Prevalence of tuberculosis and drug-resistant tuberculosis in tertiary care rural hospital in Gujarat, India: a retrospective study
Abstract
Background: Mycobacterium tuberculosis causes tuberculosis (TB), the most lethal infectious disease in the world that affects people of all ages. The aim of our study was to estimate the prevalence of TB in the Gujarat state (India).
Material and methods: This was a retrospective study conducted at the Pandit Dindayal Upadhyay Government Medical Hospital (PDUGMH) in Gujarat between 2018 and 2022.
Results: A total of 5624 TB notification records were reviewed from the TB & Chest Department of the PDUGMH. 5207 of them reported TB-positive results, majority of which concerned pulmonary TB (57.27%, n = 2982). 3586 (68.87%) of the TB-positive patients were male and 1621 (31.13%) were female. The most group most affected by TB was 15-29 years of age, with a high peak in 2019. Amongst the TB-positive patients, 215 suffered from diabetes and 454 were HIV-positive. Majority of patients with an infected lymph node suffered from extrapulmonary TB. Rifampicin-resistant TB was observed in 0.8% (n = 42) of patients and 3 patients were treated with the longer oral regimen for fluoroquinolone-resistant multi-drug resistant TB (0.1%).
Conclusions: Our findings indicate that during 2018-2022, patients treated at PDUGMH had a TB prevalence of 92.59%, with a corresponding rate of multidrug-resistant TB standing at 1.48%. A comprehensive study is required to accurately assess the TB burden in India and to guide national strategies for TB eradication.
Citation
Ughreja R, Bhatt V, Shah S, Boxa D. Prevalence of tuberculosis and drug-resistant tuberculosis in tertiary care rural hospital in Gujarat, India: a retrospective study. Eur J Transl Clin Med. 2023;6(2):36-44Introduction
Mycobacterium tuberculosis, an infectious bacterial disease that most frequently affects the lungs but can harm other tissue, is the cause of tuberculosis. M. tuberculosis spreads between people through the respiratory system [1]. The global incidence of tuberculosis was 127 per 100000 people [2]. Tuberculosis (TB) is a leading cause of mortality and a global health issue among several mycobacterial illnesses. Based on the World Health Organization’s (WHO) annual TB report for 2021, around 9.9 million people are ill with TB, with an anticipated 1.3 million deaths. It necessitates the development of new medicines, as well as improved diagnostics and healthcare coverage. The WHO internationally endorsed the directly observed treatment, short course (DOTS) strategy in 1970. It has been acknowledged as a highly efficient and cost-effective technique for tuberculosis control [3]. Furthermore, the End TB strategy by WHO aims for a 95% reduction in TB mortality by the year 2035 [4].
M. tuberculosis is a sessile, noncapsulated, nonspore-forming bacterium which although acid-fast staining, it requires an additional stain (Ziehl Neelsen, ZN). It is because of mycolic acid, which is an unsaponifiable wax. It creates a semipermeable wall surrounding the cell, making it acid-fast. Children are immunized with the Bacillus Calmette-Guerin (BCG) vaccine to protect them from severe forms of TB. Sometimes BCG is advised for adults with drug-resistant TB [5]. Numerous diagnostic techniques are available now for identifying TB, but sputum smear microscopy still is the most common, which is ineffective in cases of medication resistance [6-8].
M. tuberculosis is transmitted via mucous droplets during activities such as coughing, laughing, sneezing, spitting, and even breathing. The infected airborne droplets make their way through the oral and nasal passages, eventually infecting the alveoli of the lungs [9]. Although M. tuberculosis typically infects the lungs and causes pulmonary TB, it can also spread to other bodily areas, including the kidney, spine, and brain, where it can cause extrapulmonary TB [10]. Samples from the respiratory system (e.g. sputum, induced sputum, bronchoalveolar lavage or lung biopsy) are typically analyzed to diagnose pulmonary TB, which is more prevalent. Whereas the extrapulmonary TB can be detected through various samples, including biopsies, urine, pus, aspirates and sterile body fluids (e.g. pleural, abdominal, cerebrospinal, synovial, genitourinary, pericardial and peritoneal fluids). For HIV-positive patients, the diagnosis of extrapulmonary TB will depend on the site of infection. For that, the stool sample is examined for intestinal TB to detect M. avium [11-12].
India is among the countries with high burden of TB [13- 14]. Whereas the Gujarat state has a moderate case notification rate and in 2021 it achieved a > 20% reduction in TB incidence (received “Bronze” category award). As shown in Figure 1, the data released by TB India revealed that Nikshay-Gujarat stands third in contributing to total TB notification in April, 2020 [15]. TB burden estimation must be carried out annually in order to re-calibrate the state- and district-wide TB strategies.
As a Tertiary Care Institute, the TB and Chest Department of the PDUGMH receives sputum and other body fluid samples from the Rajkot district and 11 other districts to investigate cases of TB infections. The aim of our study was to estimate the prevalence of TB in the Gujarat state (India).
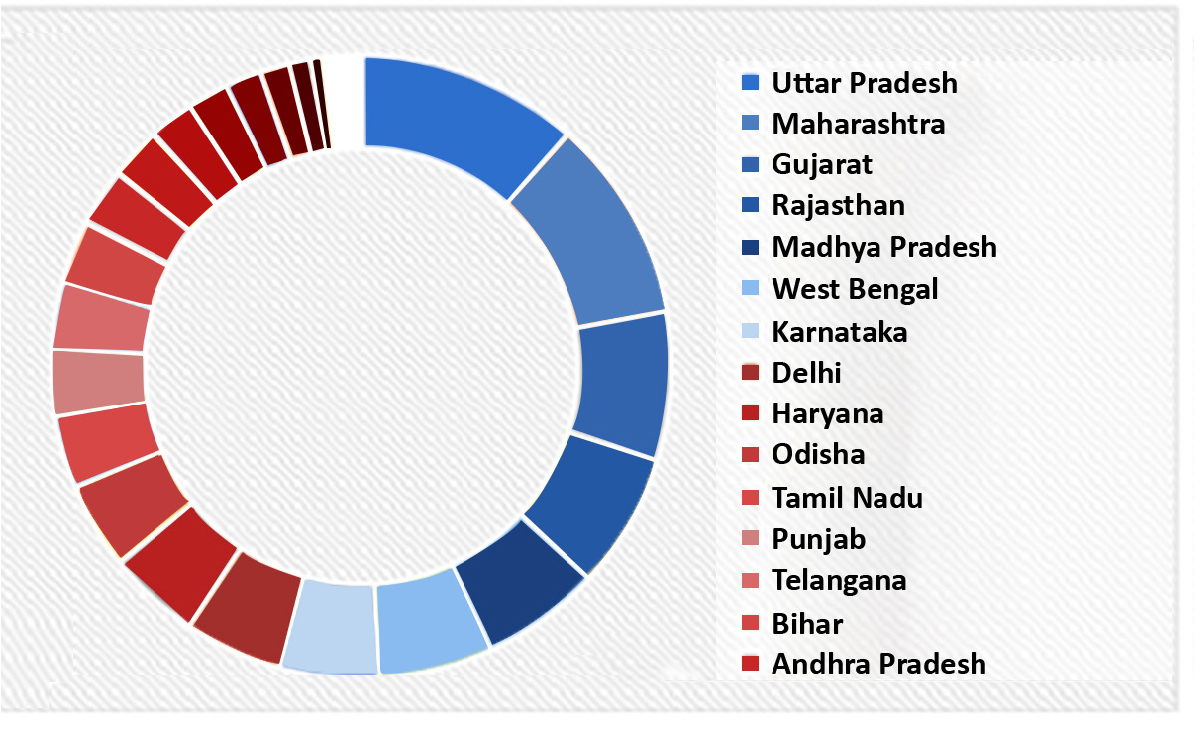
Figure 1. Nikshay-Gujarat stands third in contributing to the total TB notifications in April, 2020
Material and methods
This study was conducted at the PDUGMH in, from where the notification records were collected between January 2018 to December 2022.
Data collection
From TB & Chest Department at PDUGMH (a Tertiary Care Teaching Institute in Rajkot), the TB notification records were gathered. These included patient demographic information such as age, sex, weight, HIV status, diabetes and drug susceptibility test results. The number of patients who died during the treatment was also noted.
Patients
Participants who reported cough lasting 2 weeks or longer, fever, weight loss or night sweats of any duration were categorized as having TB symptoms and their sputum samples were collected. Two sputum samples were gathered: one immediately upon admission and the second on the following morning. The sputum samples were collected in sterile, single-use disposable containers within the PDUGMH laboratory in Rajkot on the same day for testing. The sputum from patients underwent testing for acid fast bacilli (AFB) through smear microscopy using a fluorescent stain. Patients were diagnosed with smear-positive TB if at least one sputum sample tested positive for AFB. The microscopic test employs an exceptionally sensitive microscope, capable of detecting individual live bacilli. The sputum was cultured on Lowenstein-Jensen medium and examined for M. tuberculosis growth once a week for eight weeks. A patient was diagnosed with sputum-positive pulmonary TB if found positive through smear and/or culture.
Patients underwent chest X-rays, chest ultrasonography, brain magnetic resonance imaging (MRI) and Cartridge Based Nucleic Acid Amplification Test (CBNAAT) based on their current condition and diagnostic results. For CBNAAT, specimens were transported at 2-8°C to the nearest diagnostic site using triple packaging. Extrapulmonary TB symptomatic patients had their body fluids (e.g. abdominal fluids, pleural fluids, miliary fluids, cerebrospinal fluid, pericardial fluids, genitourinary fluids) examined. Drug susceptibility tests were conducted, categorizing patients into H mono-resistant, H poly-resistant, rifampicin-resistant (RR) TB and longer multidrug-resistant (MDR) regimen cases.
Statistical analysis
The data were analyzed using Excel software (Microsoft, Redmond, WA, USA) and exported to IBM SPSS Statistics, Version 19 (IBM, Armonk, USA) for the descriptive statistics e.g. mean, total, and percent distribution. A Chi-Square statistic was applied to calculate the difference between variables like subject sex and age. The P-value of < 0.05 determined statistical significance. The percentage of resistance was estimated by dividing the total number of resistant isolates detected for a single drug or a combination by the total number of positive TB patients examined.
Results
A total of 5624 records from January 1st 2018 and December 31st 2022 were examined, with an annual range of 954-1389. Majority of these, (92.58%, n = 5207) contained TB-positive results, with the annual positive rate fluctuating between 89.31% and 96.61%. Notably, there was a dip in the annual rate in 2021, reaching 89.31%. The year 2019 exhibited the highest case count (n = 1297), as visualized in Figure 2. Among the 5207 TB-positive patients, 3,586 (68.87%) were identified as males, while 1621 (31.13%) were females. Within this TB-positive group, 215 (4.13%) patients (mostly male) were diagnosed with diabetes and 454 (8.72%) were HIV-positive. The distribution of patient ages is illustrated in Table 1. When segmented by age, the group of patients were 15-29 years of age (n = 1692), while the lowest number of patients were in the 0-14 age bracket (n = 378), as portrayed in Figure 3.
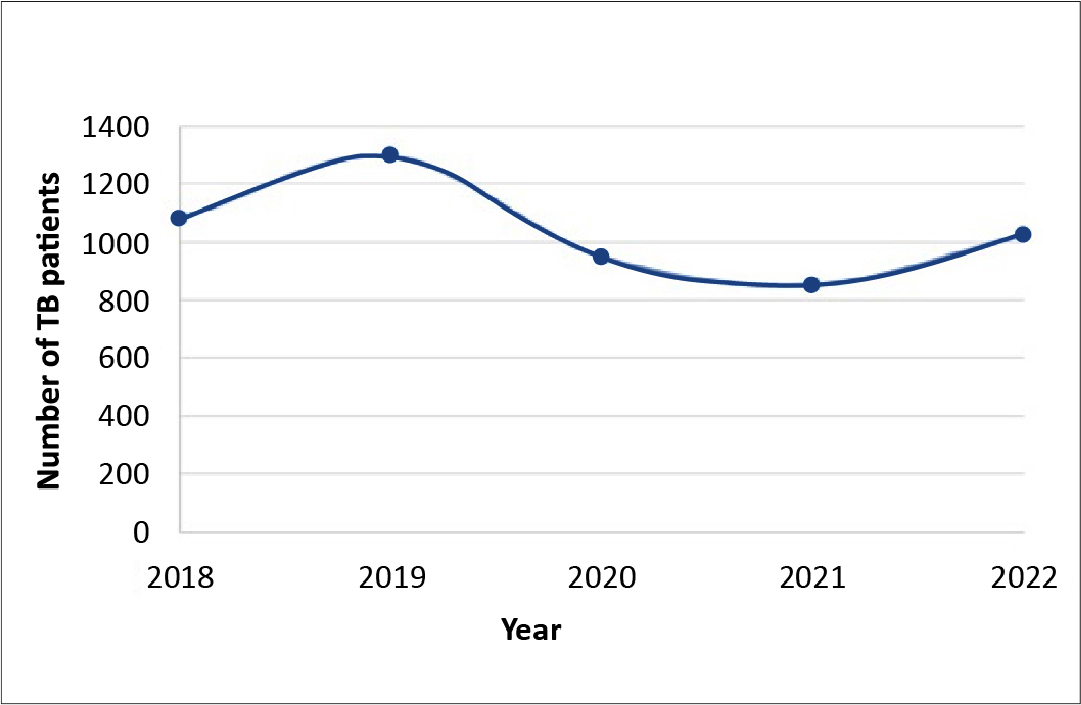
Figure 2. Number of positive TB cases
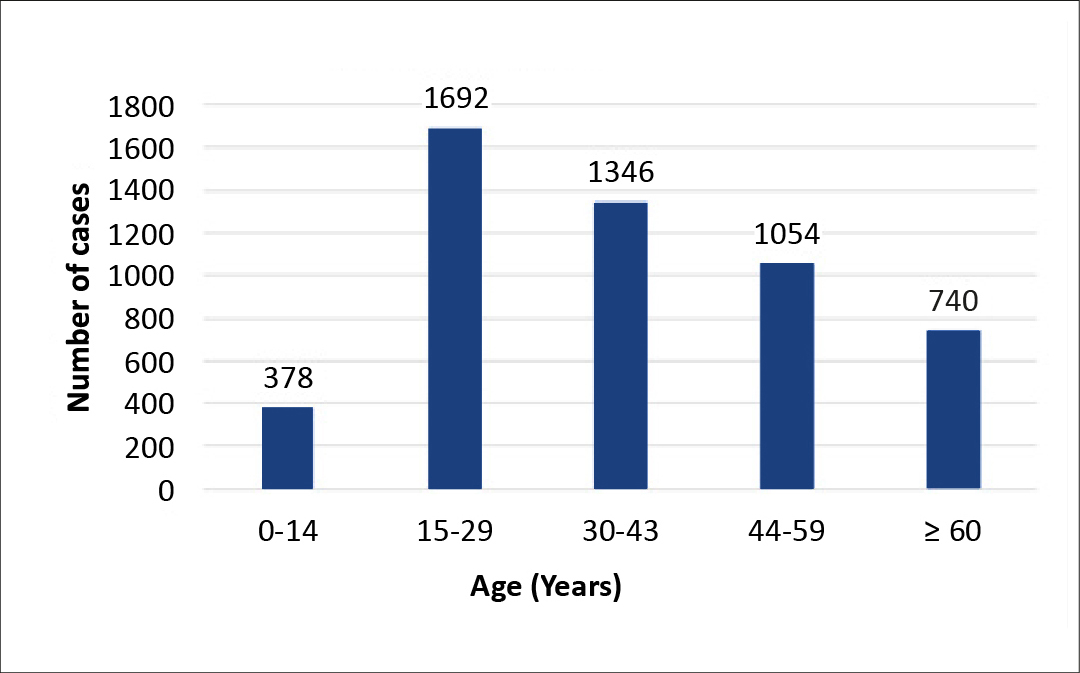
Figure 3. Age-wise distribution of TB-positive cases
Table 1. Age distribution of TB-positive patients of the Pandit Dindayal Upadhyay Government Medical Hospital during 2018-2022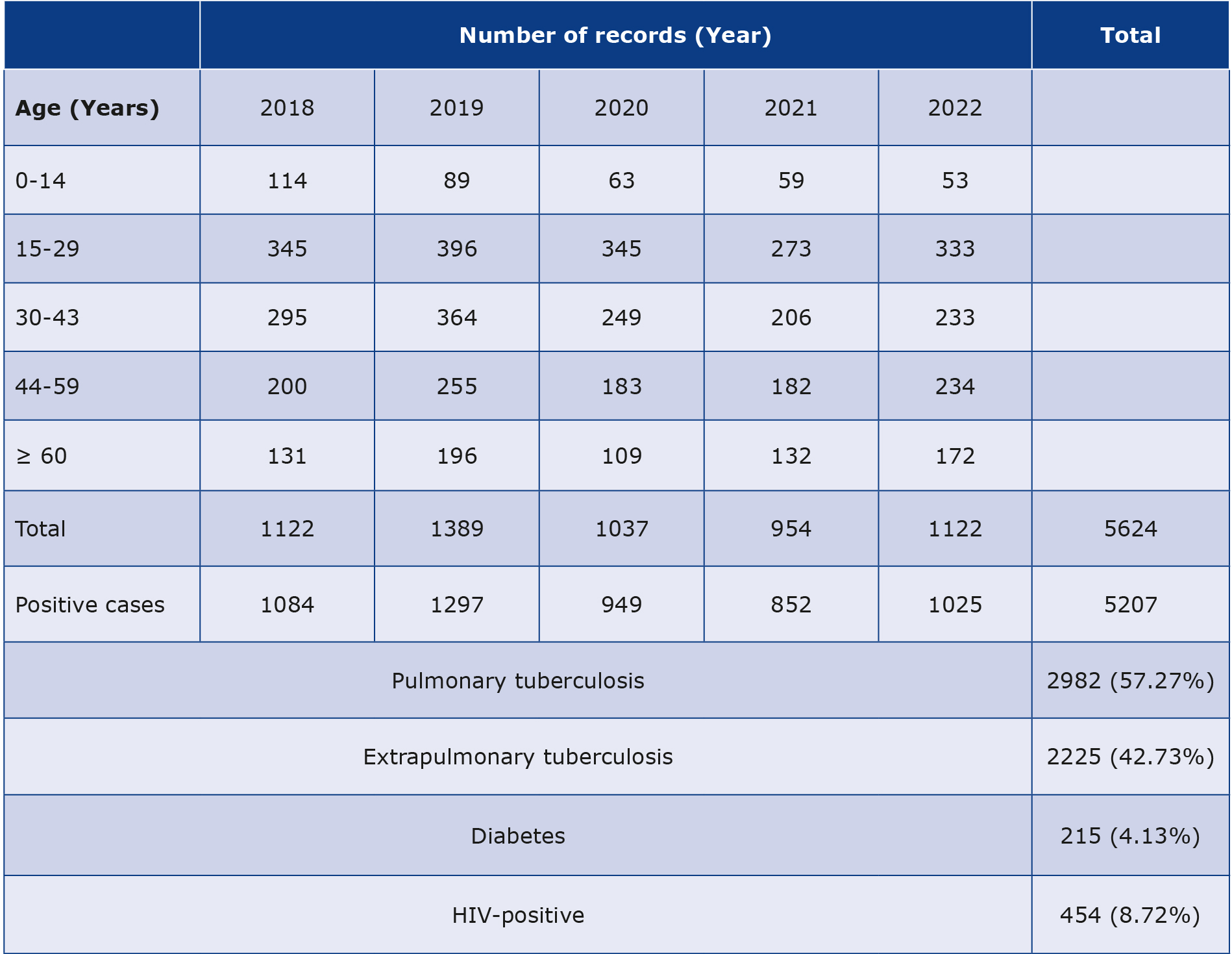
Among the 5207 TB-positive patients, majority (n = 2982, 57.27%) were pulmonary TB-positive, and 2225 (42.73%) were extrapulmonary TB-positive. Majority of these patients were male, cases, A high prevalence of pulmonary TB and extrapulmonary TB was found in males: n = 2193 and n = 1393, respectively [13], as shown in Figure 4.
Figure 4. Specific diagnoses of TB-positive patients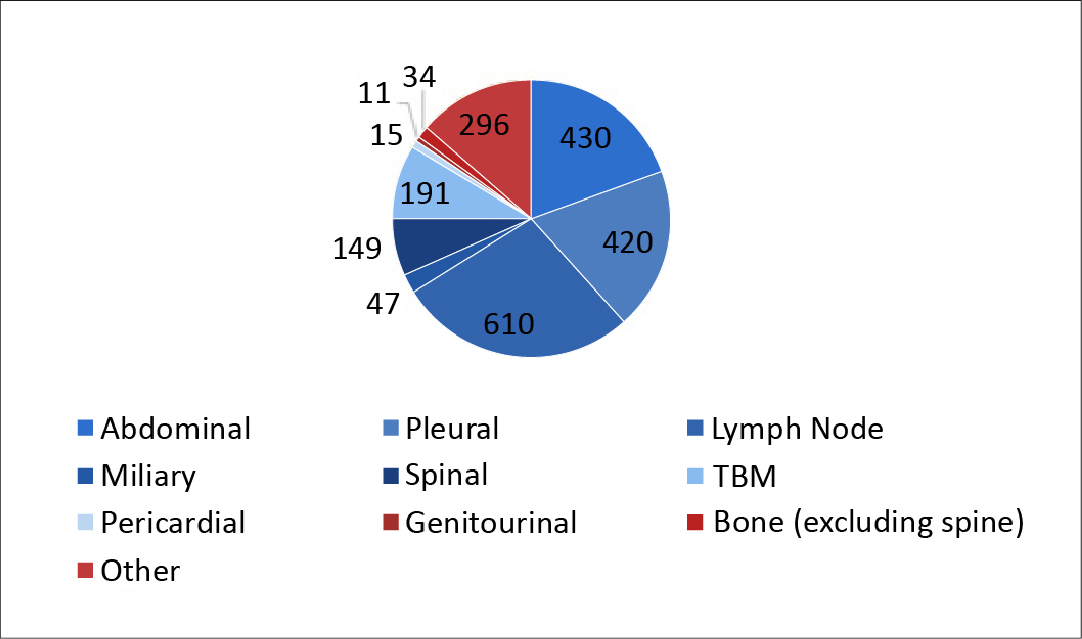
Positive pulmonary TB cases were detected through sputum analysis by fluorescent stain microscopy [16]. Positive extrapulmonary TB cases were detected in the abdominal fluid, pleural fluid, lymph node, miliary, spinal fluid, genitourinary fluid, pericardial fluid, TBM, and bone (excluding spine). Apart from these sites, the positive extrapulmonary TB patients were classified as “others”. The highest number of extrapulmonary TB cases were detected through the lymph nodes (n = 610) and the least with genitourinary fluid (n = 11), as shown in Figure 5.
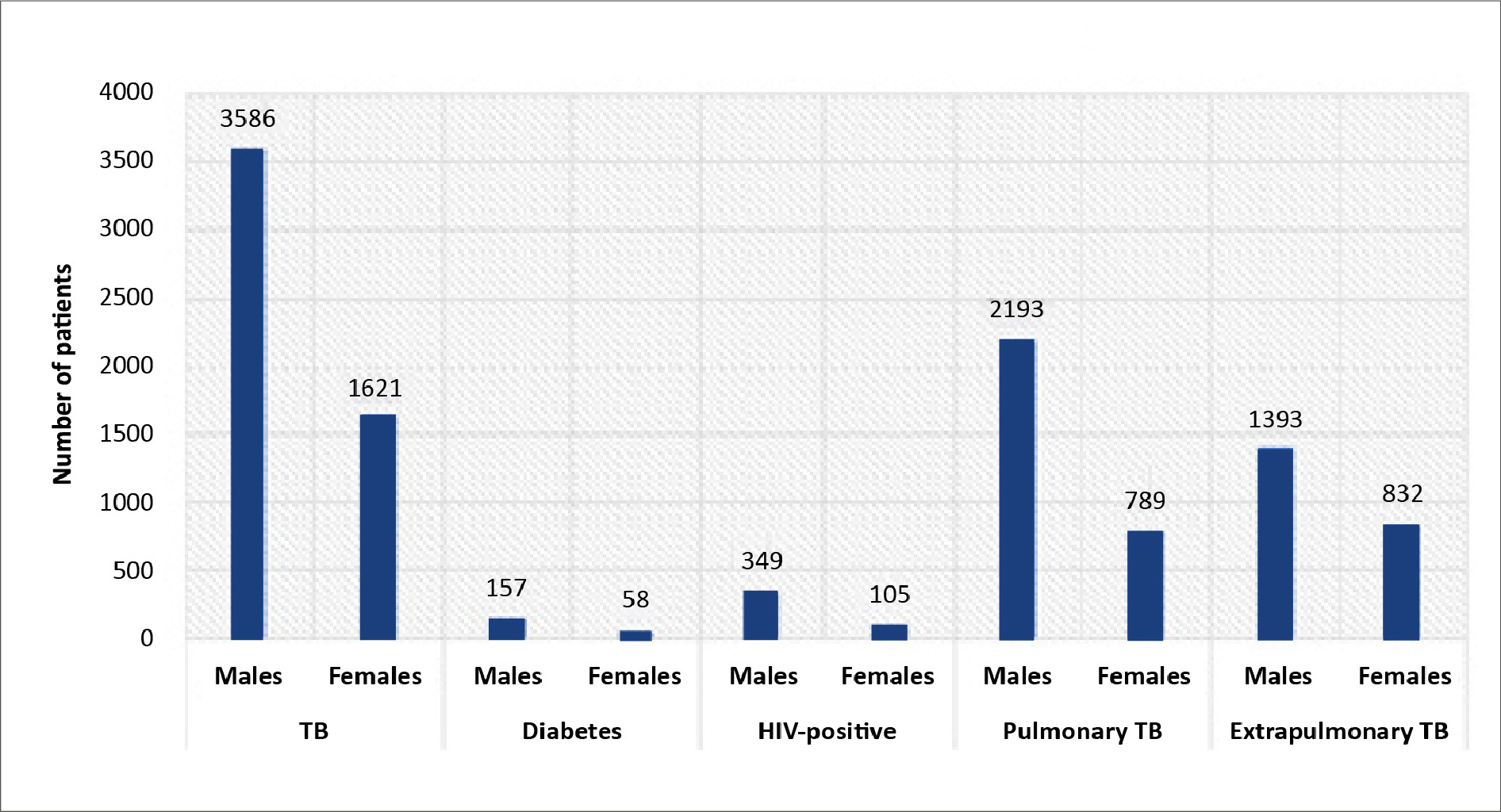
Figure 5. Distribution of extrapulmonary TB cases
Among the 5207 TB-positive cultures, 5130 (98.52%) were susceptible to all the TB medications, while 77 (1.48%) were resistant to at least one drug. Of the 77 drug-resistant cultures, most (n = 42) were RR-TB cases (Figure 6).
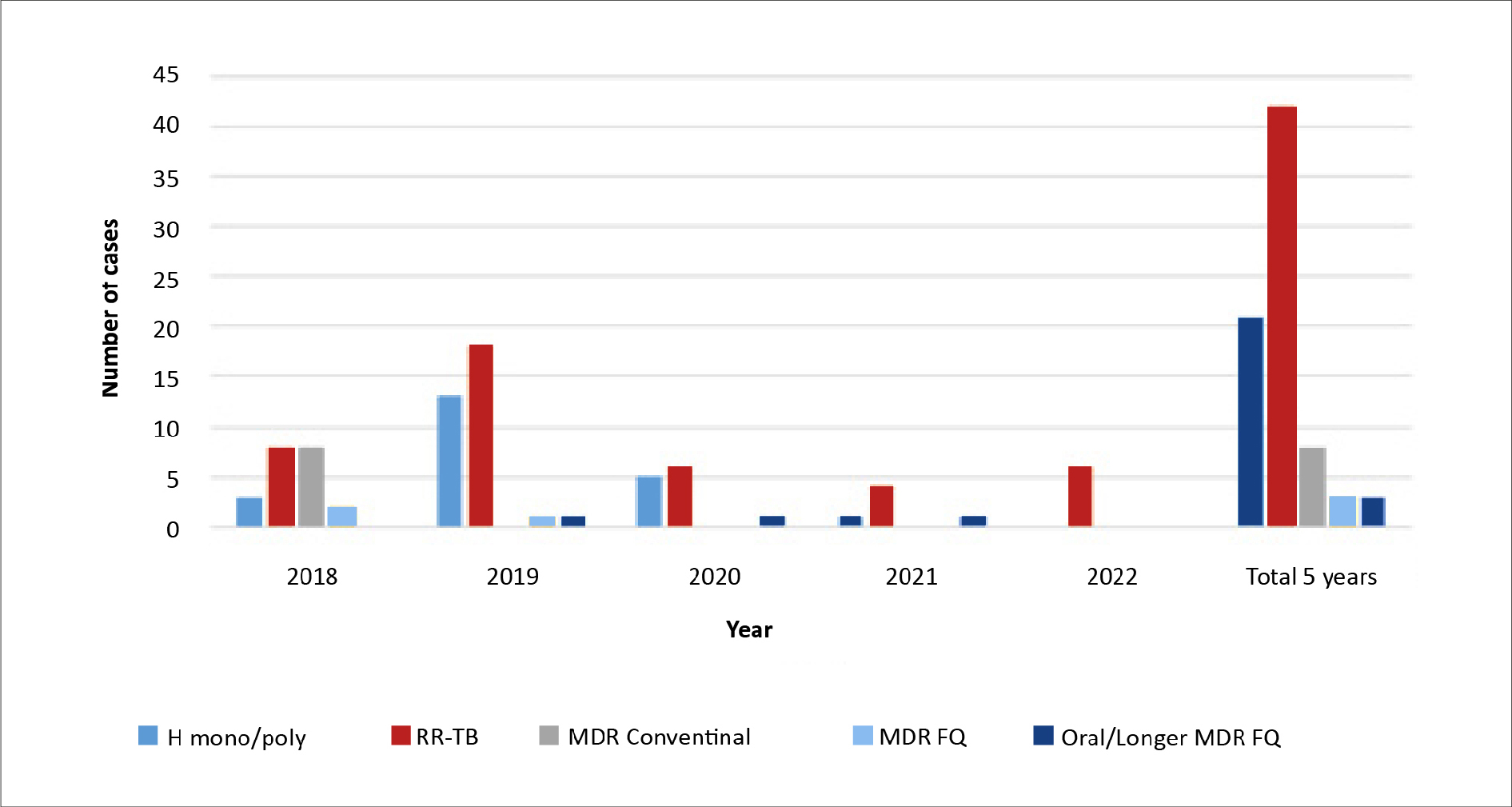
Figure 6. Distribution of drug resistant cases
FQ – fluoroquinolone-resistant, MDR – multidrug-resistant, RR – rifampicin-resistant, TB – tuberculosis
The total of 683 deaths occurred while undergoing treatment, with the lowest count observed in the year 2022 (n = 97).
Discussion
This retrospective study aimed to determine the prevalence of TB in a particular region of India and to gauge the incidence of drug-resistant tuberculosis. We found that during the 5-year period (2018-2022), the prevalence of TB among the patients treated at this institute was 92.59%. 2019 was the year with the most remarkable rate of TB positive cases, probably due to the hospital laboratory receiving samples from various districts throughout the year.
Our results also showed males were more frequently infected with TB (p < 0.05), both the pulmonary and extrapulmonary presentation, as reported in the WHO’s global surveillance report [17] and also seen in other studies [18-19]. The reasons behind this gender difference are behavioural (e.g. the patient’s activities, tobacco smoking and alcohol use) and physiological e.g. sex hormones might modulate the immune response required for defending against TB and other infections [20-22].
In some of the published studies pulmonary TB and extrapulmonary TB were found equally often with no significant difference (p > 0.05) [23-24], which is so seen in the results of the current study. We noted that the majority of pulmonary TB cases were confirmed with microscopy tests, whereas lymph node specimens were the major site for extrapulmonary TB.
A high score of positive TB infection is seen in the group 15-29 years of age (p < 0.05). The cause could be attributed to either disregarding the symptoms or delaying the seeking of medical attention upon their emergence. There might be a lack of awareness regarding the high transmission rate of the disease within the patients’ own family [25]. The rise in co-morbidities or immunosenescence may also be one of the reasons for the reactivation of latent M. tuberculosis infection and the increase in susceptibility to TB disease in older people [26-27].
The rates of drug resistant TB in other regions of India is the subject of several studies [14, 28]. Similar studies are carried out which involved the estimation of rends in TB prevalence in different communities in south India [29-30] and north India [31]. The study conducted in Chennai [30] revealed a high prevalence among individuals aged 55-64 years, whereas we found a high prevalence among those aged 15-29 years which is consistent with the report from the Thiruvallur district of the Tamil Nadu state [32]. A study conducted in northern Indian districts [31] reported a high male representation among the TB-positive cases, which aligns with our own study’s findings. Another study showed high TB burden in the states of Delhi and Tamil Nadu [33]. Also a 2005 study showed a comparative TB prevalence in India focusing rural and urban areas [34]. Following these studies, including the current one, there is a significant difference in rates of drug-resistant TB in India.
Furthermore, a study compared TB data from India with the WHO report where there was a decline in TB incidence which were close to estimates published in the recent Global TB report 2022 [35].
Since our study period includes the COVID-19 outbreak (2019), the TB incidence might be affected by the COVID positive cases. The data we collected did not include the COVID-19 results, therefore a correlation cannot be made on the TB positive and COVID positive cases. However, another study reports that there was a rise in May 2020 with peak in August and a decline in October 2020 [36]. Following our data from Table 1, it can be seen that there was a rise in TB positive cases from 2018 to 2019. Then it declined which might be due to the lock-down period from April to July 2020, as seen in a modelling study [35].
As seen in our results and in the literature, the drug resistance rate at the PDUGMH has dramatically decreased over the past five years (p < 0.05) [13, 37-39]. Improved compliance brought on by direct witnessed therapy and advancements in TB diagnosis and resistance testing may be responsible for the decline in antibiotic resistance. Among all the TB-positive resistant cases, the majority of cases were attributed to RR-TB cases.
Limitations
Our study has several limitations, including the fact that we analyzed data from only one center, which does not accurately reflect the demographic structure of the entire nation. The TB notification records do not include data on previous history of TB, therefore we were not able to assess the risk factors for drug-resistant TB. This analysis excluded second-line anti-TB medications and patients for whom complete data were unavailable.
Future perspectives
The overall prevalence of TB in the Gujarat state is low. Still, to understand the deadliness of TB, the pattern of the disease must be monitored periodically to do appropriate planning. Also, details of the patient’s history must be recorded, such as the spread of TB, sufficient and balanced nutrition, and cautious handling of ill family members. Healthcare providers must look into the information of patients’ close contacts and perform screening, particularly for those having cough, fever, and weight loss and treat them if required.
Conclusion
Among PDUGMH patients, the prevalence of tuberculosis, particularly MDR-TB, is still low. Adult male patients are more likely to contract M. tuberculosis. Our findings indicate that men and people over 60 are more likely to have active TB illness. Investigations into India’s nation-wide TB prevalence and antibiotic resistance are still needed. More emphasis must be placed on the importance of thorough national and international studies of the risk factors for the emergence of active TB and the treatment of its drug-resistance.
Acknowledgements
The authors thank the Superintendent of the Pandit Dindayal Upadhyay Government Medical Hospital in Rajkot for permitting the collection of data from TB and Chest Department.
Funding
This research did not receive any grant from institutions in the public, commercial or not-for-profit sectors.
Ethical considerations
The study was conducted following the principles described in the Declaration of Helsinki and was approved with a waiver of consent.
Conflicts of interest
None to report.
References
| 1. |
Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, et al. Tuberculosis. In: Major Infectious Diseases [Internet]. 3rd ed. World Bank Group; 2017. p. 233-313. Available from: https://openknowledge.worldbank.org/entities/publication/4699c-561-3dfb-5fad-a3c4-54893fa47014.
|
| 2. |
World health statistics 2022: monitoring health for the SDGs, sustainable development goals [Internet]. World Health Organization. 2022 [cited 2023 Dec 4]. Available from: https://www.who.int/publications/i/item/9789240051157.
|
| 3. |
Global tuberculosis report 2021 [Internet]. 2021 [cited 2023 Dec 4]. Available from: https://www.who.int/publications/i/item/9789240037021.
|
| 4. |
Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med [Internet]. 2020;382(10):893-902. Available from: https://doi.org/10.1056/NEJMoa1901814.
|
| 5. |
Andersen P, Doherty TM. The success and failure of BCG – implications for a novel tuberculosis vaccine. Nat Rev Microbiol [Internet]. 2005;3(8):656-62. Available from: https://doi.org/10.1038/nrmicro1211.
|
| 6. |
Kik S V, Denkinger CM, Chedore P, Pai M. Replacing smear microscopy for the diagnosis of tuberculosis: what is the market potential? Eur Respir J [Internet]. 2014;43(6):1793 LP-1796. Available from: http://erj.ersjournals.com/content/43/6/1793.abstract.
|
| 7. |
Schumacher SG, Sohn H, Qin ZZ, Gore G, Davis JL, Denkinger CM, et al. Impact of Molecular Diagnostics for Tuberculosis on Patient-Important Outcomes: A Systematic Review of Study Methodologies. Goletti D, editor. PLoS One [Internet]. 2016;11(3):e0151073. Available from: https://dx.plos.org/10.1371/journal.pone.0151073.
|
| 8. |
Lam E, Nateniyom S, Whitehead S, Anuwatnonthakate A, Monkongdee P, Kanphukiew A, et al. Use of drug-susceptibility testing for management of drug-resistant tuberculosis, Thailand, 2004–2008. Emerg Infect Dis [Internet]. 2014;20(3):400. Available from: https://wwwnc.cdc.gov/eid/article/20/3/13-0951_article.
|
| 9. |
Zevallos M, Justman JE. Tuberculosis in the elderly. Clin Geriatr Med [Internet]. 2003;19(1):121-38. Available from: https://www.sciencedirect.com/science/article/pii/S0749069002000575.
|
| 10. |
Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radio-Graphics [Internet]. 2019;39(7):2023-37. Available from: https://doi.org/10.1148/rg.2019190109.
|
| 11. |
Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis [Internet]. 2003;3(3):141-7. Available from: https://www.sciencedirect.com/science/article/pii/S1473309903005449.
|
| 12. |
Singh P, Saket VK, Kachhi R. Demethoxycurcumin ameliorates rotenone-induced toxicity in rats. FBE. 2019;11(1):38-60.
|
| 13. |
Dhamnetiya D, Patel P, Jha RP, Shri N, Singh M, Bhattacharyya K. Trends in incidence and mortality of tuberculosis in India over past three decades: a joinpoint and age–period–cohort analysis. BMC Pulm Med [Internet]. 2021;21(1):375. Available from: https://doi.org/10.1186/s12890-021-01740-y.
|
| 14. |
Isaakidis P, Das M, Kumar AM V, Peskett C, Khetarpal M, Bamne A, et al. Alarming Levels of Drug-Resistant Tuberculosis in HIV-Infected Patients in Metropolitan Mumbai, India. Tyagi AK, editor. PLoS One [Internet]. 2014;9(10):e110461. Available from: https://dx.plos.org/10.1371/journal.pone.0110461.
|
| 15. |
Ministry of Health and Family Welfare CTD. India TB Report 2023 [Internet]. 2023. Available from: https://tbcindia.gov.in/showfile.php?lid=3680.
|
| 16. |
Masali HT, Takpere A, Shahapur P. A Comparative Study of Ziehl-Neelsen Stain and Fluorescent Stain Microscopy in the Diagnosis of Pulmonary Tuberculosis. J Pure Appl Microbiol. 15 (4): 2027-2033, 2021. 2021; Available from: http://103.139.156.196/jspui/bitstream/123456789/4119/1/119_2021.pdf.
|
| 17. |
Global Tuberculosis Report 2022 [Internet]. [cited 2023 Dec 4]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
|
| 18. |
Marçôa R, Ribeiro AI, Zão I, Duarte R. Tuberculosis and gender – Factors influencing the risk of tuberculosis among men and women by age group. Pulmonology [Internet]. 2018;24(3):199-202. Available from: https://www.sciencedirect.com/science/article/pii/S2531043718300667.
|
| 19. |
Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis [Internet]. 2013;93(1):104-7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1472979212001990.
|
| 20. |
Watkins RE, Plant AJ. Does smoking explain sex differences in the global tuberculosis epidemic? Epidemiol Infect [Internet]. 2005/08/19.2006;134(2):333-9. Available from: https://www.cambridge.org/core/article/does-smoking-explain-sex-differences-in-the-global-tuberculosis-epidemic/D87B9C5AE7F2B623225919286C803887.
|
| 21. |
Randall D. Responses of fish to hypoxia. Comp Biochem Physiol Part C Toxicol Pharmacol [Internet]. 2008;148(4):461-2. Available from: https://www.sciencedirect.com/science/article/pii/S1532045608002469.
|
| 22. |
Nhamoyebonde S, Leslie A. Biological Differences Between the Sexes and Susceptibility to Tuberculosis. J Infect Dis [Internet]. 2014;209(suppl_3):S100-6. Available from: https://doi.org/10.1093/infdis/jiu147.
|
| 23. |
Alateah SM, Othman MW, Ahmed M, Al Amro MS, Al Sherbini N, Ajlan HH. A retrospective study of tuberculosis prevalence amongst patients attending a tertiary hospital in Riyadh, Saudi Arabia. J Clin Tuberc Other Mycobact Dis [Internet]. 2020;21:100185. Available from: https://www.sciencedirect.com/science/article/pii/S2405579420300498.
|
| 24. |
Sunnetcioglu A, Sunnetcioglu M, Binici I, Baran AI, Karahocagil MK, Saydan MR. Comparative analysis of pulmonary and extrapulmonary tuberculosis of 411 cases. Ann Clin Microbiol Antimicrob [Internet]. 2015;14(1):34. Available from: https://doi.org/10.1186/s12941-015-0092-2.
|
| 25. |
de Andrade HLP, Gomes D, Ramos ACV, Arroyo LH, Santos-Neto M, Palha PF, et al. Tuberculosis forecasting and temporal trends by sex and age in a high endemic city in northeastern Brazil: where were we before the Covid-19 pandemic? BMC Infect Dis [Internet]. 2021;21(1):1260. Available from: https://doi.org/10.1186/s12879-021-06978-9.
|
| 26. |
Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC Infect Dis [Internet]. 2016;16(1):119. Available from: https://doi.org/10.1186/s12879-016-1451-0.
|
| 27. |
Murali S, Krishnamoorthy Y, Knudsen S, Roy G, Ellner J, Horsburgh CR, et al. Comparison of profile and treatment outcomes between elderly and non-elderly tuberculosis patients in Puducherry and Tamil Nadu, South India. Quinn F, editor. PLoS One [Internet]. 2021;16(8):e0256773. Available from: https://dx.plos.org/10.1371/journal.pone.0256773.
|
| 28. |
Ramachandran R, Nalini S, Chandrasekar V, Dave P V, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis [Internet]. 2009;13(9):1154-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19723407.
|
| 29. |
Kolappan C, Subramani R, Radhakrishna S, Santha T, Wares F, Baskaran D, et al. Trends in the prevalence of pulmonary tuberculosis over a period of seven and half years in a rural community in south India with DOTS. Indian J Tuberc [Internet]. 2013;60(3):168-76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24000495.
|
| 30. |
Dhanaraj B, Papanna MK, Adinarayanan S, Vedachalam C, Sundaram V, Shanmugam S, et al. Prevalence and Risk Factors for Adult Pulmonary Tuberculosis in a Metropolitan City of South India. Tyagi AK, editor. PLoS One [Internet]. 2015;10(4):e0124260. Available from: https://dx.plos.org/10.1371/journal.pone.0124260.
|
| 31. |
Aggarwal AN, Gupta D, Agarwal R, Sethi S, Thakur JS, Anjinappa SM, et al. Prevalence of Pulmonary Tuberculosis among Adults in a North Indian District. Herrmann JL, editor. PLoS One [Internet]. 2015;10(2):e0117363. Available from: https://dx.plos.org/10.1371/journal.pone.0117363.
|
| 32. |
Chadha VK, Kumar P, Anjinappa SM, Singh S, Narasimhaiah S, Joshi M V., et al. Prevalence of Pulmonary Tuberculosis among Adults in a Rural Sub-District of South India. Pai M, editor. PLoS One [Internet]. 2012;7(8):e42625. Available from: https://dx.plos.org/10.1371/journal.pone.0042625.
|
| 33. |
Chauhan A, Parmar M, Dash GC, Solanki H, Chauhan S, Sharma J, et al. The prevalence of tuberculosis infection in India: A systematic review and meta-analysis. Indian J Med Res [Internet]. 2023;157(2&3). Available from: https://journals.lww.com/ijmr/fulltext/2023/02000/the_prevalence_of_tuberculosis_infection_in_india_.6.aspx.
|
| 34. |
Paralkar V. Worlds Apart — Tuberculosis in India and the United States. N Engl J Med [Internet]. 2008 Mar 13;358(11):1092-5. Available from: https://doi.org/10.1056/NEJMp0707933.
|
| 35. |
Mandal S, Rao R, Joshi R. Estimating the Burden of Tuberculosis in India: A Modelling Study. Indian J Community Med [Internet]. 2023;48(3). Available from: https://journals.lww.com/ijcm/fulltext/2023/48030/estimating_the_burden_of_tuberculosis_in_india__a.11.aspx.
|
| 36. |
Parikh H, Savani N. Effect of COVID-19 on Tuberculosis Care in a District of Western Gujarat. Indian J Respir Care [Internet]. 2022;11(4):369-72. Available from: https://www.ijrc.in/doi/10.4103/ijrc.ijrc_105_22.
|
| 37. |
Al-Tawfiq JA, Al-Muraikhy AA, Abed MS. Susceptibility Pattern and Epidemiology of Mycobacterium tuberculosis in a Saudi Arabian Hospital: A 15-Year Study From 1989 to 2003. Chest [Internet]. 2005;128(5):3229-32. Available from: https://www.sciencedirect.com/science/article/pii/S0012369215528821.
|
| 38. |
Al-Awaidy ST, Al-Hamdan N. Drug-susceptibility pattern of mycobacterium tuberculosis among pulmonary tuberculosis patients in riyadh, saudi arabia. J Family Community Med [Internet]. 1997;4(2):65-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23008575.
|
| 39. |
Alrajhi AA, Al-Barrak AM. Mycobacterium tuberculosis susceptibility in Saudi Arabia. Saudi Med J [Internet]. 2002;23(10):1227-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12436127.
|













