, Wang S, et al. Early prevention of radial artery occlusion via distal transradial access for primary percutaneous coronary intervention. Front Cardiovasc Med [Internet]. 2022;9. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1071575/full.
|
| 52. |
Xiong J, Hui K, Xu M, Zhou J, Zhang J, Duan M. Distal radial artery as an alternative approach to forearm radial artery for perioperative blood pressure monitoring: a randomized, controlled, noninferiority trial. BMC Anesthesiol [Internet]. 2022;22(1):67. Available from: https://bmcanesthesiol.biomedcentral.com/articles/10.1186/s12871-022-01609-5.
|
| 53. |
Dadarwal A, Garg N, Kapoor A, Tewari S, Kumar S, Khanna R, et al. Randomized comparison of proximal and distal radial access for coronary angiography and interventions. Eur Heart J [Internet]. 2022;43(Supplement_1). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehab849.128/6521101.
|
| 54. |
Lucreziotti S, Persampieri S, Gentile D, Barbieri L, Salerno-Uriarte D, Valli F, et al. Access-site hematoma in distal and conventional transradial access: a randomized trial. Minerva Cardiol Angiol [Internet]. 2022;70(2):129–37. Available from: https://www.minervamedica.it/index2.php?show=R05Y2022N02A0129.
|
| 55. |
Tsigkas G, Papageorgiou A, Moulias A, Kalogeropoulos AP, Papageorgopoulou C, Apostolos A, et al. Distal or Traditional Transradial Access Site for Coronary Procedures. JACC Cardiovasc Interv [Internet]. 2022;15(1):22–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879821018227.
|
| 56. |
Sanhoury MI, Sobhy MA, Saddaka MA, Nassar MA, Elwany MN. Distal radial approach between theory and clinical practice.. Time to go distal! Egypt Hear J [Internet]. 2022;74(1):8. Available from: https://tehj.springeropen.com/articles/10.1186/s43044-022-00243-3.
|
| 57. |
Mokbel M, Sinescu C, Florescu N. P4398Snuff-box versus distal forearm for trans-radial access: performance and radial patency. Eur Heart J [Internet]. 2018;39(suppl_1). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehy563.P4398/5083543.
|
| 58. |
Koledinskiy A., Mikheeva YU., Ogurtsov P., Kurtasov D., Vyazova N. Hospital results of endovascular treatment of patients with Acute Coronary Syndrome (ACS) through distal radial access. Eur Heart J [Internet]. 2020;41(Supplement_2). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/ehjci/ehaa946.2498/6004231.
|
| 59. |
Daralammouri Y, Nazzal Z, Mosleh YS, Abdulhaq HK, Khayyat ZY, Hamshary Y El, et al. Distal Radial Artery Access in comparison to Forearm Radial Artery Access for Cardiac Catheterization: A Randomized Controlled Trial (DARFORA Trial). Rigattieri S, editor. J Interv Cardiol [Internet]. 2022 Jul;2022:1–9. Available from: https://www.hindawi.com/journals/jitc/2022/7698583/.
|
| 60. |
Aminian A, Sgueglia GA, Wiemer M, Kefer J, Gasparini GL, Ruzsa Z, et al. Distal Versus Conventional Radial Access for Coronary Angiography and Intervention. JACC Cardiovasc Interv [Internet]. 2022;15(12):1191–201. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879822008974.
|
| 61. |
Oliveira MD, Navarro EC, Caixeta A. Distal transradial access for coronary procedures: a prospective cohort of 3,683 all-comers patients from the DISTRACTION registry. Cardiovasc Diagn Ther [Internet]. 2022;12(2):208–19. Available from: https://cdt.amegroups.com/article/view/91862/html.
|
| 62. |
Korotkikh A, Babunashvili A, Kaledin A, Akhramovich R, Derkach V, Portnov R, et al. Distal Radiation Access as an Alternative to Conventional Radial Access for Coronary Angiography and Percutaneous Coronary Interventions (According to TENDERA Trial). Curr Probl Cardiol [Internet]. 2023;48(4):101546. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0146280622004431.
|
| 63. |
Al‐Azizi K, Moubarak G, Dib C, Sayfo S, Szerlip M, Thomas S, et al. Distal Versus Proximal Radial Artery Access for Cardiac Catheterization: 30‐Day Outcomes of the DIPRA Study. J Am Heart Assoc [Internet]. 2023;12(21). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.123.030774.
|
| 64. |
Acar E, Izci S, Donmez I, Yilmaz MF, Ozgul N, Kayabası O, et al. The L eft D i stal t ransradial a ccess site co u ld give a safe alter n ate sit e for tra n sradial coronary in t ervention (The Litaunent Study). Angiology [Internet]. 2024;75(5):425–33. Available from: https://journals.sagepub.com/doi/10.1177/00033197231183226.
|
| 65. |
Koziński Ł, Orzałkiewicz Z, Dąbrowska-Kugacka A. Feasibility and Safety of the Routine Distal Transradial Approach in the Anatomical Snuffbox for Coronary Procedures: The ANTARES Randomized Trial. J Clin Med [Internet]. 2023;12(24):7608. Available from: https://www.mdpi.com/2077-0383/12/24/7608.
|
| 66. |
Kozinski L, Dabrowska-Kugacka A, Orzalkiewicz Z. Routine snuffbox approach do not reduce radial artery occlusion in comparison with conventional transradial access. Eur Heart J [Internet]. 2021;42(Supplement_1). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/eurheartj/ehab724.2129/6394067.
|
| 67. |
Kozinski L, Dabrowska-Kugacka A, Orzalkiewicz Z. Distal radial approach via anatomical snuffbox is associated with higer rate of radial spasm and greater patient discomfort. Eur Heart J [Internet]. 2020;41(Supplement_2). Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/ehjci/ehaa946.2502/6004267.
|
| 68. |
Lee J-W, Kim Y, Lee B-K, Yoo S-Y, Lee SY, Kim CJ, et al. Distal Radial Access for Coronary Procedures in a Large Prospective Multicenter Registry. JACC Cardiovasc Interv [Internet]. 2024;17(3):329–40. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879823015479.
|
| 69. |
Tehrani BN, Sherwood MW, Damluji AA, Epps KC, Bakhshi H, Cilia L, et al. A Randomized Comparison of Radial Artery Intimal Hyperplasia Following Distal Versus Proximal Transradial Access for Coronary Angiography: PRESERVE RADIAL. J Am Heart Assoc [Internet]. 2024;13(4). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.123.031504.
|
| 70. |
Chen T, Li L, Li F, Lu W, Shi G, Li W, et al. Comparison of long-term radial artery occlusion via distal vs. conventional transradial access (CONDITION): a randomized controlled trial. BMC Med [Internet]. 2024;22(1):62. Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-024-03281-7.
|
| 71. |
Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, et al. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention. JACC Cardiovasc Interv [Internet]. 2019;12(22):2235–46. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879819316516.
|
| 72. |
Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta‐Analysis. J Am Heart Assoc [Internet]. 2016;5(1). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.115.002686.
|
| 73. |
Mamas MA, Fraser DG, Ratib K, Fath-Ordoubadi F, El-Omar M, Nolan J, et al. Minimising radial injury: prevention is better than cure. EuroIntervention [Internet]. 2014;10(7):824–32. Available from: http://www.pcronline.com/eurointervention/78th_issue/142.
|
| 74. |
Yonetsu T, Kakuta T, Lee T, Takayama K, Kakita K, Iwamoto T, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J [Internet]. 2010;31(13):1608–15. Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehq102.
|
| 75. |
Pancholy S, Coppola J, Patel T, Roke‐Thomas M. Prevention of radial artery occlusion—Patent hemostasis evaluation trial (PROPHET study): A randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv [Internet]. 2008;72(3):335–40. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.21639.
|
| 76. |
Pancholy SB, Bernat I, Bertrand OF, Patel TM. Prevention of Radial Artery Occlusion After Transradial Catheterization. JACC Cardiovasc Interv [Internet]. 2016;9(19):1992–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879816310962.
|
| 77. |
van Leeuwen MAH, Hollander MR, van der Heijden DJ, van de Ven PM, Opmeer KHM, Taverne YJHJ, et al. The ACRA Anatomy Study (Assessment of Disability After Coronary Procedures Using Radial Access). Circ Cardiovasc Interv [Internet]. 2017;10(11). Available from: https://www.ahajournals.org/doi/10.1161/CIRCINTERVENTIONS.117.005753.
|
| 78. |
Koziński Ł, Dąbrowska-Kugacka A, Orzałkiewicz Z. Coronary intervention via novel distal radial artery approach. Adv Interv Cardiol [Internet]. 2019;15(1):123–4. Available from: https://www.termedia.pl/doi/10.5114/aic.2019.83779.
|
| 79. |
Aoi S, Htun WW, Freeo S, Lee S, Kyaw H, Alfaro V, et al. Distal transradial artery access in the anatomical snuffbox for coronary angiography as an alternative access site for faster hemostasis. Catheter Cardiovasc Interv [Internet]. 2019;94(5):651–7. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.28155.
|
| 80. |
Oliveira MDP, Navarro EC, Kiemeneij F. Distal transradial access as default approach for coronary angiography and interventions. Cardiovasc Diagn Ther [Internet]. 2019;9(5):513–9. Available from: http://cdt.amegroups.com/article/view/30250/26116.
|
| 81. |
Kim Y, Lee J-W, Lee SY, Bae J-W, Lee SJ, Jeong MH, et al. Feasibility of primary percutaneous coronary intervention via the distal radial approach in patients with ST-elevation myocardial infarction. Korean J Intern Med [Internet]. 2021;36(Suppl 1):S53–61. Available from: http://kjim.org/journal/view.php?doi=10.3904/kjim.2019.420.
|
| 82. |
Achim A, Szigethy T, Olajos D, Molnár L, Papp R, Bárczi G, et al. Switching From Proximal to Distal Radial Artery Access for Coronary Chronic Total Occlusion Recanalization. Front Cardiovasc Med [Internet]. 2022;9. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2022.895457/full.
|
| 83. |
Sgueglia GA, Santoliquido A, Gaspardone A, Di Giorgio A. First Results of the Distal Radial Access Doppler Study. JACC Cardiovasc Imaging [Internet]. 2021;14(6):1281–3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936878X20310949.
|
| 84. |
Lu W, Chen T, Wang H, Yang A, Li L, Shi G, et al. Comparison of the Effect of a 6-French Glidesheath Slender and a Conventional Sheath on Distal Radial Artery Occlusion: A Randomized Controlled Trial. Can J Cardiol [Internet]. 2024; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0828282X24005634.
|
| 85. |
Inanc IH, Mutlu D, Efe ZN, Kulaksızoglu S, Marmagkiolis K, Iliescu C, et al. Open Radial Artery Study. Am J Cardiol [Internet]. 2024;211:130–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914923010342.
|
| 86. |
Mehran R, Rao S V., Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. Circulation [Internet]. 2011;123(23):2736–47. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.110.009449.
|
| 87. |
Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: Prevention is better than treatment! Catheter Cardiovasc Interv [Internet]. 2010;75(3):366–8. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ccd.22448.
|
| 88. |
Koziński Ł, Dąbrowska-Kugacka A, Orzalkiewicz Z. Successful management of arteriovenous fistula after coronary catheterization via the snuffbox approach. Cardiol J [Internet]. 2020;27(2):200–1. Available from: https://journals.viamedica.pl/cardiology_journal/article/view/65172.
|
| 89. |
Oliveira MDP, Alves de Sá G, Navarro EC, Caixeta A. Pseudoaneurysm After Distal Transradial Coronary Intervention Successfully Managed by Prolonged Pneumatic Compression: Simple Solution for a Rare and Challenging Problem. J Invasive Cardiol [Internet]. 2021;33(10):E836–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34609331.
|
| 90. |
Murai K, Fujino M, Iwai T, Sawada K, Matama H, Miura H, et al. Distal Radial Approach in Coronary Angiography Using a Transdermal Nitroglycerin Patch: Double-Blinded Randomized Trial. Am J Cardiol [Internet]. 2023;203:325–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914923005982.
|
| 91. |
Al-Azizi K, Moubarak G, Dib C, Sayfo S, Szerlip M, Thomas S, et al. Distal Versus Proximal Radial Artery Access for Cardiac Catheterization: 1-Year Outcomes. Am J Cardiol [Internet]. 2024;220:102–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914924001590.
|
| 92. |
Sgueglia GA, Hassan A, Harb S, Ford TJ, Koliastasis L, Milkas A, et al. International Hand Function Study Following Distal Radial Access. JACC Cardiovasc Interv [Internet]. 2022;15(12):1205–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S193687982200841X.
|
| 93. |
Roh JW, Kim Y, Takahata M, Shiono Y, Kim H-Y, Jeong MH, et al. Optimal hemostasis duration for percutaneous coronary intervention via the snuffbox approach: A prospective, multi-center, observational study (HEMOBOX). Int J Cardiol [Internet]. 2021;338:79–82. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167527321010330.
|
| 94. |
Gasparini GL, Garbo R, Gagnor A, Oreglia J, Mazzarotto P. First prospective multicentre experience with left distal transradial approach for coronary chronic total occlusion interventions using a 7 Fr Glidesheath Slender. EuroIntervention [Internet]. 2019;15(1):126–8. Available from: http://www.pcronline.com/eurointervention/152nd_issue/23.
|
| 95. |
Soydan E, Akın M. Applicability of left distal radial artery access site in ST-segment elevation myocardial infarction; A comparative evaluation with the conventional transfemoral approach. J Vasc Access [Internet]. 2022;23(1):81–7. Available from: http://journals.sagepub.com/doi/10.1177/1129729820983138.
|
| 96. |
Cao G, Cai H-X, Cao J. Advancement in Coronary Angiography or Percutaneous Coronary Intervention Using the Distal Transradial Artery Access in Acute Coronary Syndrome and Complex Coronary Artery Disease. Anatol J Cardiol [Internet]. 2022;26(3):163–71. Available from: https://anatoljcardiol.com/article/AJC-65069.
|
| 97. |
Chu HH, Kim JW, Shin JH, Cho SB. Update on Transradial Access for Percutaneous Transcatheter Visceral Artery Embolization. Korean J Radiol [Internet]. 2021;22(1):72. Available from: https://kjronline.org/DOIx.php?id=10.3348/kjr.2020.0209.
|
| 98. |
Manzoor MU, Alrashed AA, Almulhim IA, Althubait S, Al-Qahtani SM, Al-Senani F, et al. Exploring the path less traveled: Distal radial access for diagnostic and interventional neuroradiology procedures. J Clin Neurosci [Internet]. 2021;90:279–83. Available from: https://linkinghub.elsevier.com/retrieve/pii/S096758682100309X.
|
| 99. |
Kühn AL, Singh J, Moholkar VM, Satti SR, Rodrigues K de M, Massari F, et al. Distal radial artery (snuffbox) access for carotid artery stenting – Technical pearls and procedural set-up. Interv Neuroradiol [Internet]. 2021;27(2):241–8. Available from: http://journals.sagepub.com/doi/10.1177/1591019920959537.
|
| 100. |
Achim A, Szűcsborus T, Sasi V, Nagy F, Jambrik Z, Nemes A, et al. Safety and Feasibility of Distal Radial Balloon Aortic Valvuloplasty. JACC Cardiovasc Interv [Internet]. 2022;15(6):679–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936879821022251.
|











