His bundle pacing in patients with permanent atrial fibrillation and heart failure with non-reduced ejection fraction – retrospective study
Abstract
Background: Heart failure (HF) constitutes a complex clinical entity and often coexists with atrial fibrillation (AF). There is a scarcity of evidence-based therapies for those with ejection fraction (EF) ≥ 40%. The effect restoring regular ventricular response in patients with HF with EF ≥ 40% and concomitant permanent AF is unknown.
Methods: This was a retrospective case-series study. 14 patients with symptomatic HF with EF ≥ 40% and permanent AF who had undergone permanent His bundle pacing (pHBP) were identified and enrolled. For 9 patients pHBP was a primary strategy, for the remaining patients it was an upgrade from right single chamber ventricular pacing. All patients underwent a follow-up visit 3 months after the procedure. Results: The severity of HF based on the New York Heart Association (NYHA) class was significantly reduced post-pHBP (mean 2.5 vs. 1.0, p-value < 0.001). Left ventricular ejection fraction significantly increased (mean increase 8.5%, p < 0.001) Similarly, significant decrease in the left ventricular end-diastolic diameter was observed after pHBP (mean decrease 5.4 mm, p < 0.001). The degree of mitral regurgitation after three months was lower (mean grade 2.4 vs. 1.2, p < 0.001). Conclusions: Permanent HBP might be beneficial in the setting of permanent AF and HF with EF ≥ 40%.
Citation
Skonieczny B, Sławuta A, Radziejewska J, Jagielski D, Gajek J, Kozłowski D. His bundle pacing in patients with permanent atrial fibrillation and heart failure with non-reduced ejection fraction – retrospective study. Eur J Transl Clin Med. 2023;6(2):45-50Introduction
Heart failure (HF) constitutes a complex clinical entity. While there are many drugs and devices with proven benefit for HF with reduced ejection fraction (EF) < 40%, there is a scarcity of evidence for beneficial therapies for those with EF ≥ 40%. European Society of Cardiology (ESC) 2021 guidelines for HF with their 2023 Focused Update contain only one class I recommendation for drug therapy with proven benefit on prognosis: SGLT2 inhibitors [1-2]. As such, treatment of HF with mildly reduced or preserved ejection fraction remains difficult.
Numerous pathophysiological processes can lead to impaired left ventricular (LV) filling, with increased pressure in the pulmonary vascular bed and symptoms of HF regardless of preserved or mildly reduced left ventricular systolic function [3]. The clinical course of HF with preserved EF is commonly further complicated by coexistence of atrial fibrillation (AF). The lack of mechanical function of the atria, in particular of the left atrium, influences negatively the filling of the ventricles increasing the pressure in both venous vascular beds thus contributing to the symptoms of HF. AF contributes furthermore to the development of mitral regurgitation (MR) or exacerbation of the present one which facilitates HF symptoms.
The aim of our study was to assess the influence of permanent His bundle pacing (pHBP) in patients with permanent AF (PAF) and concomitant symptomatic HF with EF ≥ 40%.
Material and methods Patients were selected and the data were gathered retrospectively from the medical records of the Kłodzko County Hospital. Baseline characteristics were extracted from admission clinical data collected prior to the procedure. Outcomes assessment was based on data collected during first follow-up visit after the device implantation, scheduled 3 months after the implantation.
The study group consisted of 14 patients who have undergone pHBP device implantation due to PAF with slow ventricular conduction or were upgradedfrom single chamber right ventricular pacing to pHBP. The inclusion criteria were as follows: age >18 years, symptomatic HF with NYHA class II-III, EF ≥ 40%, concomitant PAF, pHBP as part of treatment. All identified eligible patients were enrolled into the study. Written informed consent was given by every patient prior to the device implantation. Local institutional review board approved the study protocol.
Devices and pacing
All patients were provided with pHBP in the course of their therapy. Intrinsic QRS complexes were narrow in all of their electrocardiograms. For 9 patients, a pacemaker (PM) with the pHBP lead was the primary treatment strategy. One received single chamber pacemaker with pHBP lead only. The rest underwent dual chamber PM implantation with the HBP lead connected to atrial channel and RV lead connected to ventricular channel serving as a back-up in case of failure of HBP lead. Five patients had pre-existing single chamber VVI device with RV lead. They underwent an upgrade procedure – implantation of pHBP lead connected to atrial channel. The devices were set into the DDD (VVI in the single case with single chamber device) mode with very low atrial sensitivity level to functionally blind the atrial channel, so that the actual programmed functional mode was DVI. In 9 of them selective HBP was achieved. Whereas in the rest of the patients myocardial activation via nonselective HBP was also noted.
Statistical analysis
Continuous variables were compared with Student’s t-test. Ordinal variables, such as mitral regurgitation, were compared with the Wilcoxon signed ranks test. Pearson correlation coefficient was used to assess changes in the left ventricular end-diastolic diameter (LVEDD) and EF values at baseline and after pHBP. All calculations and data analysis were performed using the JASP software (Version 0.13.1, JASP Team, University of Amsterdam). P-value < 0.05 was considered to be statistically significant and all tests were two-tailed.
Results
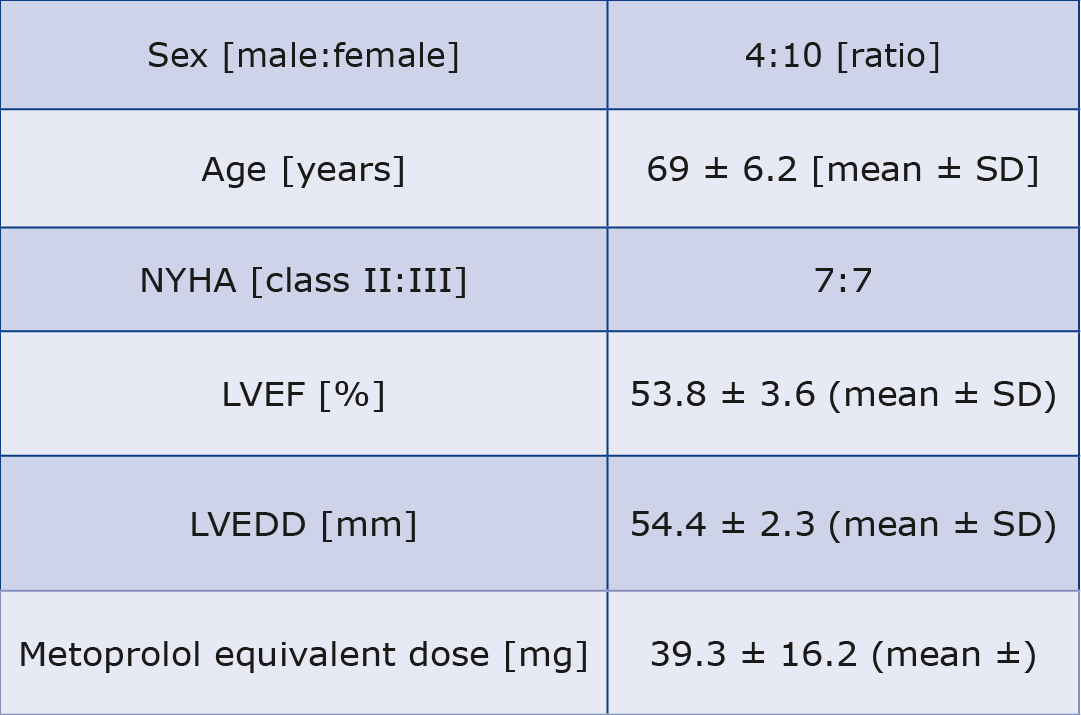
Baseline characteristics of patients are shown in Table 1. The follow-up visit took place 14.1 ± 4.6 (mean ± standard deviation) weeks after the procedure.
Table 1. Baseline characteristics
Procedure and pacing details
In 10 patients selective pHBP was achieved and nonselective pHBP in the remaining 4. The mean pHBP percentage, as assessed during the device follow-up, was 82.1% (minimum 66% – maximum 99%).
Outcomes
LVEF significantly increased from the mean 53.8% at baseline to 62.3% at follow-up (mean increase by 8.5%, 95% confidence interval 7.7-9.2, p < 0.001). Increase in EF was negatively correlated with the baseline values, as shown in Figure 1. Similarly, significant decrease in LVEDD was observed – from mean 54.4 mm at baseline to 49.6 mm at follow-up (mean decrease by 5.4 mm, 95% confidence interval 5.0-5.8, p < 0.001). However, in this case, no correlation between the baseline values and degree of diameter’s reduction was observed (Pearson’s r = -0.306, p = 0.287). The degree of MR after three months was lower (meangrade at the baseline 2.4 vs. 1.2 at follow-up, p < 0.001). NYHA class was significantly reduced (mean class 2.5 vs. 1.0 p < 0.001). All ofthese findings are summarized in Table 2. Moreover, at follow-up prescribed doses of beta blocker were higher by 69.6 ± 29.7 (mean ± standard deviation) mg in metoprolol equivalents.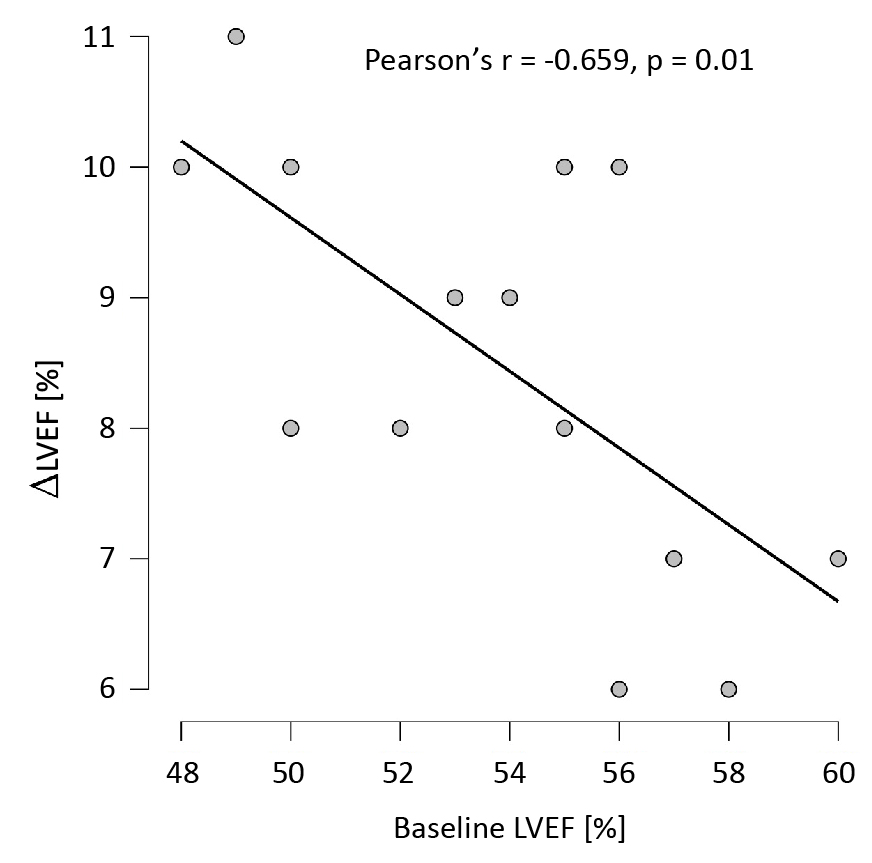
Figure 1. Scatter plot of baseline LVEF and difference in LVEF at follow-up.
Table 2. Outcomes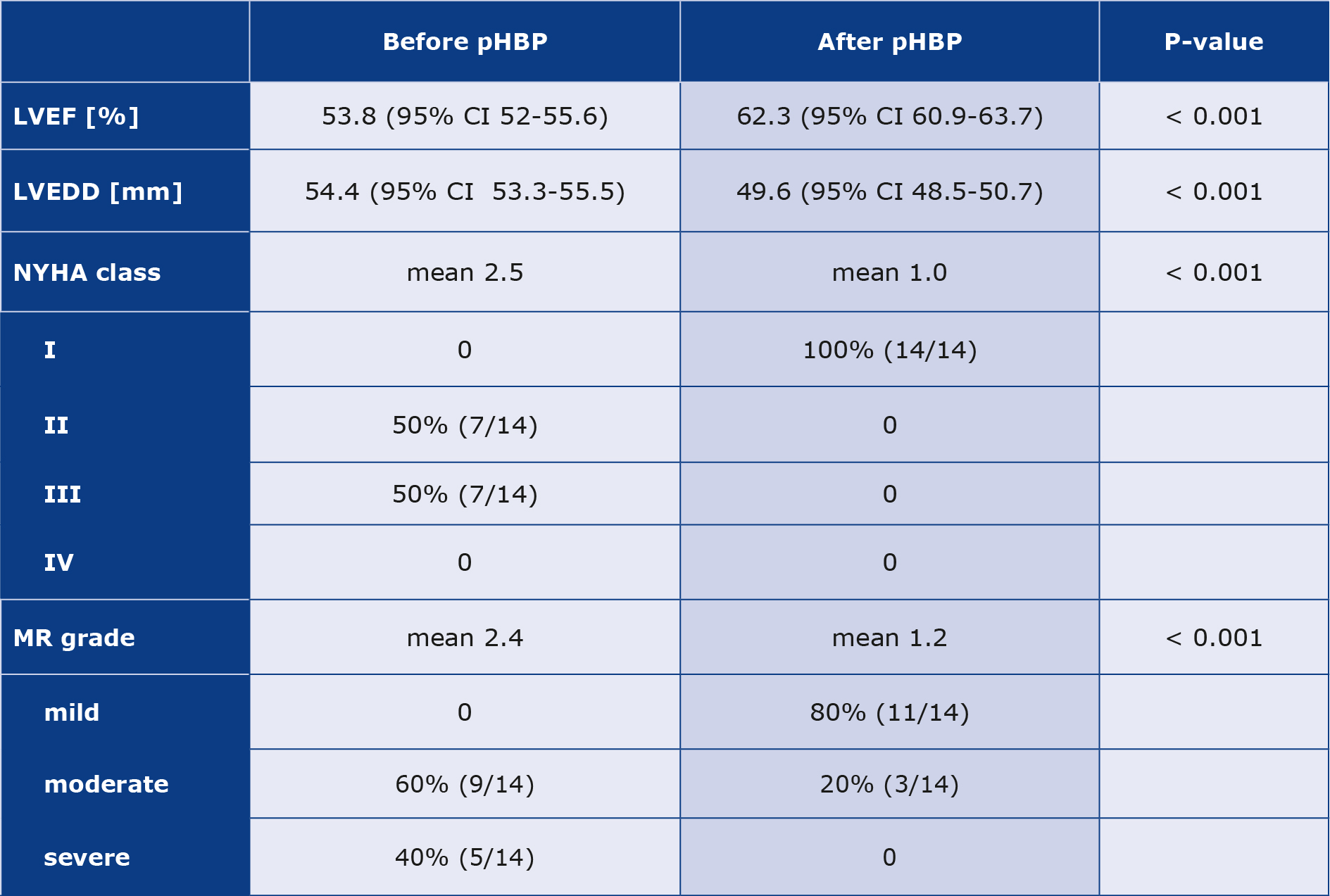
Discussion
AF and HF commonly coexist. This is no surprise as they share the same risk factors. Moreover, they influence each other so one can precede another. HF is the strongest predictor of AF in the future [4]. AF is precipitated by HF in many ways. AF can also affect HF and worsen LV function making this two conditions a vicious circle [5]. Clearly, the loss of atrial contractile function leads to a decrease in global heart contractile function. However, peculiar ventricular response in AF causes some unfavorable phenomena. Tachycardia-induced cardiomyopathy (TCM) is the long recognized clinical entity which was depicted in the setting of AF over 100 years ago [6]. Recently new clinical concept started to emerge: AF induced cardiomyopathy (AFCM) [7]. Basic assumption is that AF can lead to cardiomyopathy and worsening of LV function not only by rapid ventricular response, budue to the irregular rhythm. Clark et al. elegantly showed that irregularity of ventricular rhythm has an adverse impact on hemodynamics, irrespectively of the heart rate [8]. Similar results were observed in a study of patients with HF with reduced EF during biventricular pacing. However, detrimental effects of irregularity were seen only at higher heart rates [9]. Far bigger and clinically more important was the APAF-CRT trial. It involved patients with AF, symptomatic HF and narrow QRS. Patients were randomly assigned to the AV node ablation and biventricular pacing or to the optimal pharmacological rate-control therapy. Most of the trial participants were patients with HF and EF > 35%. That trial demonstrated the superiority of the AV node ablation and BiV pacing over the rate-control approach in matters of combined primary endpoint of death due to HF or hospitalization for HF or worsening of HF. This statistically significant effect was seen also among the patientswith EF > 35% [10].
In recent years a few trials demonstrated that sinus rhythm restoration by pulmonary vein isolation (PVI) might be beneficial in HF patients. In 2013 a small, observational study showed augmentation of LV dysfunction following AF ablation, despite optimal medical therapy and well controlled ventricular rate [11]. The CASTLE-AF randomized controlled trial involving HF patients with EF ≤ 35% and AF, showed that PVI lowers risk of death and hospitalization for worsening HF compared to the rate control strategy [12]. These two trials were conducted among patients with severely reduced LVEF, however the benefit of PVI in patients with HF and EF > 40% was noted in the post-hoc analysis of the CABANA-AF trial. In the subgroup of patients with an established HF, PVI resulted in improved survival, freedom from AF recurrence and higher quality of life. Most importantly, only 11.7% of population had EF < 40% [13].
As expected, shift in management of AF in HF patients can be seen and in the recent ESC guidelines gave PVI in patients with AF and HF with reduced LVEF has a class IIa recommendation [1]. PVI might be beneficial for patients with HF due to the restoration of atrial contractility, of regular ventricular rhythm or both. Some insights came from the PABA-CHF trial. In this randomized clinical trial, PVI was compared with AV node ablation and biventricular pacing (BiV) in patients with AF and concomitant HF. Results favoured PVI owing to the fact that at follow-up the patients in the PVI arm presented with higher EF and lower Minnesota Living with Heart Failure questionnaire scores [14]. While achieving regularity of ventricular rhythm by means of pacing clearly lacks the potential benefit of restoring atrial contractile function, nearly half of AF patients have PAF, therefore restoring the sinus rhythmis no longer considered suitable [15]. Moreover, nowadays a revolution in pacing is unfolding – conduction system pacing. Two studies published in 2017 explored role of pHBP in patients with AF and HF who had undergone AV node ablation. Vijayaraman et al. proved pHBP to be feasible in the context of AV node ablation due to symptomatic AF. Echocardiographic improvement was also noted, however driven mainly by the EF < 40% subgroup. Unfortunately, little data on baseline characteristics (e.g. prior rate control) were provided [16]. Huang et al. pulishwdthe results of a prospective registryin which patients with HF (regardless of EF) and permanent AF with satisfying rate control (mean 83 beats perminute) underwent pHBP and AV node ablation [17]. During follow-up the authors noted improvements in EF, NYHA class and diuretic usein patients with EF ≤ 40% as well as EF > 40% [17]. In accordance with our results, the improvement in EF noted in both aforementioned studies was correlated with baseline EF.
We hypothesize that irregular ventricular response facilitates the worsening of heart function which might be reversed by physiological ventricular pacing achieved by pHBP. Our results provide further data to support that thesis.
However, we identify serious limitations of our study, e.g. it was a retrospective study on a small population. Data collection was conducted in a non-blinded, unsystematic manner. We couldn’t identify data neither on prior rate control nor on QRS duration after pHBP. Percentage of pHBP pacing was far from recommended for BiV percentage.
Conclusions
Our study has shown that patients with HF with EF ≥ 40% and concomitant PAF may benefit from restoring regular heart rate with pHBP. All patients had significant clinical improvement as shown by lower NYHA class. Moreover, LVEF increase in every case, (at least by 7%) and a decrease in LVEDD suggests that the restoration of a regular heart rhythm with narrow QRS reverses adverse LV remodeling. Due to the limitations of our study, more research is needed to draw firm conclusions.
Conflicts of interest
None to report.
Funding
Not applicable.
-----------------------
(Image source: https://pixabay.com/illustrations/heart-medicine-health-disease-665092/)
References
| 1. |
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contributio. Eur Heart J [Internet]. 2021;42(36):3599-726. Available from: https://doi.org/10.1093/eurheartj/ehab368.
|
| 2. |
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J [Internet]. 2023;44(37):3627-39. Available from: https://academic.oup.com/eurheartj/article/44/37/3627/7246292.
|
| 3. |
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J [Internet]. 2011;32(6):670-9. Available from: https://doi.org/10.1093/eurheartj/ehq426.
|
| 4. |
Benjamin EJ. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. JAMA [Internet]. 1994;271(11):840. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.1994.03510350050036.
|
| 5. |
Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol [Internet]. 2003;91(6):2-8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914902033738.
|
| 6. |
Gossage AM, Braxton Hicks JA. On auricular fibrillation. QJM An Int J Med [Internet]. 1913;6:435-40. Available from: https://academic.oup.com/qjmed/article/os6/4/435/1579375/ON-AURICULAR-FIBRILLATION.
|
| 7. |
Huizar J, Ellenbogen K, Tan A, Kaszala K. Arrhythmia-Induced Cardiomyopathy. J Am Coll Cardiol [Internet]. 2019;73(18):2328-44. Available from: https://doi.org/10.1016/j.jacc.2019.02.045.
|
| 8. |
Clark D, Plumb V, Epstein A, Kay, GN. Hemodynamic Effects of an Irregular Sequence of Ventricular Cycle Lengths During Atrial Fibrillation. J Am Coll Cardiol [Internet]. 1997;30(4):1039-45. Available from: https://doi.org/10.1016/S0735-1097(97)00254-4.
|
| 9. |
Melenovsky V, Hay I, Fetics BJ, Borlaug BA, Kramer A, Pastore JM, et al. Functional impact of rate irregularity in patients with heart failure and atrial fibrillation receiving cardiac resynchronization therapy. Eur Heart J [Internet]. 2005;26(7):705-11. Available from: https://doi.org/10.1093/eurheartj/ehi066.
|
| 10. |
Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J [Internet]. 2018;39(45):3999-4008. Available from: https://doi.org/10.1093/eurheartj/ehy555.
|
| 11. |
Ling L-H, Taylor AJ, Ellims AH, Iles LM, McLellan AJA, Lee G, et al. Sinus rhythm restores ventricular function in patients with cardiomyopathy and no late gadolinium enhancement on cardiac magnetic resonance imaging who undergo catheter ablation for atrial fibrillation. Hear Rhythm [Internet]. 2013;10(9):1334-9. Available from: https://www.sciencedirect.com/science/article/pii/S1547527113006607
|
| 12. |
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med [Internet]. 2018;378(5):417-27. Available from: https://doi.org/10.1056/NEJMoa1707855.
|
| 13. |
Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation [Internet]. 2021;143(14):1377-90. Available from: https://doi.org/10.1161/CIRCULATIONAHA.120.050991.
|
| 14. |
Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-Vein Isolation for Atrial Fibrillation in Patients with Heart Failure. N Engl J Med [Internet]. 2008;359(17):1778-85. Available from: https://doi.org/10.1056/NEJMoa0708234.
|
| 15. |
Chiang C-E, Naditch-Brûlé L, Murin J, Goethals M, Inoue H, O’Neill J, et al. Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice. Circ Arrhythmia Electrophysiol [Internet]. 2012;5(4):632-9. Available from: https://doi.org/10.1161/CIRCEP.112.970749.
|
| 16. |
Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. EP Eur [Internet]. 2017;19(suppl_4):iv10-6. Available from: https://doi.org/10.1093/europace/eux263.
|
| 17. |
Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. Benefits of Permanent His Bundle Pacing Combined With Atrioventricular Node Ablation in Atrial Fibrillation Patients With Heart Failure With Both Preserved and Reduced Left Ventricular Ejection Fraction. J Am Heart Assoc [Internet]. 2023;6(4):e005309. Available from: https://doi.org/10.1161/JAHA.116.005309.
|










