Abstract
Gamma knife radiosurgery (GKRS) is a minimally invasive technique frequently employed in neurosurgery or oncology, and it has applications in psychiatric patients as well. While it is commonly used in conditions like obsessive-compulsive disorder, GKRS also has efficacy in treating major depression disorder, generalized anxiety disorder, autism spectrum disorder and anorexia nervosa. Promising outcomes have been observed, particularly in cases resistant to conventional treatment, leading to significant improvements in the patients' quality of life. Severe adverse effects from GKRS procedures are rare. To enhance our understanding of the utilization of GKRS in psychiatry, further extensive research, especially through double-blinded studies involving larger cohorts, is imperative. Determining the ideal volume and radiation dose for radiosurgical capsulotomy remains a key topic of research. When it comes to psychiatric neurosurgical procedures, the decision-making process should be personalized for each patient, taking all relevant factors into consideration.
Citation
Kwinta R, Kopcik K, Koberling A. Gamma knife radiosurgery in psychiatry: a review. Eur J Transl Clin Med. 2024;7(1):87-96Introduction
The incidence of mental disorders in the present century is increasing and the effectiveness of treatment of mental disorders is a significant clinical problem, as the therapy mechanisms are not fully discovered [1-2]. A wider knowledge of the neurophysiology and the influence of stereotactic procedures on neural tissue, coupled with advances in modern neuroimaging, has resulted in increased interest in radiosurgery [3]. Stereotactic radiosurgery (SRS) was created in the mid-20th century and involves the administration of a high dose of single ionizing radiation through the intact skull into a critically located small intracranial target [4]. Radiosurgery has been developed over decades by clinicians, physicists and engineers [5]. SRS involves exposing the intracranial target to focused radiation beams from many angles using the exact correlation of the virtual target visible on radiological images. The gamma knife (GK) procedure is a stereotactic method based on frames with Cobalt-60 sources. It combines high precision of convergence of numerous gamma rays emitted by multiple sources of Cobalt-60 with image guidance [4]. The final effective dose rate is predictable because the activity of each Cobalt-60 source at the time of manufacture and its deterioration over time are well-estabilished [6]. GK radiosurgery (GKRS) has clinical application including both benign and malignant tumors of the brain and skull base, functional and psychiatric disorders and vascular malformations [1, 7]. The radiological diagnosis is vital before starting treatment with GKRS [4].
Lars Leksell invented the GK and first used it clinically in 1967 to treat a patient with craniopharyngioma in 1967 [5]. The first use of GKRS in psychiatry was also described by Leksell in 1985: he treated a 29 years old male with resistant obsessive-compulsive disorder. Researchers used a maximum radiation dose of 100-120 Gy and the results after one month were unsatisfactory [8]. Currently, GKRS is being explored as a potential treatment method, for the severe and treatment-resistant cases of obsessive compulsive disorder (OCD), major depression disorder, generalized anxiety disorder (GAD) and autism spectrum disorder (ASD). The main GKRS procedures performed in psychiatry are anterior capsulotomy (making small changes to the anterior cingulate gyrus and the anterior limb of the internal capsule), subcaudate tractomy (disrupting the continuous white matter fibers found in the corpus callosum) and cingulotomy [1-2]. Main aim of such psychosurgery is improvement of the patient’s quality of life, reducing compulsions, obsessions, anxiety and depression by delivering precise and targeted radiation to specific brain areas [1]. Severe complications in SRS procedures are rare [9]. While research is ongoing and its long-term effects are still being studied, this innovative approach underscores the evolving intersection between technology and psychiatric care.
The aim of this study was to summarize current knowledge regarding the use of GKRS in patients with psychiatric indications.
Material and methods
To conduct this review, we searched the PubMed and Google Scholar databases for articles related to application of GK use in psychiatry. We used key words such as ‘gamma knife psychiatry’, ‘radiosurgery psychiatry’, ‘gamma knife OCD’, ‘gamma ventral capsulotomy’. Our focus was on articles published in between 2017 and 2023, including their references, as we wanted to focus on most recent publicatons. We assessed the papers by titles, abstracts and full texts, with the main inclusion criteria being that they addressed the use of GKRS in psychiatry. Among 83 papers from databases, after removing duplicates, 35 articles were identified, including review articles, case series and case reports from the field of neurosurgery and psychiatry. After evaluation, 14 papers were included to the paper, as they were raising the problem of the use of GK surgery in psychiatric disorders, while 16 publications were excluded. The most important criteria of exclusion was if the paper is describing radiosurgery methods with GK device implementation, as majority of the works described other methods of radiosurgery. Accepted papers were divided into two groups: 10 articles about GK use in the treatment of OCD and 4 articles describing 5 studies about its use in other psychiatric conditions.
Results
Despite the fact that GK surgery in psychiatry is most commonly used in treatment of OCD, we found reports about the implementation of this method in the treatment of generalized anxiety disorder (GAD), autism spectrum disorder (ASD) and major depressive disorder (MDD). Studies show that GKRS is in most cases implemented in long-lasting conditions resistant for pharmacological treatment [10]. For clarity, all the introductory information about the particular psychiatric conditions is presented here in the Results section, together with the outcomes of using GKRS in their treatment.
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is based on recurring, unwanted thoughts (obsessions) or reiterative actions (compulsions) designed to reduce or prevent fear and anxiety [11-12]. It is estimated that this disorder affects between 1% and 3% of the population worldwide, with symptoms of varying severity. Drug treatment and the use of behavioral therapy is ineffective in 3-5% of patients with OCD [13]. Drug resistance may be caused by both environmental factors and personality disorders. Other treatment methods include deep-brain stimulation, neurosurgical ablation or transcranial magnetic stimulation [14-16]. It is important to remember that OCD varies in severity and drug resistance can have different easons, e.g. psychiatric psychiatric comorbidity or environmental factors [13]. In such cases, radiosurgical methods can be used, including the use of GK and its anterior capsulotomy (GVC) approach, but it is important to remember that this method is still experimental and may need further research or ethics comittees approval in some countries. This procedure involves creating tiny lesions in the ventral part of the anterior limbic internal capsule (ALIC), whose fibers transmit information from the prefrontal cortex to the structures of the basal ganglia structures [12-13, 17-20]. GVC outcomes can be measured using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). It consists of two parts – for obsessive thoughts and for compulsive behavior. The minimum score that can be obtained is zero, while the maximum score is 40. It is possible to interpret the Y-BOCS score as follows: 1) < 8 (subclinical symptoms), 2) > 16 (clinically significant symptoms), 3) ≥ to 24 (moderately severe OCD) [17-18]. Patients are considered responsive to GVC if there is a ≥ 35% reduction Y-BOCS scores after treatment [17, 19].
Due to the large number of reports on the implementation of GK in the management of refractory OCD, selected cases described in ten papers are briefly described in Table 1 [11-13, 17, 19, 21-25].
Table 1. Summary of selected studies regarding GVC in OCD treatment
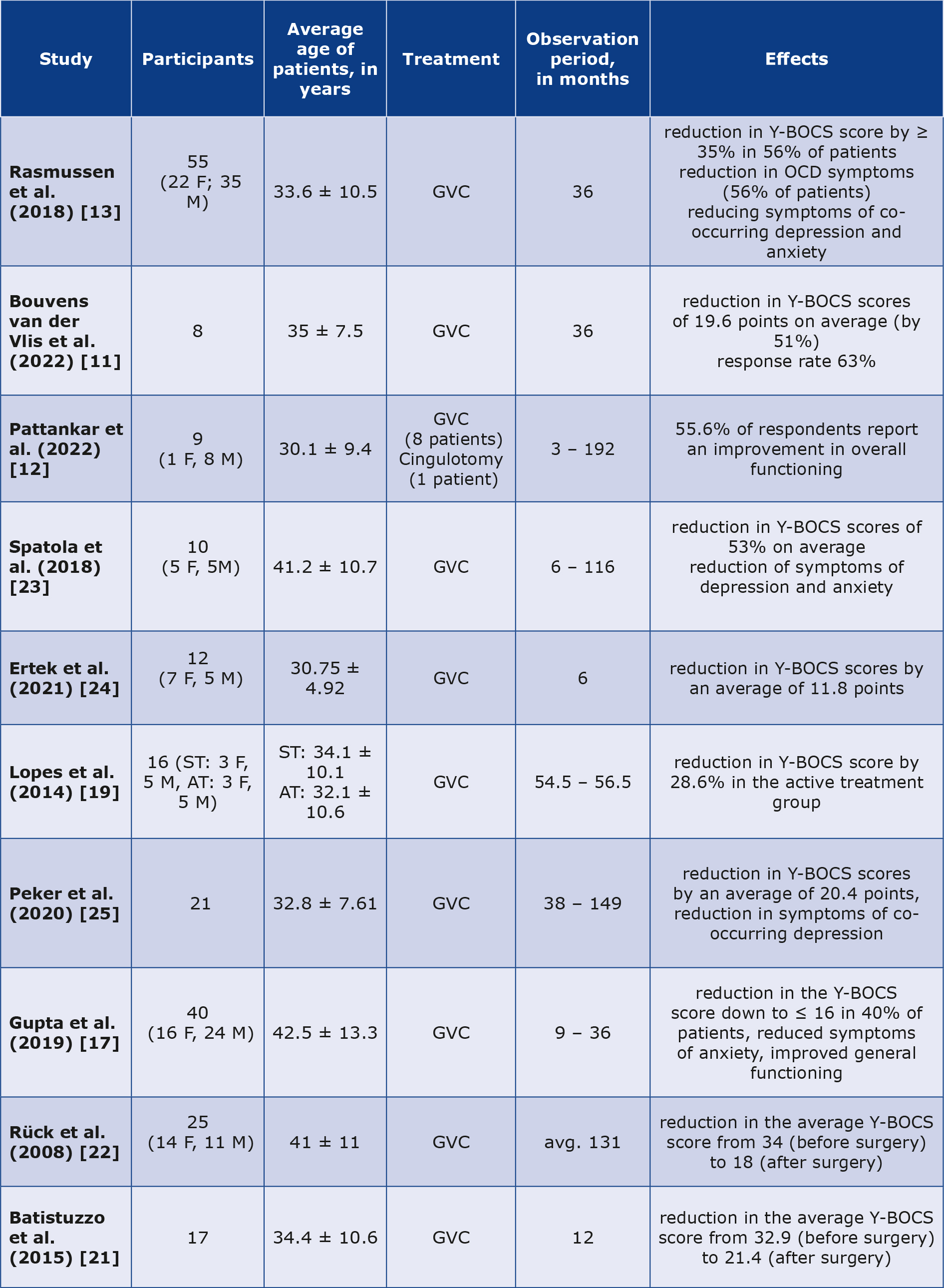
AT – active treatment, F – female, GVC – gamma ventral capsulotomy, M – male, OCD – obsessive-compulsive disorder, ST – sham treatment, Y-BOCS – Yale-Brown Obsessive-Compulsive Scale
A randomized study by Lopes et al. included 16 patients with resistant OCD, who were randomly divided into active and sham group, both containing 8 patients [19]. After 1 year, the median Y-BOCS score was 23.5 in the active group and 31 in the sham group. At month 12, the two groups were not statistically different in terms of anxiety (assessed using the Beck Anxiety Inventory, BAI) and depression (via the Beck Depression Inventory (BDI)). At month 24 after GVC three additional patients responded [19].
The paper by Rasmussen et al. presents the results of a follow-up study in patients with refractory OCD after GVC using the Leksell Gamma Knife [13]. The subjects were divided into two groups, which consisted of 15 and 40 patients. Patients in the first group were subjected to repeated single shots. The target site of the second stage (also a repeated single shot) was set directly ventral to the first stage shot. The second group of subjects received two shots bilaterally. Thirty-one patients (56%) improved by more than 35% over the 3-year follow-up period using the Y-BOCS scale [13]. The study used the Sickness Impact Profile to compare baseline and follow-up measures of functional status and quality of life. After three years, improvements were noted in emotional behavior and social interactions in the group that received repeated single injections. Nonetheless, there were improvements observed in the second group in terms of emotional behavior, recreational activities, sleep and rest, home management, mobility, and work. At 6 months, the group, which included 15 patients, showed no significant improvement in the Y-BOCS, Clinical Global Impression Scale (CGI-I), or Global Assessment of Functioning (GAF) scales. The group that received the double injection showed improvement in OCD symptoms (based on the Y-BOCS), depressive symptoms (based on the Hamilton Depression Scale) and anxiety (based on the Hamilton Anxiety Scale) [13].
A study by Bouvens van der Vlis et al. analyzed magnetic resonance (MR) images of 8 OCD patients with at least 3 years of follow-up after GVC surgery [11]. The strongest correlation with a reduction in symptom severity was found for a reduction in left ventral diencephalon volume. The largest decrease in Y-BOCS scores was observed between 6 months and 1 year, of -7.1 ± 5.6. The mean total reduction in Y-BOCS was 19.6 after 3 years of follow-up, with equal reductions for obsessions and compulsions [11].
A retrospective study by Pattankar et al. included patients with refractory OCD [11]. The following psychiatric comorbidities were present in the study group: generalized anxiety disorder (55.6%), depression (44.4%), and the occurrence of self-destructive ideations was confirmed by 33.3% of the patients. The mean Y-BOCS score at the last follow-up was 23.8 ± 7.7. Four patients showed a complete or fractional response (≥ 25% reduction in Y-BOCS score) at last follow-up. For GVC, 4 out of 5 patients with moderate or severe OCD presented favourable outcomes, while all of the three patients with extreme cases of OCD remained nonresponders (< 25% reduction in Y-BOCS score) [12]. One patient after cingulotomy remained resistant to treatment (< 25% reduction in Y-BOCS score) [12].
The study described by Spatola et al. involved patients whose previous treatment had been unsuccessful. Comorbidities in this group included bipolar disorder, depression, generalized anxiety disorder, personality disorder and tics. Before radiosurgery using GKRS, the mean Y-BOCS score was 32.7 ± 4.8 (17.3 ± 1 for obsession score; 16.3 ± 3.6 for compulsion score). Seven patients presented positive effects of GVC and two were classified as non-responders. The mean score of Y-BOCS was 14.7 ± 8.8 [23]. It is noteworthy that bipolar disease as a comorbidity is an important limitation.
A retrospective study by Ertek et al. (2021) included patients who underwent radiosurgery with Elekta Gamma-Knife between 2005 and 2020 [24]. GVC was performed on both sides, with a maximum radiation dose varying from 140 to 180 Gy. The mean Y-BOCS score decreased from 32.3 to 20.5 at 6 months after surgery. Half of the patients met the criteria for achieving a full response, which is typically defined in the literature as an improvement of more than 35% in the Y-BOCS score [24].
A single-centre retrospective study performed by Peker et al. included patients with OCD unresponsive to current treatment [25]. A bilateral single shot or a bilateral double shot technique was used. The radiation was between 140 and 150 Gy. Fifteen patients (75%) achieved a complete positive reaction and five were described as non-responders. The final status of one of the patients is not known because he was not followed up after 6 months. The mean pre-GVC Beck Depression Inventory-II scale score of 35.1 reduced to 13.8 after 36 months of follow-up [25].
An international, multicentre, retrospective cohort study was performed by Gupta et al. A group of patients with Y-BOCS scores ≥ 24 (for this group: 35 ± 3.6) underwent GVC using 1 or 2 isocentres with a dose between 120 and 180 Gy [17]. The range of Y-BOCS test values after GVC was 27.5 ± 10.8. According to the authors, eighteen patients (45%) were considered responsive to the applied treatment and 16 (40%) of them were in remission at the last post-event check. Nineteen patients (47.5%) remained stable with Y-BOCS in the range of 26-36 after GVC and three patients (7.5%) had worse Y-BOCS scores [17].
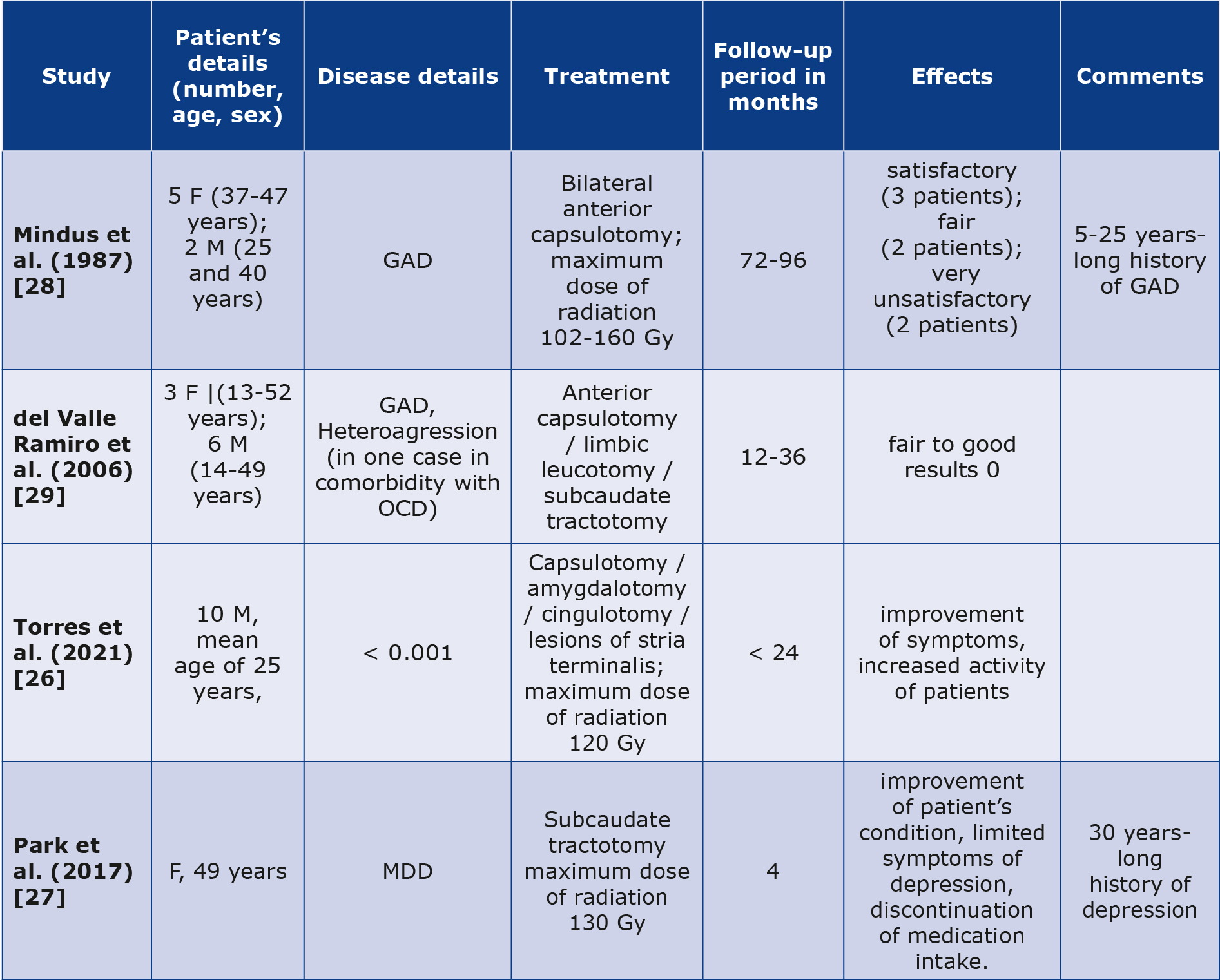
Brief description of GK radiosurgery outcomes in patients suffering from diseases other than OCD is presented in Table 2 [8, 26-29].
Table 2. Brief summary of selected cases of GKRS in psychiatric disorders other than OCD
GAD – generalized anxiety disorder, F – female, M – male, MDD – major depressive disorder, OCD – obsessive-compulsive disorder
Generalized anxiety disorder
Generalized anxiety disorder (GAD) is a mental condition typically presenting with persevering and uncontrollable fear about various aspects of life. Patients suffering from GAD experience worry or fear to an extreme and it significantly limits their daily functioning and overall mental state [30]. According to Mindus et al., the results of GKRS in GAD were not satisfactory and unfortunately were not specified [30]. The primary point made by Mindus et al. is that magnetic resonance imaging could assist in establishing a radiation threshold that is effective for clinical purposes in radiosurgery. This information would be valuable for designing future studies related to GK capsulotomy. More recently, del Valle Ramiro et al. also evaluated patients with GAD [29]. Reseachers focused on different mental conditions, including GAD, OCD and schizophrenia, but their work faced criticism, as indications was unclear and controversial, with no evauation based on clinical scales presented, technique was not described and there were no follow-up reports stated, what should be considered as an important limitation [8].
Major depressive disorder
Major depressive disorder (MDD) is a mental health condition characterized by constant feelings of sadness, hopelessness, and a lack of interest or pleasure in activities that were once enjoyable. It feels more severe than normal feelings of sadness, but it is vital to remember that presentation may differ significantly from one individual to another [31- 32]. MDD can significantly impact daily life and functioning of the patient [32-33]. While GK treatment is initially used for various neurological and mental conditions, its application in treating depression is not as common as pharmacotherapy, psychotherapy, electroconvulsive therapy, magnetic seisure therapy, direct current stimulation (DCS), vagus nerve stimulation (VNS), deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), phototherapy and relaxation or meditation techniques [34]. A potential patient must fulfill clinical criteria related to the severity, chronicity and treatment resistance as determined by a multidisciplinary team’s decision-making process [27]. Severity may be assessed with scales such as HAMD-17, BDI or MADRS [35]. Case report narrated by Park et al. was based on subcaudate tractotomy in case of a long-term, refractory depression followed by nine suicidal attempts [27]. Other surgical method for patients with resistant MDD may be cingulotomy, but case studies and evidence are limited [27]. Their case revealed that depression burden decreased after GKRS, with discontinuation of pharmacotherapy 4 months postoperatively [27].
Autism spectrum disorder
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder affecting communication skills, social interactions, behavior and motor or sensory experiences with a wide range of symptoms and levels of severity [26, 36-37]. Management methods can be based on a combination of behavioral therapy, speech and language therapy, occupational therapy and social skills training [36-37]. Treatment methods for pediatric and adult patients vary and their further description exceedes the scope of this paper [36-37]. The main aim is to help patients develop essential life and social skills, as it will increase their quality of life and level of independence [26, 36-37]. Torres et al. described the implementation of radiofrequency and GKRS on patients with ASD, who were refractory to traditional treatment. After the procedure, patients were evaluated in three behavioral scales: The Overt Aggression Scale (OAS), Parental Concern Questionnaire (PCQ), Children’s Yale-Brown Obsessive Compulsive Scale for Autism Spectrum Disorder (CYBOCS-ASD). MR scans were obtained (at 6 months and one year after the procedure, then once a year for the next 5 years post-surgery). Results were positive, with improvement in every patient since the first week after the procedure. The symptoms of the 10 patients showed a notable decrease (PCQ 39.9 to 33, OAS 11.8 to 5, CYBOCS-ASD 30.4 to 20) both before the surgery and at the latest follow-up. In all cases there was an almost total reduction of agressiveness and significant decrease of restrictive-repetitive actions, which was confirmed by patient’s caregivers The authors did not specify examples of improvement in the functioning of patients with autism, other than improvement according to the mentioned scales [26].
Discussion
Modern psychosurgery is significantly safer and more effective than invasive procedures from the past, e.g. leucotomy, lobotomy, lesioning surgeries of the hypothalamus or amygdala [26, 38-39]. Ablative techniques are connected with lower costs and no need to implement artificial devices. Also, as craniotomy is not necessary, the risk of infection or hemorrage are excluded, as far as the risks connected with anaesthesiological procedures [38]. The unsatisfactory results of previous OCD treatment with GK capsulotomy led to a change in the initial treatment target to the most ventral part of the internal capsule and the ventral part of the striatum [20]. Based on the studies presented in this paper, it can be concluded that GKRS is a safe and effective procedure that can be implemented in selected cases of resistant OCD [12-13, 25]. However, it is not possible to predict clinical outcome after GVC treatment in patients with refractory OCD. This is suggested by the variability of ALIC fibre organization between patients, which can be detected by neuroimaging studies, allowing the precise target of the treatment to be determined [11, 20]. It is noteworthy that patients undergoing GVC showed significant reduction of symptoms such as depression, anxiety, followed by improvement of the quality of life and better overall functioning [13]. Creating and implementing strict guidelines for using surgical methods in the treatment of psychiatric disorders is crucial and should be taken under consideration.
Adverse effects after GVC are rare and patients tolerated the procedure well. Rasmussen et al. observed transient oedema (9% of patients), cysts (5%) and radiation necrosis leading to a state of minimal consciousness (1.8%) [13]. Adverse effects observed by Peker et al. included transient headache (14.3% of patients), persistent headache (9.5%), and brain cyst (10%) [25]. No clinically significant abnormalities on neurological examination were reported in this group. For the group studied by Gupta et al., mood disorders occurred after GVC in 25% of patients, neurological complications in 7.5% and one patient developed radiation necrosis [17]. In contrast, Ertek et al. described the occurrence of headaches in 16.7% of patients [24]. Placebo effect seems to be absent or weak, according to the literature [8].
Among the neuropsychiatric adverse effects one can distinguish cognitive decline, insomnia or anxiety. It is important to remember that unsatisfactory treatment results, combined with inadequate pharmacological management, may lead to suicidality. In light of this, it seems justified to maintain pharmacological treatment and optimize it to prevent suicidal tendencies in patients [27].
Implementation of GKRS in psychiatric disorders other than OCD seems to have promising outcomes. Studies held in the 1980s revealed unsatisfactory results, but modern evaluations have results ranging from fair to good. The main reason for this difference is the improvement in GKRS techniques and the associated increased safety of these procedures.[8, 10, 26-27, 40]. Randomized controlled trials (RCT) with GK are potentially possible, because patients may not be aware whether they are being treated or not (they may not be informed if they are receiving radiation doses while in the operating room). The major limitation of RCTs using GK is the radiation exposure, which creates additional technical obstacles for researchers. So far, only one RCT using GK was conducted among psychiatric patients: a 2014 study by Lopes et al. regarding patients with OCD [19, 41]. It is vital to remember that modern GK devices seem not to emit radiation, as its sources remain covered. This fact may create an opportunity to widening the research using tools such as randomized clinical trials [41]. Double-blinded studies of larger cohorts are needed to extend the knowledge about psychiatric use of GK.
In spite of improvements in GK technology and radiological imaging techniques over time, the optimal volume and dose of radiation appropriate for radiosurgical capsulotomy remains a subject of research. Currently, two major trends in these issues stand out; lowering the radiation dose accompanied by a decrease in adverse events and the use of more refined analytical techniques to draw conclusions (incidence of adverse events, radiation dosage, location) [20, 42]. In view of this, further research is needed on the effective radiation dose and targeting methods that maximize the effectiveness of GVC and reduce the risk of adverse effects [13]. Furthermore, a reduction in the severity of OCD symptoms may result not only from direct modulation of neuronal pathways, but also from the increased efficacy of pharmacological and psychological therapies acting synergistically with GVC [42].
Conclusions
Decision-making regarding neurosurgical procedures to treat a mental illness should be individualized for each patient. Many factors should be considered, such as patient preferences and attitudes, risks, psychosocial support, quality of life (current and anticipated), comorbidity, socio-economic situation and follow-up. The role of the multidisciplinary team is to assist patients in deciding on treatment options. It should include clinicians specialized in all therapies under consideration. Restricted access to the centers experienced in performing GKRS procedures is an important limitation. More data from randomized clinical trials, preferrably from multiple centers, are needed to extend the use of GKRS in psychosurgery. More research is needed regarding effective radiation doses.
Conflicts of interest
None to report.
Funding
None to report.
References
| 1. |
Sadashiva N, Tripathi M, De Salles A. Contemporary Role of Stereotactic Radiosurgery for Psychiatric Disorders. Neurol India [Internet]. 2023;71(7):31. Available from: https://journals.lww.com/10.4103/0028-3886.373648.
|
| 2. |
Basiaga B, Bednarz K, Trojan S, Leśniak M, Kwieciński J, Miszuda S, et al. Invasive treatment of selected psychiatric diseases. Qual Sport [Internet]. 2023;12(1):43–51. Available from: https://apcz.umk.pl/QS/article/view/43579.
|
| 3. |
Martínez-Álvarez R, Torres-Diaz C. Modern Gamma Knife radiosurgery for management of psychiatric disorders. In: Progress in brain research [Internet]. Prog Brain Res; 2022. p. 171–83. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079612321002454.
|
| 4. |
Sadashiva N, Tripathi M. Safety checklist for gamma knife radiosurgery. Asian J Neurosurg [Internet]. 2019;14(04):1308–11. Available from: http://www.thieme-connect.de/DOI/DOI?10.4103/ajns.AJNS_237_19.
|
| 5. |
Knisely JPS, Apuzzo MLJ. Historical Aspects of Stereotactic Radiosurgery: Concepts, People, and Devices. World Neurosurg [Internet]. 2019;130:593–607. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875019310228.
|
| 6. |
Sanders J, Nordström H, Sheehan J, Schlesinger D. Gamma Knife radiosurgery: Scenarios and support for re-irradiation. Phys Medica [Internet]. 2019;68:75–82. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1120179719304910.
|
| 7. |
China M, Vastani A, Hill CS, Tancu C, Grover PJ. Gamma Knife radiosurgery for cerebral arteriovenous malformations: a systematic review and meta-analysis. Neurosurg Rev [Internet]. 2022;45(3):1987–2004. Available from: https://link.springer.com/10.1007/s10143-022-01751-1.
|
| 8. |
Lévêque M, Carron R, Régis J. Radiosurgery for the Treatment of Psychiatric Disorders: A Review. World Neurosurg [Internet]. 2013;80(3–4):S32.e1-S32.e9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875013007754.
|
| 9. |
Cormier J, Iorio-Morin C, Mathieu D, Ducharme S. Psychiatric Neurosurgery: A Survey on the Perceptions of Psychiatrists and Residents. Can J Neurol Sci / J Can des Sci Neurol [Internet]. 2019/04/12. 2019;46(3):303–10. Available from: https://www.cambridge.org/core/product/identifier/S0317167119000052/type/journal_article.
|
| 10. |
Liu W, Li D, Sun F, Zhang X, Wang T, Zhan S, et al. Long-Term Follow-up Study of MRI-Guided Bilateral Anterior Capsulotomy in Patients With Refractory Anorexia Nervosa. Neurosurgery [Internet]. 2018;83(1):86–92. Available from: https://journals.lww.com/00006123-201807000-00013.
|
| 11. |
Bouwens van der Vlis TAM, Samanci Y, Ackermans L, Schruers KRJ, Temel Y, Leentjens AFG, et al. Network analysis in Gamma Knife capsulotomy for intractable obsessive-compulsive disorder. Brain and Spine [Internet]. 2022;2:100892. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2772529422000339.
|
| 12. |
Pattankar S, Sankhe M, Chavda K. Efficacy of Gamma Knife Radiosurgery in Refractory Obsessive-Compulsive Disorder: An Indian Experience. J Neurosci Rural Pract [Internet]. 2022;13(1):23. Available from: https://ruralneuropractice.com/efficacy-of-gamma-knife-radiosurgery-in-refractory-obsessive-compulsive-disorder-an-indian-experience/.
|
| 13. |
Rasmussen SA, Noren G, Greenberg BD, Marsland R, McLaughlin NC, Malloy PJ, et al. Gamma Ventral Capsulotomy in Intractable Obsessive-Compulsive Disorder. Biol Psychiatry [Internet]. 2018;84(5):355–64. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006322317322849.
|
| 14. |
Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-Compulsive Disorder. JAMA [Internet]. 2017;317(13):1358. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2017.2200.
|
| 15. |
Rapinesi C, Kotzalidis GD, Ferracuti S, Sani G, Girardi P, Del Casale A. Brain Stimulation in Obsessive-Compulsive Disorder (OCD): A Systematic Review. Curr Neuropharmacol [Internet]. 2019;17(8):787–807. Available from: http://www.eurekaselect.com/171490/article.
|
| 16. |
Fitzsimmons SMDD, van der Werf YD, van Campen AD, Arns M, Sack AT, Hoogendoorn AW, et al. Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. J Affect Disord [Internet]. 2022;302:302–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165032722000544.
|
| 17. |
Gupta A, Shepard MJ, Xu Z, Maiti T, Martinez-Moreno N, Silverman J, et al. An International Radiosurgery Research Foundation Multicenter Retrospective Study of Gamma Ventral Capsulotomy for Obsessive Compulsive Disorder. Neurosurgery [Internet]. 2019;85(6):808–16. Available from: https://journals.lww.com/10.1093/neuros/nyy536.
|
| 18. |
Balachander S, Arumugham S, Srinivas D. Ablative neurosurgery and deep brain stimulation for obsessive-compulsive disorder. Indian J Psychiatry [Internet]. 2019;61(7):77. Available from: https://journals.lww.com/10.4103/psychiatry.IndianJPsychiatry_523_18.
|
| 19. |
Lopes AC, Greenberg BD, Canteras MM, Batistuzzo MC, Hoexter MQ, Gentil AF, et al. Gamma Ventral Capsulotomy for Obsessive-Compulsive Disorder. JAMA Psychiatry [Internet]. 2014;71(9):1066. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2014.1193.
|
| 20. |
Kochanski RB, Slavin K V. Gamma Knife radiosurgery for obsessive compulsive disorder. In: Progress in Brain Research [Internet]. Elsevier; 2022. p. 185–95. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079612322000474.
|
| 21. |
Batistuzzo MC, Hoexter MQ, Taub A, Gentil AF, Cesar RC, Joaquim MA, et al. Visuospatial Memory Improvement after Gamma Ventral Capsulotomy in Treatment Refractory Obsessive–Compulsive Disorder Patients. Neuropsychopharmacology [Internet]. 2015;40(8):1837–45. Available from: https://www.nature.com/articles/npp201533.
|
| 22. |
Rück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, et al. Capsulotomy for Obsessive-Compulsive Disorder. Arch Gen Psychiatry [Internet]. 2008;65(8):914. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.65.8.914.
|
| 23. |
Spatola G, Martinez-Alvarez R, Martínez-Moreno N, Rey G, Linera J, Rios-Lago M, et al. Results of Gamma Knife anterior capsulotomy for refractory obsessive-compulsive disorder: results in a series of 10 consecutive patients. J Neurosurg [Internet]. 2019;131(2):376–83. Available from: https://thejns.org/view/journals/j-neurosurg/131/2/article-p376.xml.
|
| 24. |
Ekmekçi Ertek İ, Uçar Ö, Yaman ME, Emmez ÖH, Candansayar S. Treatment Outcomes of Gamma-Knife Radio Surgery in Refractory Obsessive-Compulsive Disorder. Psychiatry Clin Psychopharmacol [Internet]. 2021;31(4):401–7. Available from: https://psychiatry-psychopharmacology.com/en/treatment-outcomes-of-gamma-knife-radio-surgery-in-refractory-obsessive-compulsive-disorder-133141.
|
| 25. |
Peker S, Samanci MY, Yilmaz M, Sengoz M, Ulku N, Ogel K. Efficacy and Safety of Gamma Ventral Capsulotomy for Treatment-Resistant Obsessive-Compulsive Disorder: A Single-Center Experience. World Neurosurg [Internet]. 2020;141:e941–52. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875020313565.
|
| 26. |
Torres C V., Martínez N, Ríos-Lago M, Lara M, Alvarez-Linera J, Cabanyes J, et al. Surgery and Radiosurgery in Autism: A Retrospective Study in 10 Patients. Stereotact Funct Neurosurg [Internet]. 2021;99(6):474–83. Available from: https://karger.com/doi/10.1159/000516963.
|
| 27. |
Park S-C, Lee JK, Kim C-H, Hong JP, Lee DH. Gamma-knife subcaudate tractotomy for treatment-resistant depression and target characteristics: a case report and review. Acta Neurochir (Wien) [Internet]. 2017;159(1):113–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27900544.
|
| 28. |
Mindus P, Bergstrom K, Levander SE, Noren G, Hindmarsh T, Thuomas KA. Magnetic resonance images related to clinical outcome after psychosurgical intervention in severe anxiety disorder. J Neurol Neurosurg Psychiatry [Internet]. 1987;50(10):1288–93. Available from: https://jnnp.bmj.com/lookup/doi/10.1136/jnnp.50.10.1288.
|
| 29. |
Valle Ramiro A del, De Anda S, Garnica R, Aguilar E, Pérez-Pastenes M. Radiocirugía psiquiátrica con Gamma Knife. Salud Ment [Internet]. 2006;29(1):18–27. Available from: scielo.org.mx/scielo.php?pid=S0185-33252006000100018&script=sci_arttext.
|
| 30. |
Showraki M, Showraki T, Brown K. Generalized Anxiety Disorder: Revisited. Psychiatr Q [Internet]. 2020;91(3):905–14. Available from: https://link.springer.com/10.1007/s11126-020-09747-0.
|
| 31. |
Bains N, Abdijadid S. Major Depressive Disorder [Internet]. StatPearls. Treasure Island (FL); 2024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30396512.
|
| 32. |
Malhi GS, Mann JJ. Depression. Lancet [Internet]. 2018;392(10161):2299–312. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618319482.
|
| 33. |
Park LT, Zarate CA. Depression in the Primary Care Setting. Solomon CG, editor. N Engl J Med [Internet]. 2019;380(6):559–68. Available from: http://www.nejm.org/doi/10.1056/NEJMcp1712493.
|
| 34. |
Karrouri R, Hammani Z, Benjelloun R, Otheman Y. Major depressive disorder: Validated treatments and future challenges. World J Clin Cases [Internet]. 2021;9(31):9350–67. Available from: https://www.wjgnet.com/2307-8960/full/v9/i31/9350.htm.
|
| 35. |
Hurwitz TA, Honey CR, Sepehry AA. Ablation Surgeries for Treatment-Resistant Depression: A Meta-Analysis and Systematic Review of Reported Case Series. Stereotact Funct Neurosurg [Internet]. 2022;100(5–6):300–13. Available from: https://karger.com/doi/10.1159/000526000.
|
| 36. |
Hirota T, King BH. Autism Spectrum Disorder. JAMA [Internet]. 2023;329(2):157. Available from: https://jamanetwork.com/journals/jama/fullarticle/2800182.
|
| 37. |
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet [Internet]. 2018;392(10146):508–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618311292.
|
| 38. |
Müller S, Riedmüller R, Walter H, Christen M. An Ethical Evaluation of Stereotactic Neurosurgery for Anorexia Nervosa. AJOB Neurosci [Internet]. 2015;6(4):50–65. Available from: http://www.tandfonline.com/doi/full/10.1080/21507740.2015.1094536.
|
| 39. |
Cabrera LY, Bittlinger M, Lou H, Müller S, Illes J. The re-emergence of psychiatric neurosurgery: insights from a cross-national study of newspaper and magazine coverage. Acta Neurochir (Wien) [Internet]. 2018;160(3):625–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29264778.
|
| 40. |
He W, Shao L, Wang H, Huang H, Zhang S, Li C, et al. Bilateral Anterior Capsulotomy for the Treatment of Refractory Somatic Symptom Disorder: A Case Report. Front Integr Neurosci [Internet]. 2022;15:721833. Available from: https://www.frontiersin.org/articles/10.3389/fnint.2021.721833/full.
|
| 41. |
Müller S, van Oosterhout A, Bervoets C, Christen M, Martínez-Álvarez R, Bittlinger M. Concerns About Psychiatric Neurosurgery and How They Can Be Overcome: Recommendations for Responsible Research. Neuroethics [Internet]. 2022;15(1):6. Available from: https://link.springer.com/10.1007/s12152-022-09485-z.
|
| 42. |
Miguel EC, Lopes AC, McLaughlin NCR, Norén G, Gentil AF, Hamani C, et al. Evolution of gamma knife capsulotomy for intractable obsessive-compulsive disorder. Mol Psychiatry [Internet]. 2019;24(2):218–40. Available from: https://www.nature.com/articles/s41380-018-0054-0.
|