Association of hand grip strength with psychological stress, exercise habits and body composition amongst medical students: a cross-sectional study
Abstract
Background: The aim of this study was to assess the effectiveness of hand grip strength (HGS) test in identifying highly stressed individuals and to examine the effect of exercise and lifestyle on HGS and stress measures.
Material and method: It is cross-sectional study. Students of the Medical University of Gdańsk, Poland were asked to fill out a questionnaire, undergo body composition analysis, perform HGS test and provide a saliva sample for cortisol measurement.
Results: Self-rated stress (SRS) was significantly higher in pre-clinical years (PCY) compared to clinical years (CY). HGS was significantly lower in PCY males than CY males. Participants who performed some form of exercise had significantly higher HGS compared with those who did not exercise. A positive correlation between HGS and BMI was noted. Students with low HGS were found to have lower levels of salivary cortisol (SC). However, there was no significant difference in SC levels between PCY and CY students.
Conclusions: HGS may be a reliable method of identifying stressed individuals and promoting healthy lifestyle behaviors. HGS testing is a safe, cheap and easy to perform method for a large number of participants while being time economical.
Citation
Barre S, Inyingi L, Castellanos J O, Patel A, Ruckemann-Dziurdzinska K A, Bryl E, Witkowski J M. Association of hand grip strength with psychological stress, exercise habits and body composition amongst medical students: a cross-sectional study. Eur J Transl Clin Med. 2024;7(1):33-46Abbreviations
- BMI – body mass index
- CY – clinical years
- ELISA – enzyme-linked immunosorbent assay
- HGS – hand grip strength
- HPAA – hypothalamic-pituitary-adrenal axis
- MUG – Medical University of Gdańsk
- PCY – pre-clinical years
- SC – salivary cortisol
- SRS – self-rated stress
Introduction
It is a widely accepted that medical studies are challenging and can put medical students under high stress. Several factors contribute to the psychological stress amongst medical students, such as their competitive environment, hierarchical student-teacher/consultant relationships, personal and social expectations, reduced leisure time, facing death and suffering [1-3]. According to the literature, nearly a 1/3 of medical students are suffering from psychological distress, depression and/or anxiety [4-6]. Exposure to chronic levels of stress may have detrimental effects on the individual’s physical, mental and immunological functioning and predispose them to chronic illnesses, e.g. cardiovascular diseases, diabetes, depression, metabolic syndrome and autoimmune diseases [7-8].
Hand grip strength (HGS) has become an increasingly popular measurement to assess the physical fitness and well-being, particularly in the elderly population [9]. It is defined as the force generated by squeezing one’s hand. HGS indicates strength , muscle mass and protein levels, thereby may be used clinically as a proxy for overall health profile [10]. A hand-held dynamometer is a simple, inexpensive and reliable tool for HGS measurement [11]. In the elderly population, HGS was found to predict the risk of all-cause mortality as well as disease-specific causes such as cardiovascular diseases, cancer, strokes and others [10-12]. HGS is also associated with injury from falls, cognitive function, depression, age-related disability, severity of several diseases, comorbidities and hospitalization rates [9, 12-15]. The associations of HGS seen in the elderly are primarily derived from the effect of sarcopenia (low muscle mass), which is a major issue in this population. Musculoskeletal aging is a public health concern, which results in sarcopenia and is also associated with the factors mentioned above [16]. Recently, the assessment of HGS was extended into younger populations, including children, with the goal of finding normative reference values in different regions of world [17]. According to Doods et al., HGS increased during 4-30 years of age and slowly decreased starting from 40 years of age, independently of sex and country. The impact of HGS on the general health of young people is not fully known. Most recently, an analysis of the correlations between muscle mass, HGS and cardiovascular markers in young adults indicated that gaining body muscle is an important factor for avoiding heart disease [18].
Chronic levels of psychological stress are known to cause dysregulation in the functioning of the hypothalamic-pituitary-adrenal axis (HPAA) [19-20]. Repeated or prolonged exposure to a similar stressor, over a period of time, may result in changes of baseline HPAA activity as well as the body’s responsiveness to the stressor, resulting in cumulative glucocorticoid burden. Although the exact mechanisms of HPAA dysregulation in chronic stress remains unclear, a new model developed by Karin et al. suggests that prolonged activation of the adrenal glands results in their hypertrophy and leads to overproduction of cortisol [21]. Stress response is also mediated by the sympathetic nervous system and its continuous stimulation may result in high systemic levels of catabolic stress hormones such as cortisol, epinephrine and glucagon [19]. The catabolic effects of cortisol, particularly when in excess, are well-known. Persistent stress may induce chronic elevation of cortisol levels, which via its catabolic effect may result in decreased muscle mass, increased fat mass, insulin resistance and other metabolic changes [22]. Moreover, hypercortisolism-induced abdominal obesity may increase the oxidative stress and inflammatory cytokine levels resulting in the development of processes such as sarcopenia [23].
Considering all of the above, we raised the question of whether HGS test may be a reliable indicator of stress levels amongst medical students. We hypothesized that low HGS may be associated with increased levels of stress in medical students with the aim of assessing the effectiveness of HGS test in identifying highly stressed individuals. Specifically we aimed to measure the HGS and stress levels subjectively, via self-reported stress (SRS) and objectively (via salivary cortisol, SC) as well as body composition of medical students in order to find any potential associations with their lifestyle factors. The secondary aim of the study was to examine the effect of exercise habits of medical students on HGS and stress measures.
Materials and methods
We implemented a cross-sectional study design and recruited medical students at the Medical University of Gdańsk (MUG) in Poland. During normal working days, the students were invited to our laboratory on campus, where they were explained the purpose and objectives of our study. All students volunteered and signed written informed consent forms. Data were collected while maintaining the students’ anonymity by providing each participant a unique code, used to label their information and samples. The students were asked to fill out an online questionnaire, undergo body composition analysis, perform HGS test and provide their saliva samples. The data was collected during the months of October-December, between 12:00-18:00 hours. The study was approved by the Independent Bioethics Committee for Scientific Research at MUG (NKBBN/120/2018).
Study group
A total of 161 students were initially recruited for the study. Three participants were excluded from the study because they did not perform HGS test, thus 158 students were included: 80 females (50.6%) and 78 males (49.4%). There were 27 1st year students, 20 2nd year students and 42 3rd year students (together included in the pre-clinical years (PCY) group of 89 students) and 38 4th year, 9 5th year and 22 6th year students (included in the clinical years (CY) group of 69 students). This grouping was based on the curriculum and workload of medical studies at the MUG. The PCY tend to involve longer class schedules per day with higher number of exams during a semester as compared to the CY of studies. Additionally, during the PCY the international students are also getting used to life on their own and in a new country, while also managing their workflow and approach towards medical studies, all of which are an additional stressor. The intention of such grouping was to establish two groups with contrasting amount of stressful environment.
Self-reported stress and lifestyle habits
We created an 18-item questionnaire, consisted of open-ended and multiple-choice questions, which included questions about demographic data of the participants (age, sex, year of study), lifestyle factors (diet, water intake, smoking, alcohol use, type and intensity of exercise, vitamin D supplementation and sleep (duration and pattern), relationship status, the number of upcoming exams in the ongoing semester and self-rated stress (SRS). The participants’ SRS was measured on a 10-point scale (1 = least stressed, 10 = extremely stressed).
Body composition
The TANITA SC-240 medical bioimpedance analyzer (Tanita Corporation, Tokyo, Japan), was used for the body composition examination (weight, fat%, fat-free% and muscle mass%). Body mass index (BMI) was calculated from the participants’ self-reported height. For each scan, the participants were asked to remove their shoes, excess clothing as well as remove all material which could affect the bioelectrical impedance analysis of the scale.
Hand grip strength test
HGS was measured using a Saehan Squeeze Dynamometer (#SH5008, Saehan Corporation, Incheon-City, South Korea). While sitting down, the participants were instructed to place their forearm parallel to the table, and to form a 90º angle with their arm, then asked to squeeze the bulb of the dynamometer as hard as possible with each hand twice. The participants also indicated their dominant hand during the data collection process. , The maximum HGS measurement (kilogram force), obtained with the dominant hand was used for statistical analysis.
Salivary cortisol measurement
The participants were asked to provide their saliva samples at least 2 hours after their last meal. Before collecting the samples, all participants were asked to rinse their oral cavity with water to remove any residual food. The saliva samples were collected using Salivette Cortisol tubes (Sarstedt, Nümbrecht, Germany). They were then processed according to the instructions provided by the manufacturer [22]. SC levels were analyzed using Salimetrics Salivary Cortisol ELISA kit (Salimetrics, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. The level of SC was used as an objective measure of stress of each participant in this study.
Statistical analysis
Normality distribution was analyzed using Shapiro-Wilk Test. This analysis did not confirm normal distribution for most of the data. Therefore, Mann-Whitney U test and Chisquare test were used to analyze these data, for continuous and categorical variables, respectively. Interestingly, when data concerning saliva cortisol, HGS, SRS and BMI were grouped based on different exercise habits of participants (Table 2) they displayed normal distribution (Shapiro-Wilk test) which allowed for use of the ANOVA test. Type of test used for each parameter is provided in the legend of each table. Significance of the analysis was assumed if two-tailed P-value was less than 0.05. Additionally, correlation analysis between stress measures, body composition and HGS were performed using Spearman’s rank correlation test. Microsoft Excel (version 16.0.1, Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism (version 8.0.1, GraphPad Software Inc., Boston, MA, USA) software were used for data computation and statistical analysis.
Results
Pre-clinical year (PCY) vs clinical year (CY) participants
A total of 158 medical university students (mean age 23.07 years) were included in this study. The demographic characteristics along with questionnaire responses, HGS, SC and body composition values dichotomized according to the groups based on the year of study (PCY vs CY) and sex are presented in Table 1.
Table 1. Demographic characteristics, hand grip strength (HGS), stress measures and body composition parameters dichotomized according to the year of study and sex 
Results are shown as mean (SD) or number (%). Statistical tests used are Mann-Whitney U test (A), Chi-square test (B).
BMI – body mass index; CY – clinical years; HGS – hand grip strength; kgf – kilogram-force; PCY – preclinical years; SRS – self-rated stress
Among the male participants, the HGS was significantly lower, while the SRS was significantly higher in the PCY as compared to the CY. The questionnaire responses indicated that during the PCY males were significantly less likely to exercises, significantly fewer of them slept 6-9 hours per night and they had significantly higher number of exams in the ongoing semester compared to the CY male participants. There was no significant difference between the two groups in terms of SC levels. In contrast, the BMI and fat mass of PCY male participants was significantly lower than that of CY male participants.
Similar to males, the female participants in their PCY of studies had significantly higher SRS than the females in their CY. However, there was no significant difference seen in the HGS and SC levels between females in PCY and CY. Based on the lifestyle questionnaire, it appeared that females in the PCY were significantly more likely to smoke, supplement vitamin D and had more exams in the ongoing semester in comparison to females in CY. Additionally, the questionnaire revealed that females in PCY were significantly more likely to be single as compared to those in CY of studies.
Effect of exercise on HGS, stress measures and body composition
Data related to exercise habits and their effect on HGS, stress measures and BMI are presented in Table 2. Regardless of gender, participants who performed some form of exercise had significantly higher HGS when compared with those who did not exercise. Additionally, male participants who exercised had significantly higher BMI than those who did not exercise. No significant difference was seen in either stress measure (SRS and SC levels) between participants who exercised and those who did not. These four parameters were also compared based on the number of days the participants exercise in a week. In this analysis, the only significant difference was seen in the HGS of males, with those who exercise 5-7 days/ week having higher HGS than those who did it 1-4 days/week.
Table 2. HGS, stress and BMI in terms of exercise habits of the male and female participants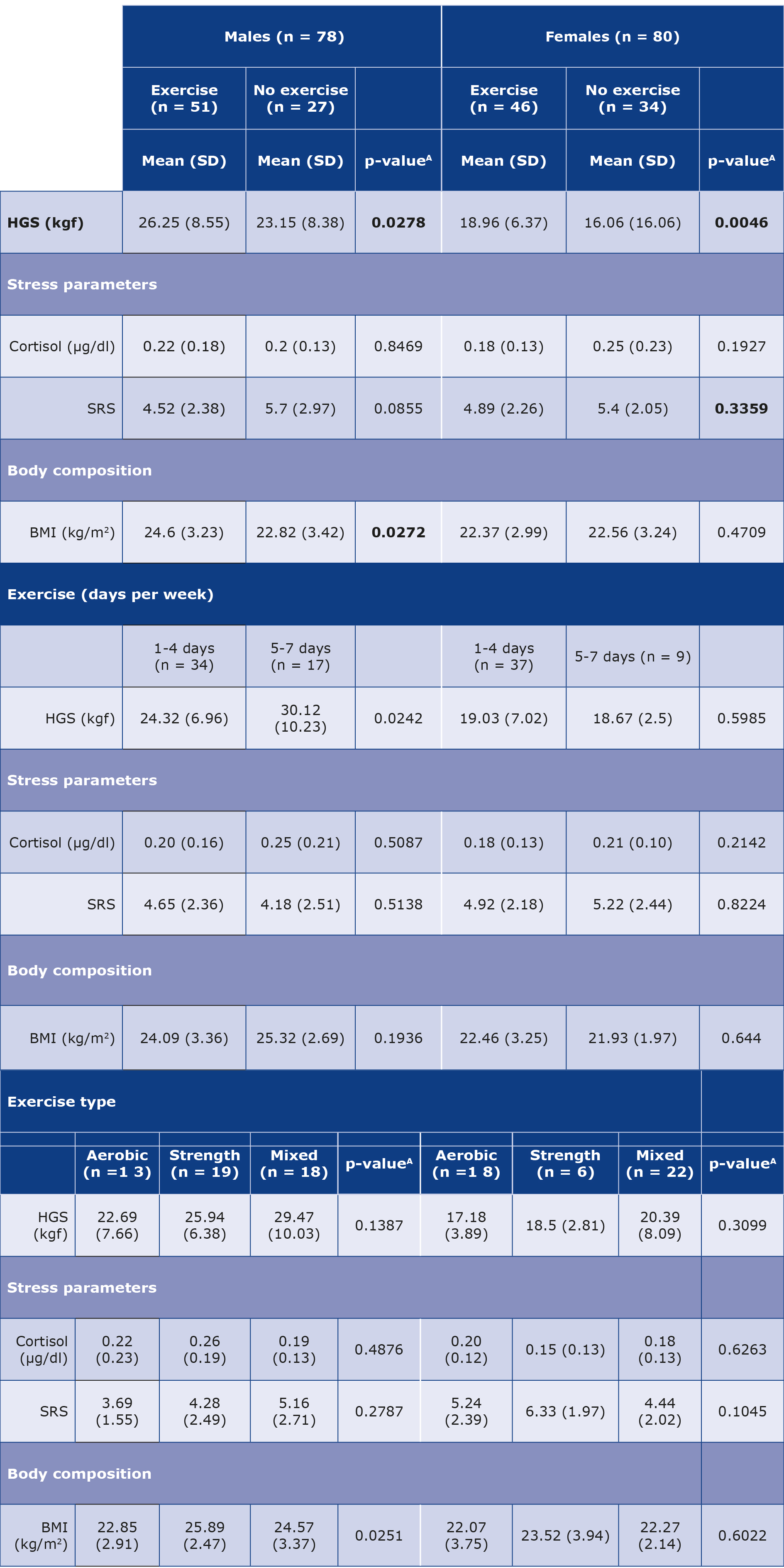
Results are reported as means (SD). Statistical tests used are Mann-Whitney U test (A) and ANOVA test (B).
BMI – body mass index; HGS – hand grip strength; kgf – kilogram force; SRS – self- rated stress
In addition, we analyzed the effect of exercise type (strength, aerobic or mixed (strength + aerobic)) on these parameters. The BMI of males who did primarily strength training was significantly higher in comparison to those who did aerobic or mixed training. Moreover, the HGS of both male and female participants was found to be stronger in those doing mixed training than only aerobic or strength training.
Correlation analyses
The univariate analyses revealed no significant correlation between the subjective stress measure (SRS) and objective stress measure (SC) in the entire study group. There was no significant correlation between SC levels and HGS SRS or BMI when the entire study group was taken into consideration (Table 3). Additionally, no significant correlation was noticed between HGS and SRS. However, it was found that HGS had a positive correlation with BMI and age of the participants, seen as the entire study group (Table 3).
Table 3. Correlation analyses of cortisol and HGS with parameters such as SRS, BMI and age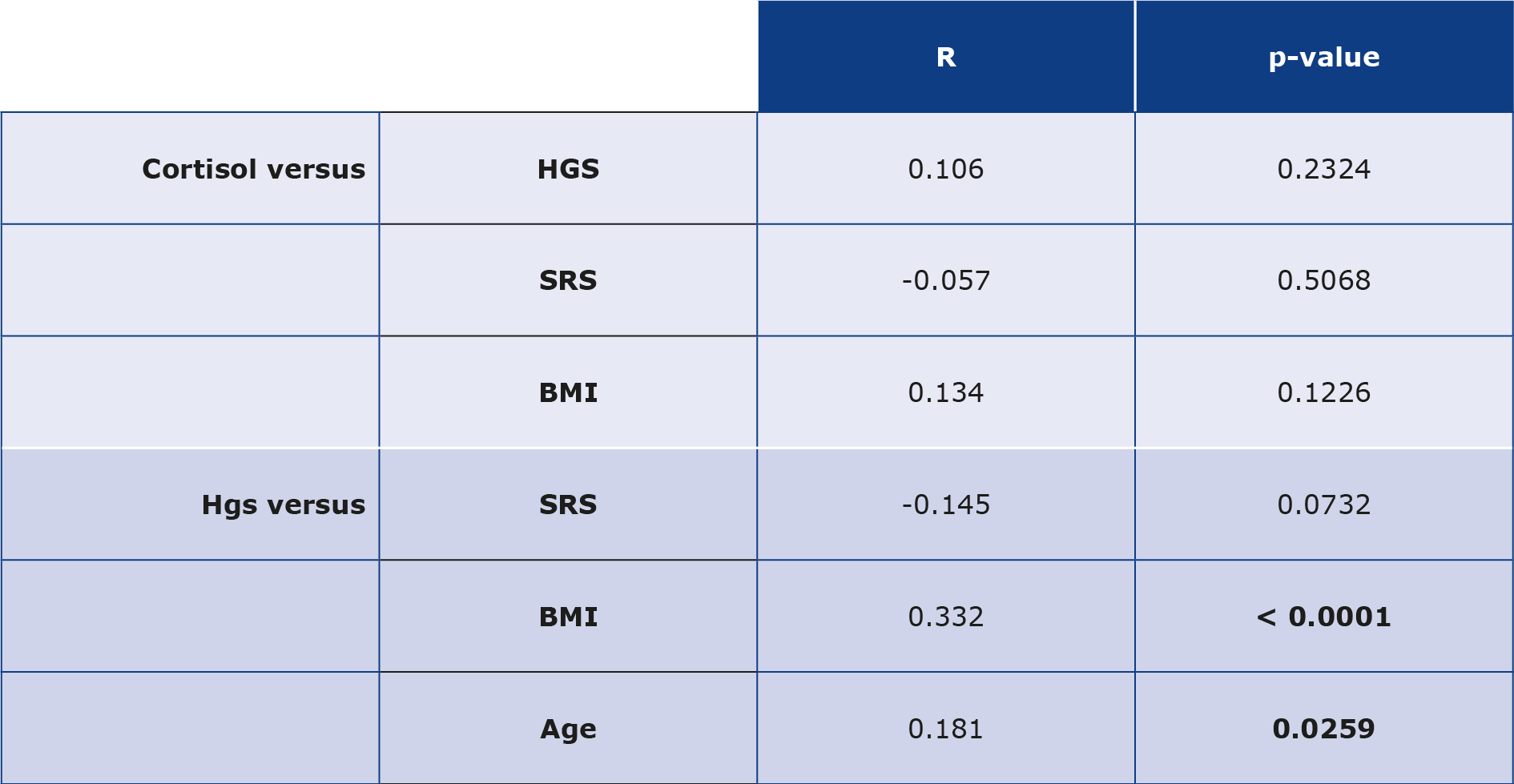
The data shown are results from the entire study group regardless of year of study and gender. Statistical analyses were performed using Spearman’s rank correlation test.
BMI – body mass index; HGS – hand grip strength; SRS – self-rated stress
The HGS of our study group was dichotomized according to sex and year of study (PCY vs CY) and compared with SC, SRS and body composition parameters (Table 4). Regardless of the group, we noticed that the HGS had a significant positive correlation with fat-free mass. Similarly, a significant correlation between HGS and muscle mass was seen in all groups except for males in the CY group. Additionally, an isolated significant correlation was noticed between HGS and BMI of males in the PCY group (R = 0.517, p = 0.0003). Finally, we analyzed the results obtained for students with high SC/low HGS in comparison to those with low SC/high HGS. These groups were identifies by analyzing students with outlying SC and HGS results. The results of this analysis are presented in Table 5.
Table 4. Correlation analyses comparing HGS with stress measures and body composition parameters and comparing fat-free mass with BMI and muscle mass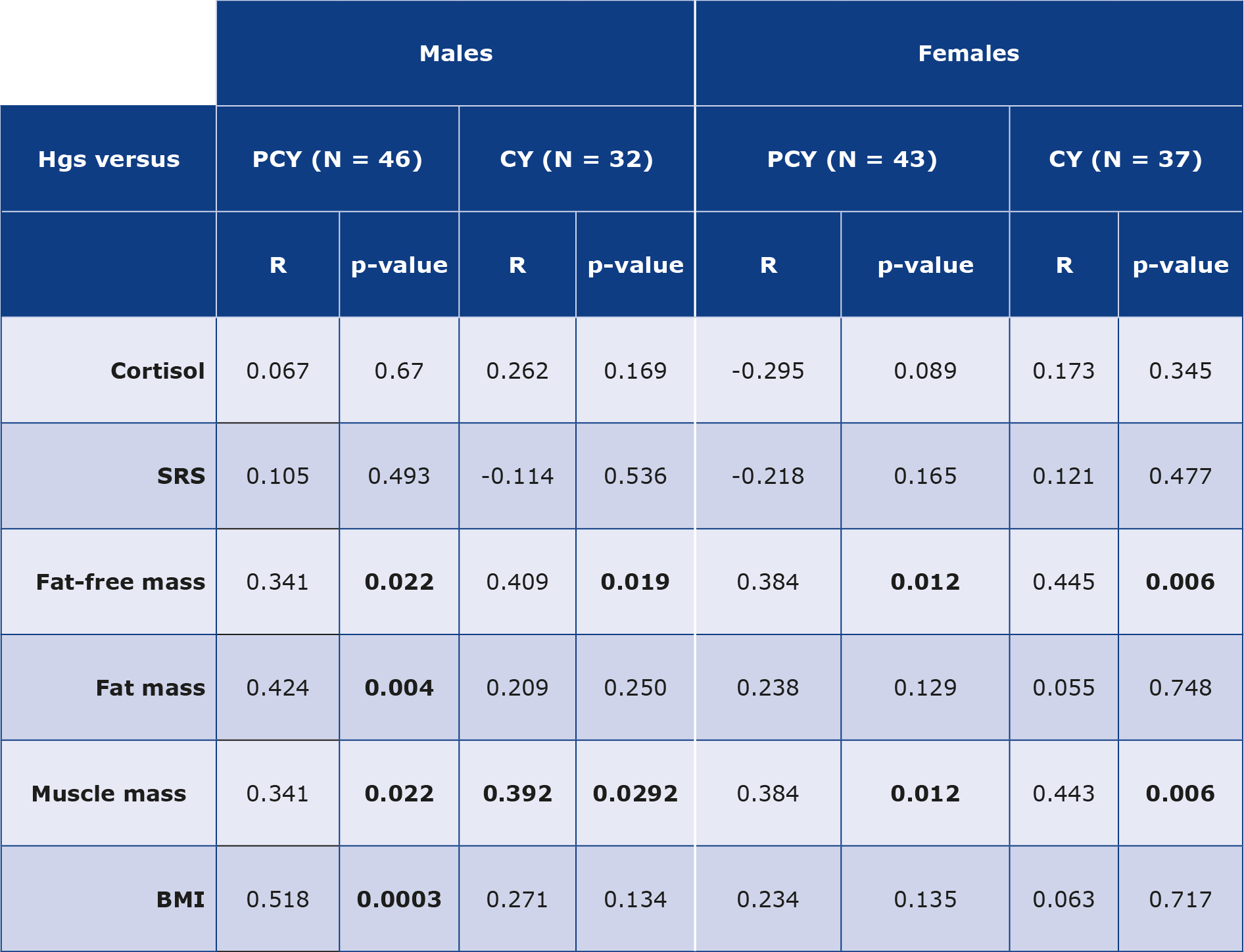
BMI – body mass index; CY – clinical years; HGS – hand grip strength; PCY – preclinical years; SRS – self-rated stress
Statistical analyses were performed using Spearman’s rank correlation test.
Table 5. Comparison of students with high cortisol-low HGS and low cortisol-high HGS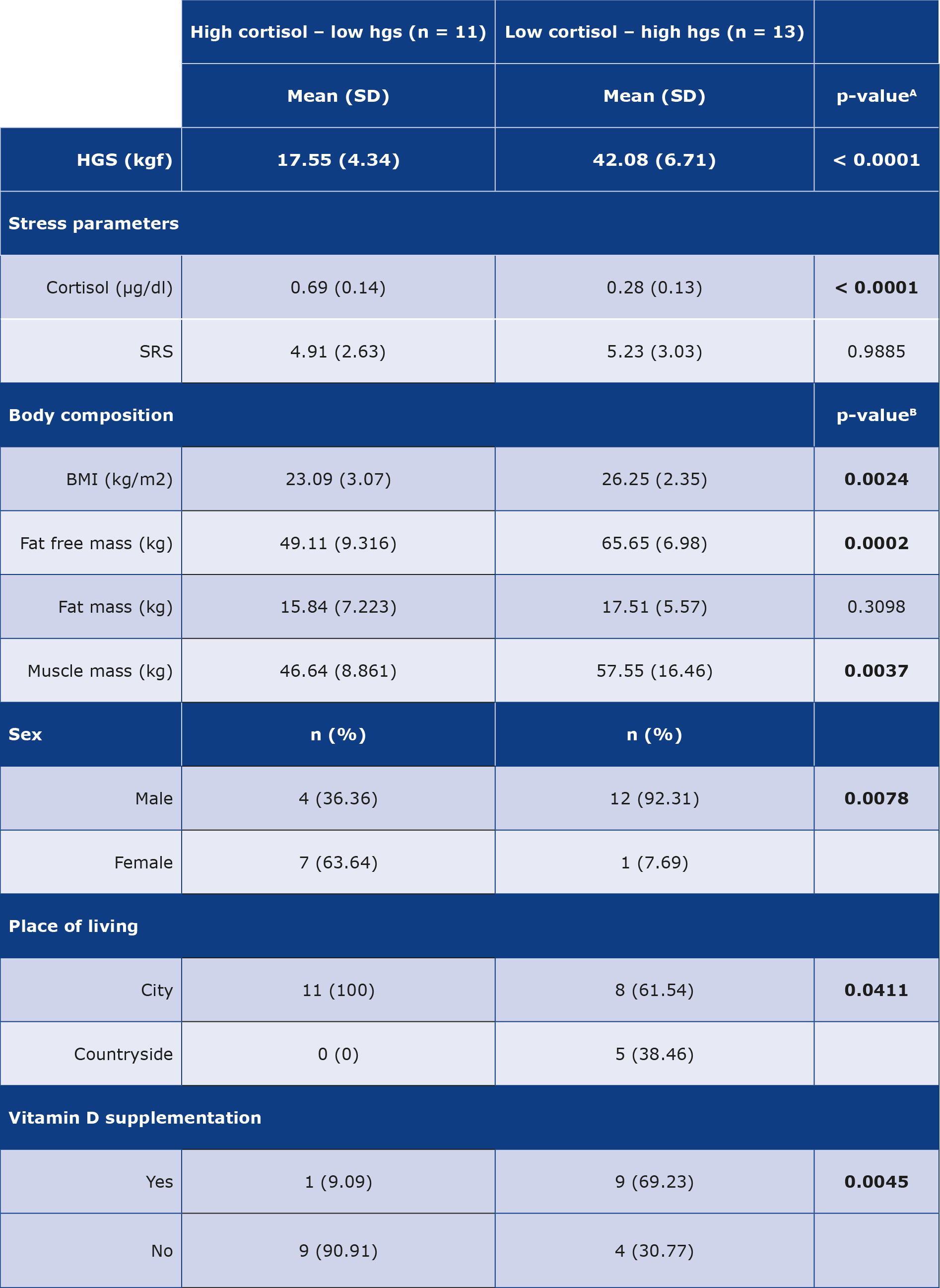
Results are reported in mean (SD) and number (%). Statistical tests used are Mann-Whitney U test (A), Chi-square test (B).
Discussion
It is well-recognized that medical school is a stressful environment [2, 24-25]. In our study, we found that the SRS was significantly higher in the PCY participants, regardless of the sex. We expected such result based on our initial assumption that PCY tend to be more stressful for MUG students, based on reasons stated in the Methods section. These assumptions were further verified by the findings of PCY participants being less likely to get adequate sleep, exercise and having more exams. Almojali et al. have previously highlighted the importance of adequate sleep and its effect on increasing stress levels in medical students [5]. Furthermore, Heinen et al. corroborated the fact that perceived stress is quite high, particularly among 1st year medical students. [24]. We found lower HGS in the “more stressed” PCY males than in the “less stressed” CY suggesting a potential relationship between HGS and the SRS. However, such associations were not observed in female participants. It is worth noting that male students in CY were older and exercised more often.
Based on the HPAA dysregulation caused by chronic stress, we expected to find higher SC levels in the PCY than in the CY group. However, our results did not show any such correlations. Additionally, we did not notice any correlation between our subjective stress measure (SRS) and objective stress measure (SC). This may bring into question the effectiveness of SC in estimating chronic stress levels. Cortisol in the saliva, as in other biological fluid (e.g. serum, plasma or urine) is modulated by several different factors such as time of testing, fluctuations due to acute stressors and recent use of stimulants such as caffeine or nicotine [26-29].
The beneficial effects of regular exercise are well-established [30-31]. It has been consistently proven that physical activity has a positive effect on psychological well-being, while also reducing the risk of cardiovascular disease, obesity, hypertension, diabetes, mental health problems as well as mortality and morbidity [30-33]. In our study, the higher HGS seen in participants who exercise may suggest lower stress levels. However, we did not see any associations between SRS, SC and exercise type and habits (e.g. diet, smoking, water intake, alcohol use, caffeine intake). Contrary to our findings, but as expected, Nakandala et al. demonstrated that physical activity in young undergraduate students was associated with higher HGS [34]. A potential explanation for higher HGS with exercise may simply be the effect of strength training and increased muscle mass. This seems to be more likely in our case given that of male participants who exercised, 37% primarily did strength training compared to only 13% female participants. Looking at the entire sample, the majority of participants with low SC levels also had low HGS. It is worth noting the outliers in this group: those who had low SC and high HGS were mostly males (92.31%), who performed exercise (77%) and rated their stress an average of 5.23. We also observed that the majority of these participants supplemented vitamin D s (69.23%). On the contrary, those with high SC and low HGS were mostly females (63.64%), less than half of them exercised (45%), who reported lower SRS (4.91) and few of them supplemented vitamin D (9%). It is worth noting that a proportion of participants (mainly females) had very low HGS (< 19), similar to the measurements seen in frail elderly [35].
Limitations
There are several limitations to our study. First, our study group was small and quite heterogenous, because it included primarily international students of different ethnicities. Regarding this element, a recent meta-analysis showed that there are significant differences in HGS of people from developed countries and underdeveloped countries [17]. Differences could be also related to differences in body size and composition including muscle mass. In our study, the majority of students had rather low muscle mass and size, which can partially explain the differences. It is noteworthy that medical students at the beginning of their studies were weaker and had lower knowledge regarding healthy lifestyle. Stress and long hours spent on studying could be responsible for not exercising enough. The CY group of participants seemed to have more knowledge regarding healthy lifestyle and more time to exercise. It is also worth to notice that this phenomenon regarding exercises occurs mostly in male students.
Second, saliva samples were collected in the afternoon (12:00-18:00), which may have potentially influenced our findings. This time period was chosen due to the time constraints of the medical students’ schedules. For these reasons, our analysis might have resulted in less significant findings regarding cortisol. Lastly, we applied only univariate analyses which prevents taking into consideration the potential confounders. Regardless of these limitations, this appears to be the first study to examine the association between HGS and stress amongst medical students and their lifestyle.
Conclusions
Our findings suggest that HGS has some association with the perceived stress of medical students as seen by the significantly lower values in the “more stressed” group (PCY) as compared to the “less stressed” group (CY), particularly in male students. Future studies are required to consolidate the association of HGS and stress in young, healthy individuals with a more focused assessment of objective stress measures, potentially hair cortisol. In conclusion, HGS may be a reliable method of identifying stressed individuals and promoting healthy lifestyle behaviors such as mixed-type (aerobic + strength) exercise and adequate sleep among others.
Acknowledgements
We would like to thank Marcin Kutek MD for introducing the topic and helping with its initial conceptualization.
Conflict of interest
None.
Funding
None.
Attachments
References
| 1. |
Firth J. Levels and sources of stress in medical students. BMJ [Internet]. 1986;292(6529):1177–80. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.292.6529.1177.
|
| 2. |
Nechita F, Nechita D, Pîrlog MC, Rogoveanu I. Stress in medical students. Rom J Morphol Embryol [Internet]. 2014;55(3 Suppl):1263–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25607418.
|
| 3. |
Chowdhury R, Mukherjee A, Mitra K, Naskar S, Karmakar P, Lahiri S. Perceived psychological stress among undergraduate medical students: Role of academic factors. Indian J Public Health [Internet]. 2017;61(1):55. Available from: https://journals.lww.com/10.4103/0019-557X.200253.
|
| 4. |
Puthran R, Zhang MWB, Tam WW, Ho RC. Prevalence of depression amongst medical students: a meta-analysis. Med Educ [Internet]. 2016;50(4):456–68. Available from: https://onlinelibrary.wiley.com/doi/10.1111/medu.12962.
|
| 5. |
Almojali AI, Almalki SA, Alothman AS, Masuadi EM, Alaqeel MK. The prevalence and association of stress with sleep quality among medical students. J Epidemiol Glob Health [Internet]. 2017;7(3):169. Available from: https://www.atlantis-press.com/article/125905819.
|
| 6. |
Dyrbye LN, Thomas MR, Shanafelt TD. Systematic Review of Depression, Anxiety, and Other Indicators of Psychological Distress Among U.S. and Canadian Medical Students. Acad Med [Internet]. 2006;81(4):354–73. Available from: http://journals.lww.com/00001888-200604000-00009.
|
| 7. |
Cohen S, Janicki-Deverts D, Miller GE. Psychological Stress and Disease. JAMA [Internet]. 2007;298(14):1685. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.298.14.1685.
|
| 8. |
Schneiderman N, Ironson G, Siegel SD. Stress and Health: Psychological, Behavioral, and Biological Determinants. Annu Rev Clin Psychol [Internet]. 2005;1(1):607–28. Available from: https://www.annualreviews.org/doi/10.1146/annurev.clinpsy.1.102803.144141.
|
| 9. |
Musalek C, Kirchengast S. Grip Strength as an Indicator of Health-Related Quality of Life in Old Age—A Pilot Study. Int J Environ Res Public Health [Internet]. 2017;14(12):1447. Available from: https://www.mdpi.com/1660-4601/14/12/1447.
|
| 10. |
Prasitsiriphon O, Pothisiri W. Associations of Grip Strength and Change in Grip Strength With All-Cause and Cardiovascular Mortality in a European Older Population. Clin Med Insights Cardiol [Internet]. 2018;12:117954681877189. Available from: http://journals.sagepub.com/doi/10.1177/1179546818771894.
|
| 11. |
Hamilton GF, McDonald C, Chenier TC. Measurement of Grip Strength: Validity and Reliability of the Sphygmomanometer and Jamar Grip Dynamometer. J Orthop Sport Phys Ther [Internet]. 1992;16(5):215–9. Available from: http://www.jospt.org/doi/10.2519/jospt.1992.16.5.215.
|
| 12. |
Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet [Internet]. 2015;386(9990):266–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673614620006.
|
| 13. |
Adamo DE, Anderson T, Koochaki M, Fritz NE. Declines in grip strength may indicate early changes in cognition in healthy middle-aged adults. Wylie GR, editor. PLoS One [Internet]. 2020;15(4):e0232021. Available from: https://dx.plos.org/10.1371/journal.pone.0232021.
|
| 14. |
Nacul LC, Mudie K, Kingdon CC, Clark TG, Lacerda EM. Hand Grip Strength as a Clinical Biomarker for ME/CFS and Disease Severity. Front Neurol [Internet]. 2018;9. Available from: https://www.frontiersin.org/article/10.3389/fneur.2018.00992/full.
|
| 15. |
Mainous AG, Tanner RJ, Anton SD, Jo A. Grip Strength as a Marker of Hypertension and Diabetes in Healthy Weight Adults. Am J Prev Med [Internet]. 2015;49(6):850–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0749379715002676.
|
| 16. |
Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol [Internet]. 2017;13(6):340–7. Available from: https://www.nature.com/articles/nrrheum.2017.60.
|
| 17. |
Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing [Internet]. 2016;45(2):209–16. Available from: https://academic.oup.com/ageing/article-lookup/doi/10.1093/ageing/afv192.
|
| 18. |
Bell JA, Carslake D, Wade KH, Richmond RC, Langdon RJ, Vincent EE, et al. Influence of puberty timing on adiposity and cardiometabolic traits: A Mendelian randomisation study. Rader DJ, editor. PLOS Med [Internet]. 2018;15(8):e1002641. Available from: https://dx.plos.org/10.1371/journal.pmed.1002641.
|
| 19. |
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the Hypothalamic‐Pituitary‐Adrenocortical Stress Response. In: Comprehensive Physiology [Internet]. Wiley; 2016. p. 603–21. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c150015.
|
| 20. |
Guilliams TG, Edwards L. Chronic stress and the HPA axis. Stand [Internet]. 2010;9(2):1–12. Available from: https://www.pointinstitute.org/wp-content/uploads/2012/10/standard_v_9.2_hpa_axis.pdf.
|
| 21. |
Karin O, Raz M, Tendler A, Bar A, Korem Kohanim Y, Milo T, et al. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol Syst Biol [Internet]. 2020;16(7). Available from: https://www.embopress.org/doi/10.15252/msb.20209510.
|
| 22. |
Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, et al. Effects of Cortisol on Carbohydrate, Lipid, and Protein Metabolism: Studies of Acute Cortisol Withdrawal in Adrenocortical Failure. J Clin Endocrinol Metab [Internet]. 2007;92(9):3553–9. Available from: https://academic.oup.com/jcem/article/92/9/3553/2597859.
|
| 23. |
Yanagita I, Fujihara Y, Kitajima Y, Tajima M, Honda M, Kawajiri T, et al. A High Serum Cortisol/DHEA-S Ratio Is a Risk Factor for Sarcopenia in Elderly Diabetic Patients. J Endocr Soc [Internet]. 2019;3(4):801–13. Available from: https://academic.oup.com/jes/article/3/4/801/5368377.
|
| 24. |
Heinen I, Bullinger M, Kocalevent R-D. Perceived stress in first year medical students - associations with personal resources and emotional distress. BMC Med Educ [Internet]. 2017;17(1):4. Available from: http://bmcmededuc.biomedcentral.com/articles/10.1186/s12909-016-0841-8.
|
| 25. |
Fares J, Al Tabosh H, Saadeddin Z, El Mouhayyar C, Aridi H. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci [Internet]. 2016;8(2):75. Available from: http://www.najms.org/text.asp?2016/8/2/75/177299.
|
| 26. |
Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology [Internet]. 2009;34(1):2–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306453008002643.
|
| 27. |
Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep [Internet]. 2015;48(4):209–16. Available from: http://koreascience.or.kr/journal/view.jsp?kj=E1MBB7&py=2015&vnc=v48n4&sp=209.
|
| 28. |
Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SHM. Hair cortisol analysis: An update on methodological considerations and clinical applications. Clin Biochem [Internet]. 2019;63:1–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009912018307306.
|
| 29. |
Wester VL, van Rossum EFC. Clinical applications of cortisol measurements in hair. Eur J Endocrinol [Internet]. 2015;173(4):M1–10. Available from: https://academic.oup.com/ejendo/article/173/4/M1/6660781.
|
| 30. |
Booth FW, Roberts CK, Laye MJ. Lack of Exercise Is a Major Cause of Chronic Diseases. In: Comprehensive Physiology [Internet]. Wiley; 2012. p. 1143–211. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c110025.
|
| 31. |
Turner JE, Lira VA, Brum PC. New Insights into the Benefits of Physical Activity and Exercise for Aging and Chronic Disease. Oxid Med Cell Longev [Internet]. 2017;2017:1–3. Available from: https://www.hindawi.com/journals/omcl/2017/2503767/.
|
| 32. |
Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm [Internet]. 2009;116(6):777–84. Available from: http://link.springer.com/10.1007/s00702-008-0092-x.
|
| 33. |
Dyrbye LN, Satele D, Shanafelt TD. Healthy Exercise Habits Are Associated With Lower Risk of Burnout and Higher Quality of Life Among U.S. Medical Students. Acad Med [Internet]. 2017;92(7):1006–11. Available from: https://journals.lww.com/00001888-201707000-00046.
|
| 34. |
Nakandala P, Manchanayake J, Narampanawa J, Neeraja T, Pavithra S, Mafahir M, et al. Descriptive Study Of Hand Grip Strength And Factors Associated With It In A Group Of Young Undergraduate Students In University Of Peradeniya, Srilanka Who Are Not Participating In Regular Physical Training. Int J Physiother [Internet]. 2019;6(3). Available from: https://ijphy.com/index.php/journal/article/view/422.
|
| 35. |
Nygård LK, Dahl L, Mundal I, Šaltytė Benth J, Rokstad AMM. Protein Intake, Protein Mealtime Distribution and Seafood Consumption in Elderly Norwegians: Associations with Physical Function and Strength. Geriatrics [Internet]. 2020;5(4):100. Available from: https://www.mdpi.com/2308-3417/5/4/100.
|











