Abstract
Introduction: This literature review explores the role of physical activity (PA) in managing and preventing type 2 diabetes mellitus (T2DM), synthesizing the latest guidelines for all T2DM patients. Following PRISMA guidelines, it identifies specific areas for further research.
Methods: Indexing services (PubMed and Scopus) were used to identify relevant studies, emphasizing original research, review articles, and updated institutional guidelines spanning 2017-2023. The following keywords were used: diabetes, physical activity, type 2 diabetes mellitus, PA, and exercise. Proposal and opinion articles, non-English papers (or those lacking full-text translation), studies involving non-human subjects, pediatric populations or with skewed gender distribution were excluded.
Results: PA improves quality of life, BMI, glycemic control and well-being. Effects vary by activity domain, such as work or leisure time. Recommendations suggest 60 daily minutes of exercise for children, 150 weekly minutes of moderate intensity exercise for adults, and screening for adverse events. A “sit-less” approach is proposed for those unable to maintain regular activity, with glycemic monitoring for those with variability.
Conclusion: PA is crucial in T2DM management. Conflicting findings regarding glycemic control warrant further investigation to ascertain causes, whether related to bias or other factors.
Citation
Burooj A. Physical activity in type 2 diabetes mellitus: a review. Eur J Transl Clin Med. 2024;7(1):97-105Introduction
For quite some time it has been known that lifestyle changes have been the primary intervention in type 2 diabetes mellitus (T2DM) [1]. The implementation of physical activity (PA) or exercise into daily life has been the preferred method of achieving adequate glycemic control, losing weight and lowering the body mass index (BMI) [1]. Although a lot of research supports the fact that an exercise regimen in patients with diabetes helps to achieve more favourable outcomes, these results are vastly heterogeneous. Intriguingly, some recent studies have shown the lack of a relationship between exercising and achieving adequate glycemic control (see the “Contradictory results” subsection) [2-5]. Nonetheless, an exercise regimen is still recommended in diabetes-related guidelines all around the world. The aim of this review was to condense the newest, most relevant studies and guidelines to guide clinicians in their formulation of individual management plans for their patients. Further aim was to temper expectations and accurately define what exactly can be expected as a result of implementing PA in terms of glycemic control and overall morbidity.
Methods
Indexing services PubMed and Scopus were used to find studies that were relevant, factually important and recent. The search was conducted using the following keywords: physical activity, diabetes, PA, T2DM, type 2 diabetes mellitus, exercise, graded regimen. Newer articles were prioritised and only those from 2017-2023 were included in the review. Articles not published in English (or without a full-text English version), research on a non-human population, research focusing entirely on a paediatric population, opinion pieces and proposals were excluded. Duplicate articles were removed. This literature review includes original research, review papers, meta analyses and institutional guidelines. The basis of this shortlisting was to not only provide the most detailed narrative with the newest information but also to discuss some interesting contradictory results found as of late. No papers with significant biases were included in this review. However, articles with potential for bias were included in the “Contradictory results” subsection, for the purpose of initiating a discussion regarding not only the direction for further research but also the initiation of similar studies to either prove or disprove said results. This review was conducted in accordance with the PRISMA 2020 guidelines.
Results
The initial search retreived a total of 11733 abstracts. Upon implementation of the exclusion criteria and removal of duplicates, 1074 abstracts remained. After screening the full-texts, a total of 46 articles were chosen for analysis in this review (Figure 1).
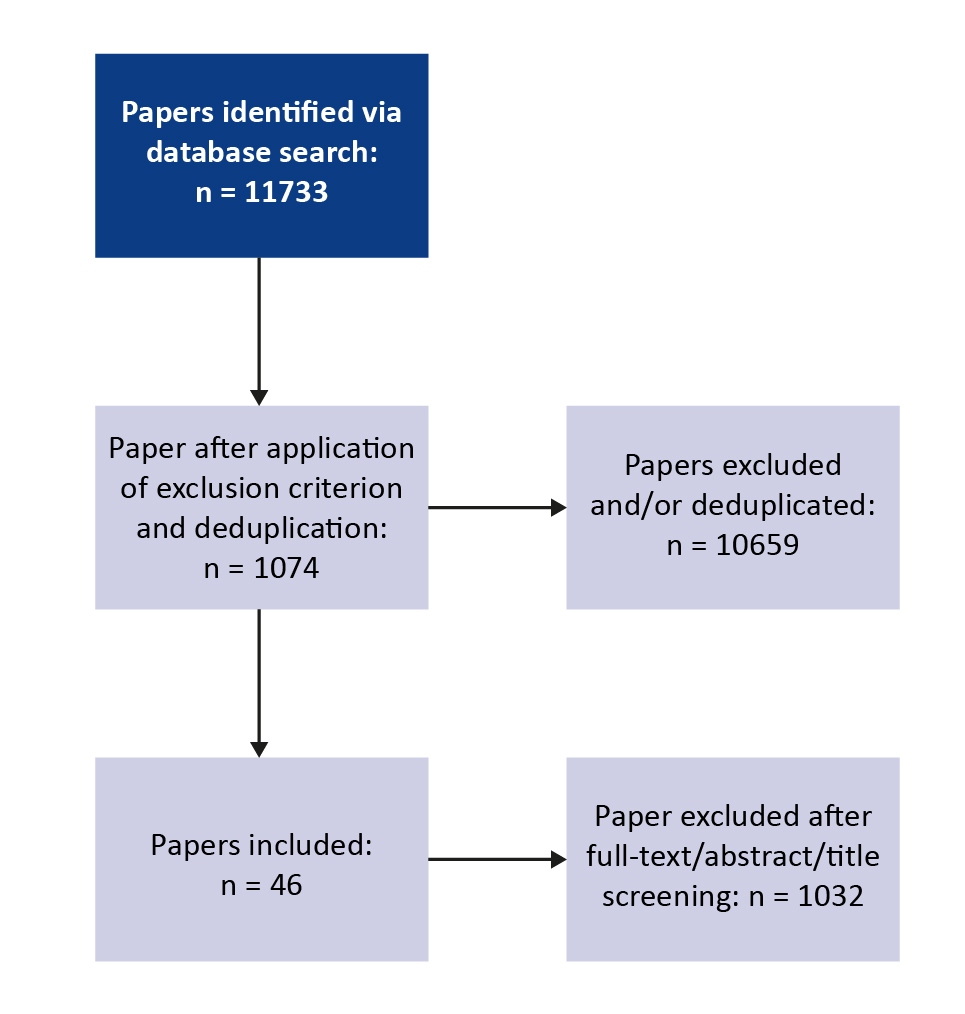
Figure 1. PRISMA flow diagram
Discussion
It is well-known that regular exercise/PA is one of the foundations to a healthy lifestyle. It was found that patients with T2DM and low PA level had a shorter life expectancy with higher risk of chronic diseases than their counterparts with moderate to high PA levels [1]. Additionally, it has been demonstrated that individuals with higher levels of weekly PA are at a significantly lower risk of developing diabetes than their counterparts with lower levels of weekly PA. It was shown that individuals who managed 150-300 minutes, 300- -600 minutes and > 600 minutes of weekly PA as compared to individuals with < 150 minutes, were respectively at a 49%, 62% and 71% lower risk of developing diabetes [6].
PA has also been shown to improve the common comorbidities that are expected in a majority of patients with T2DM. Exercise improves endurance, provides a therapeutic effect in musculoskeletal and cardiovascular comorbidities as well as reduces weight and BMI [7-8]. Exercise also improves endothelial dysfunction in patients independent of their glycemic state [9]. This cardiovascular benefit of a 3-month exercise regimen is shown to reduce adverse events up to 24 months later, even despite cessation of exercise after 3 months [9].
PA has also been shown to improve glycosylated hemoglobin (HbA1c) levels, BMI, quality of life and waist circumference in patients on long-term regimina [10]. It was in fact found that “improve activity” was by far the most effective lifestyle intervention in the prevention of T2DM, even ahead of “promote healthy diet” or “control smoking” [11].
In addition to lowering weight and BMI in those as required, PA (both short-term and long-term) has also been shown to improve the 24-hour glycemic control in patients with T2DM [12]. It has also been shown that PA can reduce the time spent in hyperglycemia in these patients [12]. That being said, there was a high amount of heterogeneity observed in the results, as reported in a meta-analysis from 2020 [13].
Demographics
It was shown that sex can not be considered separate from the discussion of PA in diabetes and might be a confounding factor in studies that have heterogeneous results [14]. Studies that included primarily men showed much different results than those that featured a diverse group of participants from both sexes. Similarly, sex, not BMI or age was found to have the most significant changes to 24-hour glycemic control [13].
Additionally, it has also been shown that women might experience a more dynamic set of barriers (including perceived barriers) to exercise as compared to men [15]. This was primarily found to be the case due to a perceived lack of time, societal or personal opinions about the benefits or reasons for exercise or family discouragement [16]. It was in fact shown that there is a strong correlation between a barrier to PA and the presence of children at home, however the number of children did not seem to increase this perceived barrier [17]. This is further affirmation that this perceived barrier is in line with the perceived lack of available time. It was also found that working women faced the same perceived barriers whether they were from higher or lower socioeconomic backgrounds with the primary ones being a lack of energy and a lack of time [18]. This is an important finding as it could imply that these barriers are perceptual and not necessarily a negative driving force as there is a homogeneity in experience between working and non-working women. Clinicians must therefore tailor their approaches to a holistic psychosocial model for each patient and understand their perceptions of own health as well as their attitudes towards the suggested treatment plan.
It was also shown that exercise carried out during leisure time was inversely related with the development of diabetes [19]. On the contrary, PA carried out domestically or at work was shown to have an astonishing additive effect to the risk for developing diabetes [20]. This is an immensely important finding with more research warranted as it may require clinicians to be more precise with their weekly exercise recommendations since equivalent amounts of exercise have a seemingly opposite effect depending on the context and setting in which they are performed [20]. Additionally, it has been shown that although leisure-time PA shows an inverse relationship with the development of diabetes, so called “transport physical activity” (the PA involved in going from one place to another) only shows this inverse relationship in men and not in women [21]. This study was carried out in a Korean cohort. As has been shown in previous studies, there is something to be said for the heterogeneity in the response of cohorts of different ethnicities and their respective glycemic responses to glucose loads implying a potential fundamental processing difference [22].
There is therefore an evident need for clinical recommendations made with domain-specific PA in mind, tailored to each individual. Clinicians must also keep this in mind whilst inquiring about patients’ average weekly mobility and amount of exercise, and must strive to organize the respective times into domain-specific categories.
Recommendations
As mentioned, patients face a variety of perceived barriers to PA. Therefore, patients should feel like an active member of the decision-making team so that they are invested in the management plan and are more likely to follow it. Additionally, the exercise regimen that unanimously chosen by the patient and clinician should be specifically tailored to suit individual needs so as to not overwhelm the patient physically or psychologically. It is shown that this is a key factor especially in the first 6 weeks of starting a training regimen and is the most likely cause behind the subsequent discontinuation [23]. Additionally, elderly patients may especially benefit from a PA regimen that places an emphasis on not just exercise but also flexibility and stretching due to their reduced age-related or comorbidity-related functional capacities [24].
It has been shown in multiple studies that patients using insulin to manage their diabetes may benefit from rigorous monitoring of their glycemia before, during and after exercise in order to prevent adverse events related to hypoglycemia [24]. Continuous glucose monitoring systems (CGMS) may be an appropriate intervention for patients with diabetes who wish to pursue an exercise regimen, as they have been shown to not only lead to lesser hypoglycemic events but also increase a patients’ time-in-range (TIR) or the time that a patient spends in a euglycemic state [25]. The use of such devices can further help individual patients by allowing them to monitor the variations in their glycemia and use this data to appropriately modify their daily diet.
It has been shown that PA has been an effective intervention in improving the severity of depression symptoms [26]. Intriguingly, it was found that on the contrary, in patients who have both T2DM and depression, exercise was not an adequate intervention in providing significant or even sufficient glycemic control [27]. Interestingly, exercise was still found to be an appropriate lifestyle intervention to reduce the severity of depression in patients with coexisting depression and diabetes. Unfortunately there is a severe lack of literature on this particular topic and more research is needed.
Sitting
It was noted that there was an increased risk of cardiovascular disorders as well as diabetes in individuals who spent longer periods during the day sitting [28]. Interestingly, the risk for the development of diabetes was not diminished even after correcting for time spent daily engaging in PA, however the risk for cardiovascular disorders was diminished all the same [29]. This is important because it is essential for the clinician to understand that there are certain factors that not even exercise can mitigate and these must be discussed and kept in mind whilst making recommendations to patients.
It was also found in a cohort of Europeans that there was a causal relationship between television viewership, a sedentary lifestyle and the development of T2DM. Conversely, there was no causal relationship between computer usage and the development of diabetes. It was also shown that being seated whilst driving was in fact not associated with an increased risk for developing diabetes [30]. This might be explained by various mechanisms including the observed differences in the health ramifications between the sedentary activities that are mentally passive versus mentally active [31].
It was also noted that interrupting sitting time with light-intensity exercise may attenuate postprandial hyperglycemia even more effectively than a continuous bout of moderate exercise, particularly in patients with insulin resistance and a higher BMI [32]. This is important as it may be an alternative regimen for patients that are unable or not motivated enough to partake in continuous exercise.
Contradictory results
Intriguingly, a meta-analysis of 28 studies showed that although PA improved 24-hour glucose concentrations in short-term studies, this effect was not statistically significant in long-term studies [2]. Although 24-hour glucose levels were not significantly improved compared to the control cohort, they were improved compared to the pre-exercise levels of the same cohort. That being said, there was a high degree of heterogeneity in these results. Although the limitations of that analysis are not completely understood, it must be stated that there is a high possibility of bias in the trial methodologies (when accounting for sex, intensity of exercise and time of day when exercise is performed), as well as the methodology used to analyse these trials [3]. A follow-up study showed that 50 minutes of walking at 3 different times of day in fact did not improve 24-hour glucose profiles in patients with T2DM [2].
Another study found that only exercise interventions in the evening had a positive impact on 24-hour glucose levels as opposed to no exercise or exercise interventions in the morning [4]. Even more interestingly, a recent study had contradictory results to the aforementioned: in a cohort of 73 patients who took part in short term PA in the form of 50 minutes of walking before dinner, it was found that there was no significant improvement in 24 hours glucose concentrations [5]. Once again, there was a high degree of heterogeneity in the results. The reasons for this contradictory finding are not understood clearly.
Guidelines
It has been shown that lack of PA negatively affects the lives of patients, particularly those with T2DM [33]. Patients need to therefore be educated and made aware about this fact so that they are more likely to actively engage with proposed therapies. PA has additionally also been shown to decrease HbA1c levels, blood pressure and lipid levels in patients [34-35]. Therefore guidelines suggest adopting a holistic, multi-factorial therapeutic approach to diabetic patients.
The International Society for Pediatric and Adolescent Diabetes suggests that children and adolescents with diabetes must be encouraged to obtain at least 60 minutes of moderate to intense aerobic PA daily [36]. Moderate PA entails activities such as walking, cycling, running, hiking etc. Moderate to intense activity may involve sports such as football, basketball, cricket and so on. Conversely, very high intensity and anaerobic exercises may cause unwanted spikes in blood glucose as seen with activities such as weightlifting and sprinting [37]. This is important because patients on insulin therapy that partake in very high intensity or anaerobic exercises might not benefit from a therapeutic pre-exercise dose reduction and may instead require a post-exercise hyperglycemic correction. Patients on insulin therapy that partake in aerobic exercise however may benefit from a pre-exercise dose reduction and this may be evaluated on a case-by-case basis. High-intensity exercise is also contraindicated in patients with recurrent adverse sequelae of diabetes or with advanced retinopathy [36]. Additionally, an episode of severe hypoglycemia in the past 24 hours or recurrent episodes of hyperglycemia may be seen as contraindications to exercise until the underlying basal metabolic status is rectified.
Further, combined nutrition, PA and behavioral therapy is recommended to patients with diabetes [38-40]. This is particularly true for patients who are overweight or obese. This regimen is to be accompanied by frequent counseling sessions (as many as 16+ sessions in 6 months) to ensure adherence and eventual accomplishment of a caloric deficit of more than 500 kcal per day [41].
The American College of Sports Medicine also recommends that individuals with diabetes that wish to or are recommended to incorporate an exercise regimen into their daily life may benefit from medical screening, particularly individuals with macrovascular disorders, cardiac dysfunction etc. The screening would take into account their current level of PA, as well as any cardiovascular, metabolic or renal pathologies [42]. Although the results of such a screening may not necessarily disqualify an individual from a PA regimen, it may guide clinicians in modifying their recommendations with respect to total time spent and intensity of said regimen.
A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends that adults obtain a weekly minimum of 150 minutes of moderate intensity anaerobic exercise [43] (classified as any activity that can be carried out whilst maintaining an uninterrupted conversation) and reduce sedentary time and break-up prolonged sitting time. Additionally, those individuals that opt to partake in aerobic activity should supplement this with 2-3 weekly sessions of resistance, flexibility or balance training (the last of which is particularly beneficial for the elderly with limited mobility) [43].
Similarly, the 2023 guidelines published by the ADA recommend that adult patients with diabetes partake in a minimum of 150 minutes of weekly moderate to vigorous intensity anaerobic exercise whilst also focusing on reducing sedentary time and breaking up prolonged seated time [44]. This weekly activity target may be spread over 3 days [45-46]. Resistance exercises in conjunction with moderate aerobic activity, particularly in the same session, were found to have an additive effect in the lowering of glycemic levels and may therefore be safely recommended to patients. It is recommended that resistance exercises be carried out at least 2 days a week [45]. Exercises that aid in increased flexibility may help older patients with diabetes that are experiencing varying forms of functional disability. Exercise-related recommendations to patients ought to be as specific as possible, mentioning the type, duration and intensity of recommended weekly activities. Such recommendations must be customized to each individual patient on the basis of their comorbidities and physical ability.
Discussion
It is therefore evident that PA is an essential intervention in the clinicians’ tool chest for patients with T2DM. An appropriate amount of weekly aerobic exercise may not only allow for better glycemic control, but may even reduce time spent in hyperglycemia [8]. Additionally, patients show improved BMI, a better quality of life and lower waist circumference, all further improving glycemic control as well as their overall health [10].
Recent studies have shown that there is some variability in the performance of exercise in different domains of life. Notably, leisure-time PA was found to have the expected directly proportional relationship to glycemic control whereas worktime PA or even transportation PA were either not shown to exhibit this same effect or even had an inversely proportional relationship [21]. Therefore, clinicians must be specific in their recommendations as well as questioning of patients whilst trying to determine current and potential future weekly activity levels.
According to the American Diabetes Association, adolescents and children would benefit from at least 60 minutes of moderate aerobic exercise daily whereas adult patients should look to striving for closer to a minimum of 150 minutes of weekly aerobic exercise interspersed with resistance training [43-46]. All individuals who are to be prescribed an exercise or activity regimen would benefit from a screening to ensure that there is minimal risk for adverse events [42]. Additionally, patients who frequently experience variability in glycemic levels may benefit from CGMS and potential dose adjustments [25]. Elderly patients may benefit from the addition of exercises that enhance flexibility thereby increasing functional mobility.
Patients who are unable to stick to an exercise regimen due to functionality or motivational issues may benefit from a “sit-less” intervention that has been shown to reduce insulin resistance in patients significantly [32].
Patients with diabetes, particularly those treated with insulin and sulfonylurea, face a heightened risk of hypoglycemia during and after exercise [24]. Engaging in PA can enhance the glucose-lowering effects of these medications, potentially leading to hypoglycemia. It is therefore imperative for individuals taking these medications to monitor their glucose levels closely before and during exercise. CGMS offer a valuable tool for real-time glucose monitoring, facilitating timely adjustments to insulin doses as needed [25]. Although some intriguing and contradicting results have been noted in recent studies, there is a lack of understanding as to whether these are a result of bias or an occult underlying phenomenon. These heterogenic results ranged from meta analyses unable to reciprocate the beneficial effects of PA in diabetes over a long period of time [2], to a lack of improvement in 24 hour glucose concentrations after implementation of a PA regimen [4-5]. Further investigation is warranted to better understand the pathophysiological mechanisms governing these findings.
Conclusion
PA has been shown to improve quality of life, BMI, overall morbidity and glycemic control. Adults with T2DM must aim to achieve at least 150 minutes of moderately intense aerobic exercise per week, interspersed with resistance training sessions. Children and adolescents with T2DM must aim to achieve at least 60 minutes of moderately intense aerobic exercise daily. All patients may benefit from a medical screening before prescription of an exercise regimen. The domain in which PA is conducted (ie. in the workplace, at leisure, during transportation, etc.) has shown a variability in results with respect to glycemic control. Patients must be advised to undertake leisure-time PA and not count work-time PA towards their weekly activity goals. Conflicting findings regarding glycemic control warrant further investigation, whether related to bias or other factors.
Funding
No funding was received for this paper.
Conflicts of interest
There are no conflicts of interest to declare.
Data availability
No datasets were generated or analysed during the current study.
References
| 1. |
Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ [Internet]. 2020;l6669. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.l6669.
|
| 2. |
Munan M, Dyck RA, Houlder S, Yardley JE, Prado CM, Snydmiller G, et al. Does Exercise Timing Affect 24-Hour Glucose Concentrations in Adults With Type 2 Diabetes? A Follow Up to the Exercise-Physical Activity and Diabetes Glucose Monitoring Study. Can J Diabetes [Internet]. 2020;44(8):711-718.e1. Available from: https://www.sciencedirect.com/science/article/pii/S1499267120301507.
|
| 3. |
Munan M, Oliveira CLP, Marcotte-Chénard A, Rees JL, Prado CM, Riesco E, et al. Acute and Chronic Effects of Exercise on Continuous Glucose Monitoring Outcomes in Type 2 Diabetes: A Meta-Analysis. Front Endocrinol (Lausanne) [Internet]. 2020 Aug 4;11. Available from: https://www.frontiersin.org/article/10.3389/fendo.2020.00495/full.
|
| 4. |
Moholdt T, Parr EB, Devlin BL, Debik J, Giskeødegård G, Hawley JA. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia [Internet]. 2021;64(9):2061-76. Available from: https://doi.org/10.1007/s00125-021-05477-5.
|
| 5. |
Rees JL, Chang CR, François ME, Marcotte-Chénard A, Fontvieille A, Klaprat ND, et al. Minimal effect of walking before dinner on glycemic responses in type 2 diabetes: outcomes from the multi-site E-PAraDiGM study. Acta Diabetol [Internet]. 2019;56(7):755-65. Available from: https://doi.org/10.1007/s00592-019-01358-x.
|
| 6. |
Boonpor J, Parra-Soto S, Petermann-Rocha F, Lynskey N, Cabanas-Sánchez V, Sattar N, et al. Dose–response relationship between device-measured physical activity and incident type 2 diabetes: findings from the UK Biobank prospective cohort study. BMC Med [Internet]. 2023;21(1):191. Available from: https://doi.org/10.1186/s12916-023-02851-5.
|
| 7. |
Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol [Internet]. 2018;15(12):731-43. Available from: https://doi.org/10.1038/s41569-018-0065-1.
|
| 8. |
Jaramillo AP, Ibrahimli S, Castells J, Jaramillo L, Moncada D, Revilla Huerta JC. Physical Activity as a Lifestyle Modification in Patients With Multiple Comorbidities: Emphasizing More on Obese, Prediabetic, and Type 2 Diabetes Mellitus Patients. Cureus [Internet]. 2023; Available from: https://www.cureus.com/articles/165669-physical-activity-as-a-lifestyle-modification-in-patients-with-multiple-comorbidities-emphasizing-more-on-obese-prediabetic-and-type-2-diabetes-mellitus-patients.
|
| 9. |
Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol [Internet]. 2019;597(19):4901-14. Available from: https://physoc.onlinelibrary.wiley.com/doi/10.1113/JP277764.
|
| 10. |
Shah SZA, Karam JA, Zeb A, Ullah R, Shah A, Haq IU, et al. Movement is Improvement: The Therapeutic Effects of Exercise and General Physical Activity on Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther [Internet]. 2021;12(3):707-32. Available from: https://doi.org/10.1007/s13300-021-01005-1.
|
| 11. |
Luo L, Pang B, Chen J, Li Y, Xie X. Assessing the Impact of Lifestyle Interventions on Diabetes Prevention in China: A Modeling Approach. Int J Environ Res Public Health [Internet]. 2019;16(10):1677. Available from: https://www.mdpi.com/1660-4601/16/10/1677.
|
| 12. |
de Oliveira Teles G, da Silva CS, Rezende VR, Rebelo ACS. Acute Effects of High-Intensity Interval Training on Diabetes Mellitus: A Systematic Review. Int J Environ Res Public Health [Internet]. 2022;19(12):7049. Available from: https://www.mdpi.com/1660-4601/19/12/7049.
|
| 13. |
Munan M, Oliveira CLP, Marcotte-Chénard A, Rees JL, Prado CM, Riesco E, et al. Acute and Chronic Effects of Exercise on Continuous Glucose Monitoring Outcomes in Type 2 Diabetes: A Meta-Analysis. Front Endocrinol (Lausanne) [Internet]. 2020;11:495. Available from: https://www.frontiersin.org/article/10.3389/fendo.2020.00495/full.
|
| 14. |
Alexandre K, Campbell J, Bugnon M, Henry C, Schaub C, Serex M, et al. Factors influencing diabetes self-management in adults: an umbrella review of systematic reviews. JBI Evid Synth [Internet]. 2021;19(5):1003-118. Available from: https://journals.lww.com/10.11124/JBIES-20-00020.
|
| 15. |
Whipple MO, Pinto AJ, Abushamat LA, Bergouignan A, Chapman K, Huebschmann AG, et al. Sex Differences in Physical Activity Among Individuals With Type 2 Diabetes Across the Life Span: A Systematic Review and Meta-analysis. Diabetes Care [Internet]. 2022;45(9):2163-77. Available from: https://diabetesjournals.org/care/article/45/9/2163/147491/Sex-Differences-in-Physical-Activity-Among.
|
| 16. |
Doherty J, Giles M, Gallagher AM, Simpson EEA. Understanding pre-, peri- and post-menopausal women’s intentions to perform muscle-strengthening activities using the Theory of Planned Behaviour. Maturitas [Internet]. 2018;109:89-96. Available from: https://www.sciencedirect.com/science/article/pii/S0378512217308551.
|
| 17. |
Abell LP, Tanase KA, Gilmore ML, Winnicki AE, Holmes VL, Hartos JL. Do physical activity levels differ by number of children at home in women aged 25–44 in the general population? Women’s Heal [Internet]. 2019;15:174550651987118. Available from: http://journals.sagepub.com/doi/10.1177/1745506519871186.
|
| 18. |
Pedersen MRL, Hansen AF, Elmose-Østerlund K. Motives and Barriers Related to Physical Activity and Sport across Social Backgrounds: Implications for Health Promotion. Int J Environ Res Public Health [Internet]. 2021;18(11):5810. Available from: https://www.mdpi.com/1660-4601/18/11/5810.
|
| 19. |
Lao XQ, Deng H-B, Liu X, Chan T-C, Zhang Z, Chang L, et al. Increased leisure-time physical activity associated with lower onset of diabetes in 44 828 adults with impaired fasting glucose: a population-based prospective cohort study. Br J Sports Med [Internet]. 2019;53(14):895-900. Available from: https://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2017-098199.
|
| 20. |
Oh HS. Opposite Effects of Work-Related Physical Activity and Leisure-Time Physical Activity on the Risk of Diabetes in Korean Adults. Int J Environ Res Public Health [Internet]. 2020;17(16):5812. Available from: https://www.mdpi.com/1660-4601/17/16/5812.
|
| 21. |
Lee E-B, Hong S, Min J, Park D-H, Cho W, Suh S-H, et al. Association between domain-specific physical activity and diabetes in Korean adults. Sci Rep [Internet]. 2021;11(1):13066. Available from: https://doi.org/10.1038/s41598-021-92560-x.
|
| 22. |
Chen M, Moran LJ, Harrison CL, Ukke GG, Sood S, Bennett CJ, et al. Ethnic differences in response to lifestyle intervention for the prevention of type 2 diabetes in adults: A systematic review and meta‐analysis. Obes Rev [Internet]. 2022;23(1). Available from: https://onlinelibrary.wiley.com/doi/10.1111/obr.13340.
|
| 23. |
Esefeld K, Heinicke V, Kress S, Behrens M, Zimmer P, Stumvoll M, et al. Diabetes, Sport und Bewegung. Der Diabetol [Internet]. 2020;16(3):292-9. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/a-1193-3901.
|
| 24. |
Pereira WVC, Vancea DMM, de Andrade Oliveira R, de Freitas YGPC, Lamounier RN, Silva Júnior WS, et al. 2022: Position of Brazilian Diabetes Society on exercise recommendations for people with type 1 and type 2 diabetes. Diabetol Metab Syndr [Internet]. 2023;15(1):2. Available from: https://doi.org/10.1186/s13098-022-00945-3.
|
| 25. |
Schubert-Olesen O, Kröger J, Siegmund T, Thurm U, Halle M. Continuous Glucose Monitoring and Physical Activity. Int J Environ Res Public Health [Internet]. 2022 Sep 28;19(19):12296. Available from: https://www.mdpi.com/1660-4601/19/19/12296.
|
| 26. |
Gujral S, Aizenstein H, Reynolds CF, Butters MA, Erickson KI. Exercise effects on depression: Possible neural mechanisms. Gen Hosp Psychiatry [Internet]. 2017;49:2-10. Available from: https://www.sciencedirect.com/science/article/pii/S0163834317301159.
|
| 27. |
Arsh A, Afaq S, Carswell C, Bhatti MM, Ullah I, Siddiqi N. Effectiveness of physical activity in managing co-morbid depression in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. J Affect Disord [Internet]. 2023;329:448-59. Available from: https://www.sciencedirect.com/science/article/pii/S0165032723002902.
|
| 28. |
Patterson R, McNamara E, Tainio M, de Sá TH, Smith AD, Sharp SJ, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol [Internet]. 2018;33(9):811-29. Available from: https://doi.org/10.1007/s10654-018-0380-1.
|
| 29. |
Bailey DP, Hewson DJ, Champion RB, Sayegh SM. Sitting Time and Risk of Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. Am J Prev Med [Internet]. 2019;57(3):408-16. Available from: https://www.sciencedirect.com/science/article/pii/S0749379719302090.
|
| 30. |
Deng M-G, Cui H-T, Lan Y-B, Nie J-Q, Liang Y-H, Chai C. Physical activity, sedentary behavior, and the risk of type 2 diabetes: A two-sample Mendelian Randomization analysis in the European population. Front Endocrinol (Lausanne) [Internet]. 2022;13. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2022.964132/full.
|
| 31. |
Hallgren M, Nguyen T-T-D, Owen N, Stubbs B, Vancampfort D, Lundin A, et al. Cross-sectional and prospective relationships of passive and mentally active sedentary behaviours and physical activity with depression. Br J Psychiatry [Internet]. 2019/03/21. 2020;217(2):413-9. Available from: https://www.cambridge.org/core/product/DBB965C7F964604B9004AEFA1C50E73B.
|
| 32. |
Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sport Med [Internet]. 2020;50(2):295-330. Available from: https://doi.org/10.1007/s40279-019-01183-w.
|
| 33. |
Katzmarzyk PT, Friedenreich C, Shiroma EJ, Lee I-M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med [Internet]. 2022;56(2):101LP-106. Available from: http://bjsm.bmj.com/content/56/2/101.abstract.
|
| 34. |
Saco‐Ledo G, Valenzuela PL, Ruiz‐Hurtado G, Ruilope LM, Lucia A. Exercise Reduces Ambulatory Blood Pressure in Patients With Hypertension: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials. J Am Heart Assoc [Internet]. 2020;9(24). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.120.018487.
|
| 35. |
He N, Ye H. Exercise and hyperlipidemia. In: Physical exercise for human health [Internet]. Springer; 2020. p. 79-90. Available from: https://link.springer.com/chapter/10.1007/978-981-15-1792-1_5.
|
| 36. |
Adolfsson P, Taplin CE, Zaharieva DP, Pemberton J, Davis EA, Riddell MC, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in children and adolescents with diabetes. Pediatr Diabetes [Internet]. 2022;23(8):1341-72. Available from: https://doi.org/10.1111/pedi.13452.
|
| 37. |
Ozaslan B, Patek SD, Fabris C, Breton MD. Automatically accounting for physical activity in insulin dosing for type 1 diabetes. Comput Methods Programs Biomed [Internet]. 2020;197:105757. Available from: https://www.sciencedirect.com/science/article/pii/S016926072031590X.
|
| 38. |
Petroni ML, Brodosi L, Marchignoli F, Sasdelli AS, Caraceni P, Marchesini G, et al. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients. 2021;13(8): 2748. Available from: https://www.mdpi.com/2072-6643/13/8/2748.
|
| 39. |
Koehler K, Drenowatz C. Integrated Role of Nutrition and Physical Activity for Lifelong Health. Vol. 11, Nutrients. 2019.
|
| 40. |
Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients. 2019;11(11):2611. Available from: https://www.mdpi.com/2072-6643/11/11/2611.
|
| 41. |
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes – 2023. Diabetes Care [Internet]. 2022;46(Supplement_1):S128-39. Available from: https://doi.org/10.2337/dc23-S008.
|
| 42. |
Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med Sci Sport Exerc [Internet]. 2022;54(2):353-68. Available from: https://journals.lww.com/10.1249/MSS.0000000000002800.
|
| 43. |
Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia [Internet]. 2022;65(12):1925-66. Available from: https://doi.org/10.1007/s00125-022-05787-2.
|
| 44. |
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes – 2023. Diabetes Care [Internet]. 2023 Jan 1;46(Supplement_1):S68-96. Available from: https://diabetesjournals.org/care/article/46/Supplement_1/S68/148055/5-Facilitating-Positive-Health-Behaviors-and-Well.
|
| 45. |
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA [Internet]. 2018;320(19):2020-8. Available from: https://doi.org/10.1001/jama.2018.14854.
|
| 46. |
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med [Internet]. 2020;54(24):1451-62. Available from: https://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2020-102955.
|







