Abstract
Background: Diabetes mellitus is a global health concern affecting 173 million adults annually that requires effective treatment. Medicinal plants such as ginger and curcumin, rich in bioactive compounds, have therapeutic potential. The aim of this study was to evaluate the therapeutic potential of ginger and curcumin extracts in diabetic nephropathy in the rat model.
Material and methods: High-performance liquid chromatography was used to examine ginger and curcumin extracts. Fifty male Sprague Dawley rats were divided into five groups: control, untreated diabetic, ginger-treated diabetic, curcumin-treated diabetic, and a ginger + curcumin combination group. Diabetes was induced with a single intraperitoneal dose of streptozotocin. Rats received daily oral doses of ginger, curcumin or the combination of both. After sixteen weeks, rats were anesthetized and various tests were conducted to evaluate treatment outcomes.
Results: The rats treated with combined ginger and curcumin extracts had superior outcome in terms of more antioxidant activity, better glycemia management and less DN-related kidney damage (reduced albuminuria and less histological changes).
Conclusions: Our findings indicate that ginger and curcumin extracts have therapeutic potential in mitigating functional and structural alterations in the kidneys of diabetic rats, possibly due to their anti-diabetic and anti-inflammatory properties.
Citation
Taha A, Ashour H, Reffat M, Elkhawaga O Y. The impact of ginger and curcumin on diabetic nephropathy induced by streptozotocin in rats. Eur J Transl Clin Med. 2023;6(2):51-65Abbreviations
- ALT – alanine aminotransferase
- AST – aspartate aminotransferase
- AOA – anti-oxidant activity
- BUN – blood urea nitrogen
- CAT – catalase
- DAB – 3,3’-diaminobenzidine tetrachloride
- DM – diabetes mellitus
- DN – diabetic nephropathy
- DPPH – 2,2-diphenyl-1-1picryhydrazyl
- GSH – reduced glutathione
- IL-6 – interleukin- 6
- MDA – malondialdehyde
- NF-κB – nuclear factor kappa B
- OS – oxidative stress
- PKC – protein kinase C
- ROS – reactive oxygen species
- SOD – superoxide dismutase
- STZ – streptozotocin
Introduction
Diabetes mellitus (DM), affecting 8.8% of people globally in 2017 and expected to rise to 9.9% by 2045 [1], often leads to diabetic nephropathy (DN) in 30-40% of individuals. DN is linked to improper glycemia management, glycosylated proteins, and renal tissue abnormalities. Researchers emphasize the role of reactive oxygen species (ROS) and oxidative stress (OS) in DN pathophysiology [2-3]. Despite recent medical trials, addressing vascular issues and poor glucose control remains a challenge [4]. Early detection and understanding the complex interplay of metabolic factors are crucial to managing this chronic kidney disease [5].
Despite therapies with anti-hyperglycemic and renin-angiotensin system blocking drugs, DN development persists. Exploring new treatment options is essential. Studies investigating the potential benefits of nutritional antioxidants in diabetes patients have been conducted. Additionally, there’s a growing demand for herbal medications derived from medicinal plants [6].
The ginger plant (Zingiber officinale Roscoe) belongs to the Zingiberaceae family, renowned for its medicinal significance and wide distribution. This family encompasses 53 genera and over 1200 medicinal plants, primarily found in tropical regions with large rhizomes. Ginger is cultivated in Southeast Asia, Australia, Brazil, West Africa, and the United States [7-8]. Its chemical analysis reveals more than 400 compounds, including phenolic and terpene compounds like gingerol, shogaol, and paradols, which contribute to its diverse biological activities. Ginger is widely used as a condiment in various cuisines around the world [9-10]. Ginger’s phytochemical composition underscores its health-enhancing properties [11]. It serves as an antioxidant, protecting the body from oxidative stress and DNA damage while counteracting free radicals [12]. Ginger’s therapeutic effect in managing diabetic complications is believed to involve reducing oxidative stress and inflammation, partly through inhibiting the NF-κB signaling pathway [13]. Studies indicate that ginger, containing compounds like Zerumbone, alleviates renal damage in diabetic rats. Thanks to its anti-inflammatory benefits, ginger can be helpful in conditions such as gout, osteoarthritis and rheumatoid arthritis. Ginger’s functions include regulating blood glucose levels, pain relief, cardiac stimulation, antiemetic, antimicrobial, and antifungal actions [14]. Ginger exhibits protective effects on diabetic liver, kidney, eye, and nervous system complications. Numerous experiments confirm ginger extract’s ability to lower blood glucose levels in both diabetes types, demonstrating a dose-dependent pattern [15].
Curcumin, extracted from the rhizome of turmeric belongs to the Zingiberaceae family, grows predominantly in India, Southeast Asia and China. Curcumin is a potent component in herbal medicine and is extensively studied for various health conditions [16]. Its primary compound, polyphenol curcumin, exhibits powerful anti-inflammatory, antioxidant, and anticarcinogenic properties. In diabetes management, curcumin’s effectiveness lies in its interaction with key molecules and pathways crucial in the disease’s progression. Studies show curcumin’s ability to alleviate insulin resistance, a factor in metabolic syndrome. Both ginger and curcumin contain antioxidants that activate redox-sensitive transcription factors, bolstering cellular antioxidant defenses [17-18]. Given the importance of dietary management in diabetes, interventions using natural substances like ginger and curcumin offer promising strategies to mitigate the renal complications of DM.
In this study we aimed to assess the impact of curcumin and ginger on kidney function, antioxidant activity (AOA) and lipid peroxidation in diabetic rats with DM induced by streptozotocin (STZ). We also explored the effects of ginger extract, curcumin extract, and their combination on treating DN in rat models with DM.
Material and methods
Chemicals and extraction
Acetonitrile and STZ were obtained from Sigma-Aldrich (St. Louis, USA). Superoxide dismutase was obtained from Biodiagnostic Company, Egypt.
The ginger and curcumin powders were obtained from the Imtenan Egypt Company. Methyl alcohol was obtained from El Nasr Pharmaceutical Chemicals Company in Egypt. The ginger powder (500g) and curcumin powder (500 g) were soaked in pure methanol for 72 hours two times. Then, the extract was clarified, and the residue was rejected. Excess alcohol was eliminated from every extract using a rotary evaporator at 50 °C and afterwards dried in a freeze-dryer. The sticky extracts were collected and stored at -20 °C before the experiment [19].
Preliminary phytochemical investigation of the plants
The presence of carbohydrates was determined by the Molisch’s test [20]. A few sodium hydroxide drops determined the presence of flavonoids according to the alkaline reagent test [21]. The presence of saponins was determined based on the presence of foam. The presence of tannins was tested using a 2% solution of FeCl3 [22]. Additionally, the presence of glycoside content was detected according to the Wagner and Hager tests [23]. The reaction of Liebermann detected the presence of steroid content [24]. The presence of terpenoids was determined according to Salkowski’s test (a reddish-brown tint appeared upon contact) [25]. The total flavonoids in each plant extract were determined by spectrophotometric analysis using the reference substances quercetin and gallic acid, respectively [26].
Analysis of the plant extracts’ anti-oxidant activity (AOA)
The obtained extracts’ ability to react with stable 2,2-diphenyl-1-1picryhydrazyl (DPPH) free radicals (measured by using a spectrophotometer at wavelength 517 nm) was used to determine the extracts’ antioxidant activity (AOA) [27-28]. The total flavonoid content (extracted from ginger and curcumin) was calculated using a colorimetric assay. A freshly prepared extract (0.5 g) was put in a tube with 5 ml of methanol (80%) in this assay. The extracts were inverted and mixed after vertexing the tubes and letting them stand for 20 minutes. A clear micro-centrifuge tube was filled with 2 ml of each extract, and then the tubes underwent a 5-minute, 4 °C, 12000 rpm centrifugation. The supernatant was analyzed within two weeks after being stored at -20 °C in a clear microcentrifuge tube. A combination of 600 μl of distilled water and 45 μl of NaNO2 was added with 150 μl of the methanol extract. After the incubation of the solution at room temperature for 5 minutes, 45 μl of AlCl3 (10%) was added, and the combination was then re-incubated for 1 minute. Finally, 300 μl of distilled water and 1M NaOH were added. In place of the extract, a blank containing 80% aqueous methanol was used to calculate the absorbance at 510 nm. Using catechin as the standard, the concentration was calculated using a calibration curve. Catechin equivalents (CE) were expressed as mg of CE/g fresh weight [29].
Spectroscopic Investigation
The ginger extracts were analyzed on a high-performance liquid chromatography (HPLC) instrument Agilent1260 (Agilent Technologies, Santa Clara, USA). The series column used was the Agilent C18 column (4.6 mm x 250 mm, i.e., 5μm). The mobile phase consisted of acetonitrile: 5% Acetic Acid (50:50, v/v). The sample solvent was methanol. The flow rate was set to 2 ml/min. The injection volume of the sample, which was dissolved in methanol, was 25 μm. The column’s temperature was 25°C, the wavelength of detection was 280 nm, and the run was 20 min [30]. The curcumin extract was analyzed using the same HPLC instrument and mobile phase as the ginger extract. For each of the curcumin sample solutions, the injection volume of the sample dissolved in methanol in the column was 20 m. At 425 nm, the multiple wavelength detector was adjusted. Finally, the column was stored at 40 °C [30].
Experimental Animals
Fifty male Sprague Dawley rats weighing between 180 and 200 g were selected. Before the experiment, the animals were given a week to get used to the lab environment in separate metal cages with five animals per cage. Clean and fresh water was made available at all times. Throughout the study, the nutritional status of the rats was monitored under standard environmental conditions (temperature 23-27 °C, 60% humidity). The animal experiment in this study were performed in compliance with the guidelines of the Guide for the Care and Use of Laboratory Animals and approved by the Suez Canal University Animal Care Committee (REC 26/2022).
Induction of T1DM
In order to induce DM, a single intraperitoneal injection of 60 mg STZ dissolved in citrate buffer (pH 4.5) / kg body weight was given to 40 of the 50 rats [31]. The glycemia of all rats was measured in blood samples obtained from the tail vein 72 hours following an overnight fast. After that, blood sugar levels were checked every three days using a GlucoDr Super Sensor glucometer (OneTouch Technology, All Medicus Co, Korea). The study comprised of 38 rats who developed DM (chronic hyperglycemia ≥ 250 mg/dl) one week after the STZ injection [32].
Experimental Design and Groups
The 50 rats were divided into 5 groups. The control group (n = 10) received 0.25 ml of citrate buffer (pH 4.5) intravenously. As mentioned above, 40 rats that were injected with STZ and we subdivided them into 4 additional groups of 10 each: DM (not treated with any substance), DM-ginger (treated with 350 mg/kg/day of ginger extract), DM-curcumin (treated with 350 mg/kg/day of curcumin) and DM ginger + curcumin (treated with combination of both extracts). An intragastric tube was used to administer both extracts orally after dissolving in water. The conversion of drug dosages to human equivalent doses was used [33]. Drug treatment began 8 weeks after the STZ injection and continued for additional 8 weeks, making the total study duration 16 weeks. At the end of week 16 of the experiment, all the rats were sacrificed via cervical dislocation and tissue samples were obtained for analysis.
Urine Samples
Rats from each group were weighed every 2 weeks and kept apart in metabolic cages to enable 24-hour urine collection. The rodents’ access to food and water remained unlimited. The total volume of urine output was measured. In addition, urine samples were collected every 24 hours, stored at -80 °C and were used to calculate the excretion of urinary albumin.
Biochemical estimation and evaluation
Glucose levels, liver function (alanine aminotransferase, ALT; aspartate aminotransferase, AST), and kidney function (blood urea nitrogen and creatinine) were examined in the serum. Albuminuria changed due to the experiment’s measurement of the inflammation markers IL6 and malondialdehyde (MDA) and the antioxidants; superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT).
To determine the amount of glucose in blood samples, glucose kits (Engineering Chemistry for Lab Technology, BioMed-Glucose L. S., Badr City, Egypt) were used [34]. Creatinine levels were measured using kits (Diamond Diagnostics Company Hannover, Germany) [35]. Berthelot enzymatic colorimetric method was used to measure blood urea nitrogen (BUN) (Diamond Diagnostics Company, Hannover, Germany) [36].
Utilizing micro-albuminuria kits from ABC Diagnostic in New Damietta, Egypt, urine samples were collected in accordance with the manufacturer’s instructions to test urinary albumin excretion (μg/ml) [37]. Using methods that considered the urine volume (ml) in 24 hours, albuminuria was computed as g/24 hours. ALT and AST weremeasured using the kits (Cat. No. 264001 and 260001) from Spectrum Diagnostics Company was used to detect ALT in serum. Additionally, AST was measured in serum according to the kit of aspartate aminotransferase from the Company of Spectrum Diagnostics (Cat. No. 260001).
Inflammatory Markers and Kidney Anti-oxidant Parameters
The right kidney of all rats was taken and homogenized in phosphate buffer to test the mean OS parameters, MDA, GSH, CAT, SOD, and IL-6 levels using specialized kits following the manufacturer’s instructions. The following kits were used: a rat IL-6 ELISA kit from Bioassay Technology Laboratory Company (Shanghai, China) and MDA, GSH, CAT, and SOD kits from the Bio-Diagnostic Company (Dokki, Giza, Egypt; MD2529, CA2517 and SD2521, respectively).
Histopathology and immunohistochemistry
Neutral buffered formalin (10%) was perfused into the left kidneys of all rats, which were then processed into 5-mm paraffin sections for histological examination. Hematoxylin and eosin (H & E), Masson, periodic acid-Schiff (PAS), and immunostaining renal sections against NF-κB were used to stain all kidney tissues [6]. NF-κB expression was assessed through the immunostaining of deparaffinized samples slides using polyclonal NF-κB antibody (sc-59103) from Santa Cruz Biotechnology Inc., CA, USA) diluted 1:50.
Data analysis
After doing a one-way analysis of variance (ANOVA), the parametric data presented as mean ± SD were examined using the post-hoc Scheffé test. The Kruskal-Wallis H, chi-square “χ2” or Fisher’s exact test were applied to examine quantitative data. P ≤ 0.05 served as the significance threshold.
Results
Ginger and curcumin extract contained the highest phytochemical content and AOA (Table 1). The two Zingiberaceae plant species were examined phytochemically to identify seven naturally occurring classes (carbohydrates, saponins, glycosides, tannins, alkaloids, flavonoids, and steroids). It was found that ginger extract had a significant amount of phenolic (flavonoids). The high glycoside content was recorded in curcumin. The high saponins content was recorded in ginger. The high carbohydrate content was recorded in ginger. Furthermore, the high terpene content was recorded in curcumin extract. The highly alkaloid content was recorded in curcumin extract.
Table 1. Phytochemistry of plant extracts
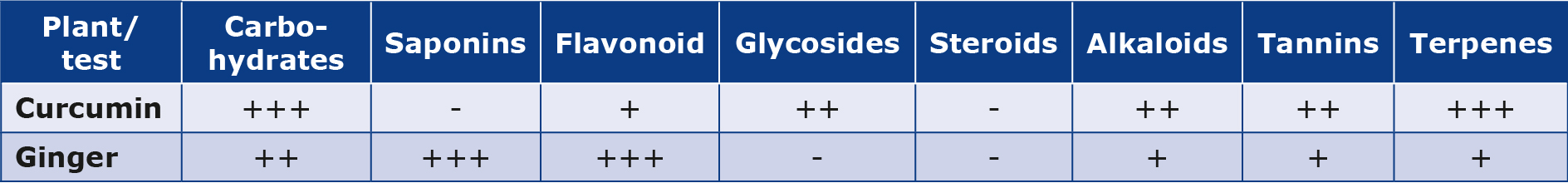
The signs (-) and (+) denote a negative test, (+) a weakly positive test, (++) a moderately positive test, and (+++) a strongly positive test, respectively.
DPPH scavenging activity
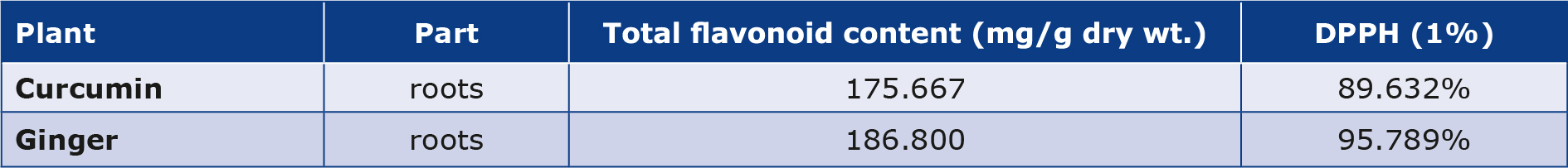
Each extract underwent quantitative analysis to estimate the outcomes of the analysis of scavenging activity (2, 2-diphenyl-1-1picryhydrazyl, DPPH). It was found that the scavenging activity of ginger was more than that of curcumin (Table 2).
Table 2. Total flavonoid contents and DPPH scavenging activity
Total Flavonoids Contents
Each extract underwent quantitative analysis to determine its estimated total flavonoid concentration. The ginger extract had a greater total flavonoid content than the curcumin extract (Table 2).
HPLC Results
HPLC chromatogram results of ginger extract interpretation are shown in (Table 3, Figure 1). The peak identification of the ginger extract component depends on retention time, peak area, and height of the ginger extract compared with the standard. It was found that the detailed levels 6-gingerol was more abundant than 6-shogaol, 10-gingerol, and 8-gingerol. The lowest peak was 10-shogaol and 8-shogaol.
Table 3. HPLC analysis of the ginger extract
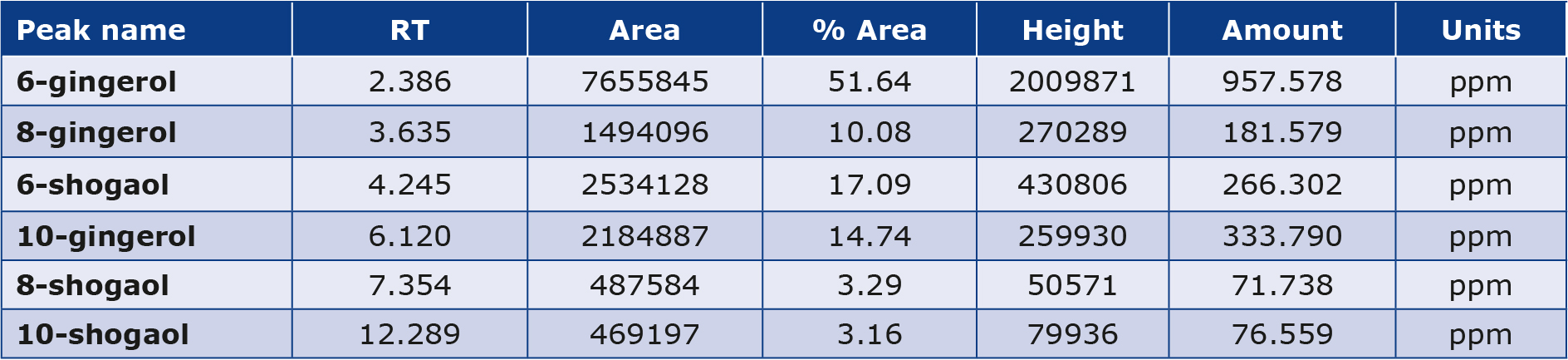
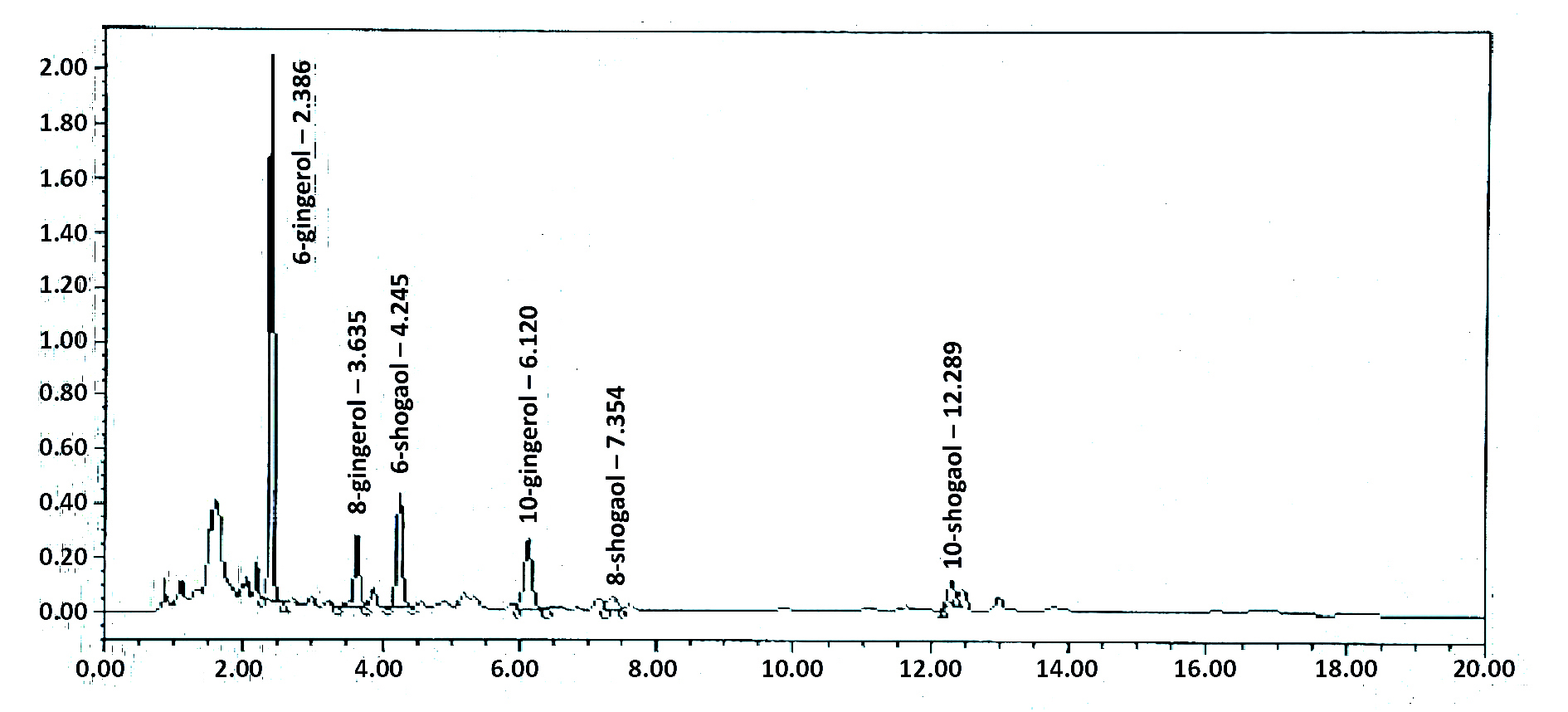
Figure 1. HPLC chromatogram of the ginger extract
The sample performed in HPLC triplicate resulted in the identification of the curcumin extract. By dividing the overall area of peaks, which included the primary curcumin peak, demethoxycurcumin, and at least bisdemethoxycurcumin, one can determine the standard or curcumin extract ratio. By dividing the standard’s ratio by the standard’s ratio times the standard’s potency, the amount of curcumin in curcumin extract was estimated (Table 4; Figures 2a and 2b).
Table 4. HPLC analysis of the curcumin extract
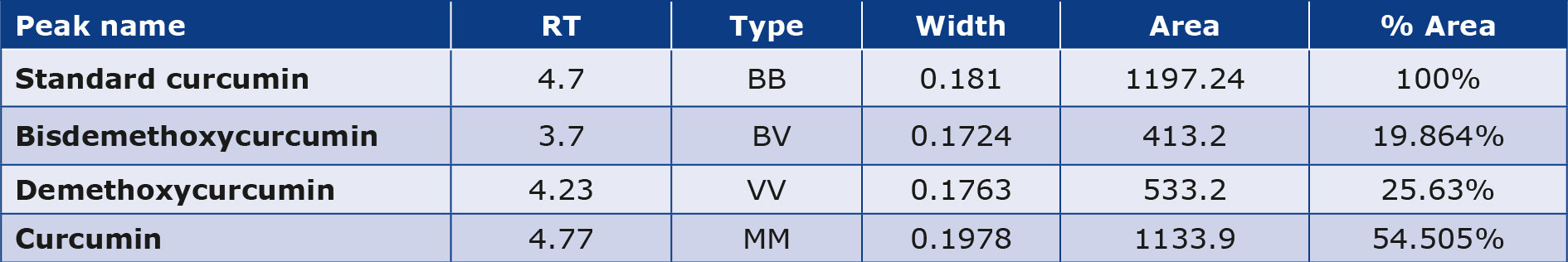
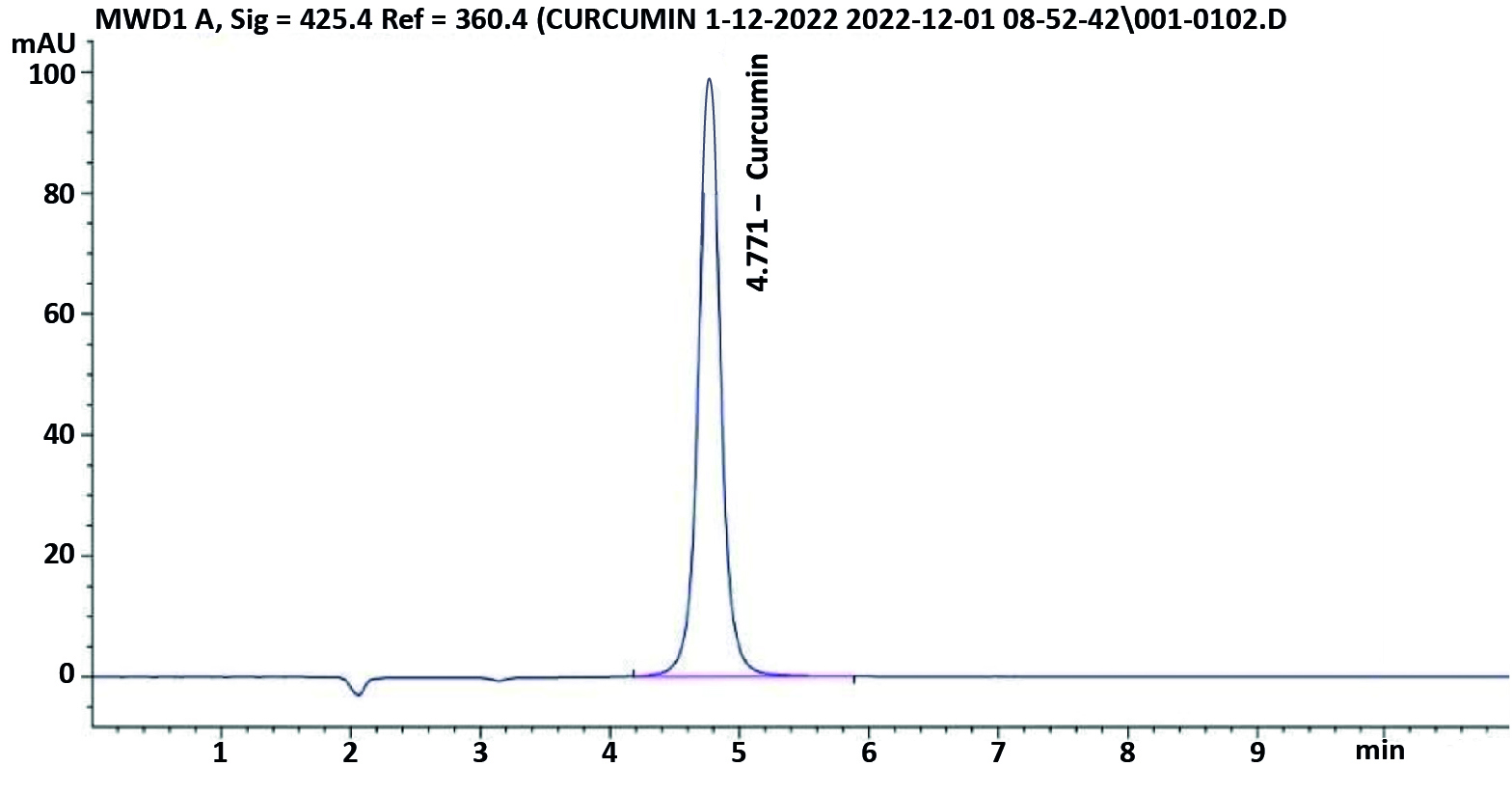
Figure 2a. HPLC chromatogram of a standard (Curcumin)
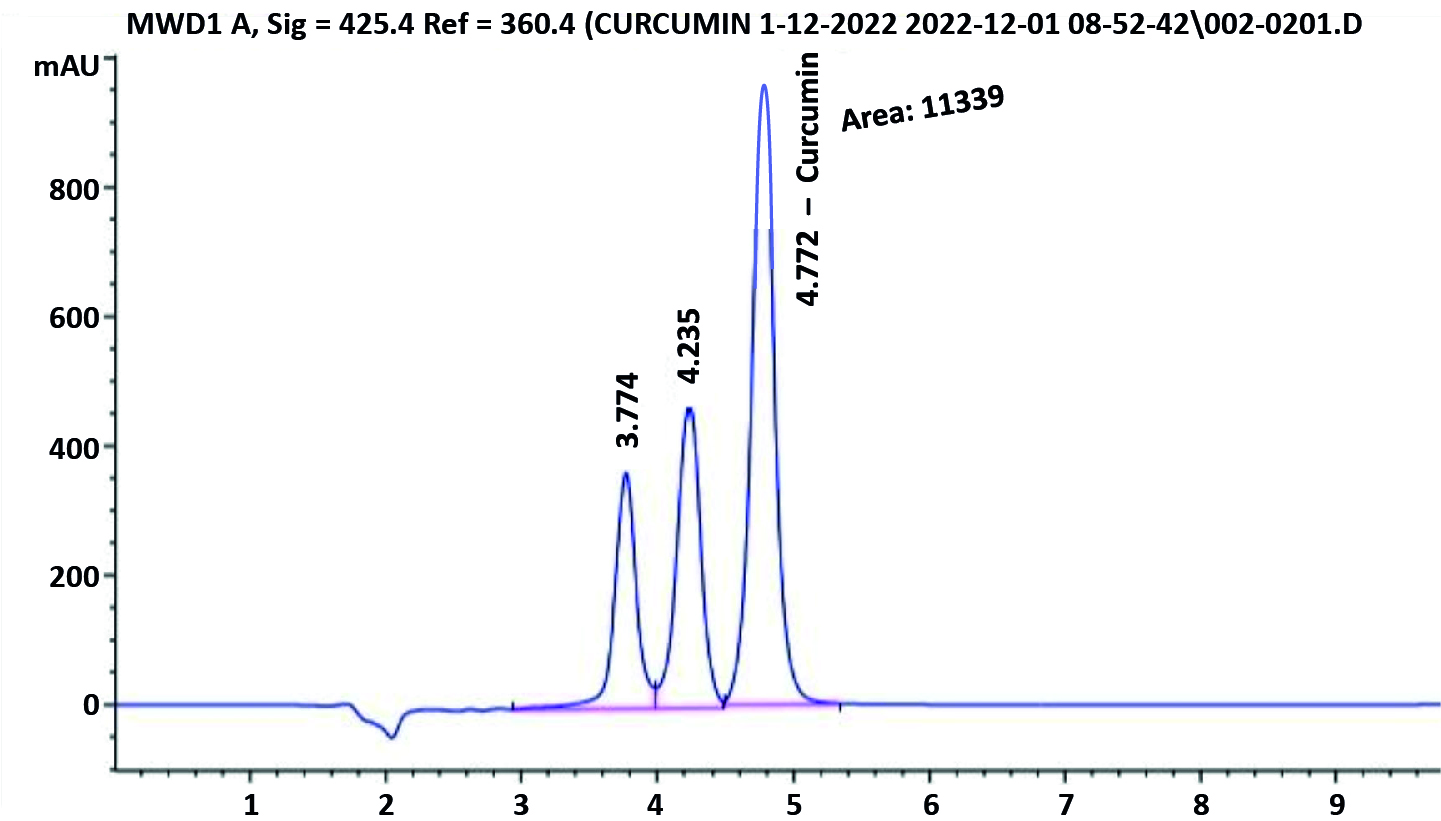
Figure 2b. HPLC chromatogram of Curcumin extract
Characterization of STZ-induced diabetes
Based on their fasting blood glucose levels, 96% of the rats developed DM one week following the STZ injection. Before the 16 week-long observation was completed, rats with DM who received no treatment had a mortality rate of 30% (n = 3). The same mortality rate was observed in the curcumin group (30 %, n = 3), whereas in the ginger group it was 20% (n = 2). No mortality was noted in the control group.
Glycemia, kidney function and liver function
We noted a significantly increased glycemia in the control group and the untreated diabetic group (p < 0.05). In contrast, glycemia was significantly lower after treatment with the extracts of ginger, curcumin or their combination (p < 0.05) (Table 5). It was found that there was a more significant increase in renal function parameters (creatinine and blood urea nitrogen) in the control group and untreated diabetic group (p < 0.05). However, there were significantly lower levels of creatinine and blood urea nitrogen) after treatment with either of the extracts or their combination (p < 0.05). Combining ginger and curcumin extracts achieved reduced blood urea nitrogen and creatinine levels (p < 0.05) (Table 5). There was a highly significant increase in liver function parameters (ALT, AST and albumin levels) in the untreated diabetic group compared to the control group (p < 0.05). After the treatment of the diabetic group with ginger extract, curcumin extract, or their combination. (ALT, AST, and albumin levels) decreased significantly (p < 0.05), with a more pronounced decline in the group treated with combined ginger and curcumin (p < 0.05) (Table 5).
Table 5. Blood glucose levels, kidney function and liver function tests
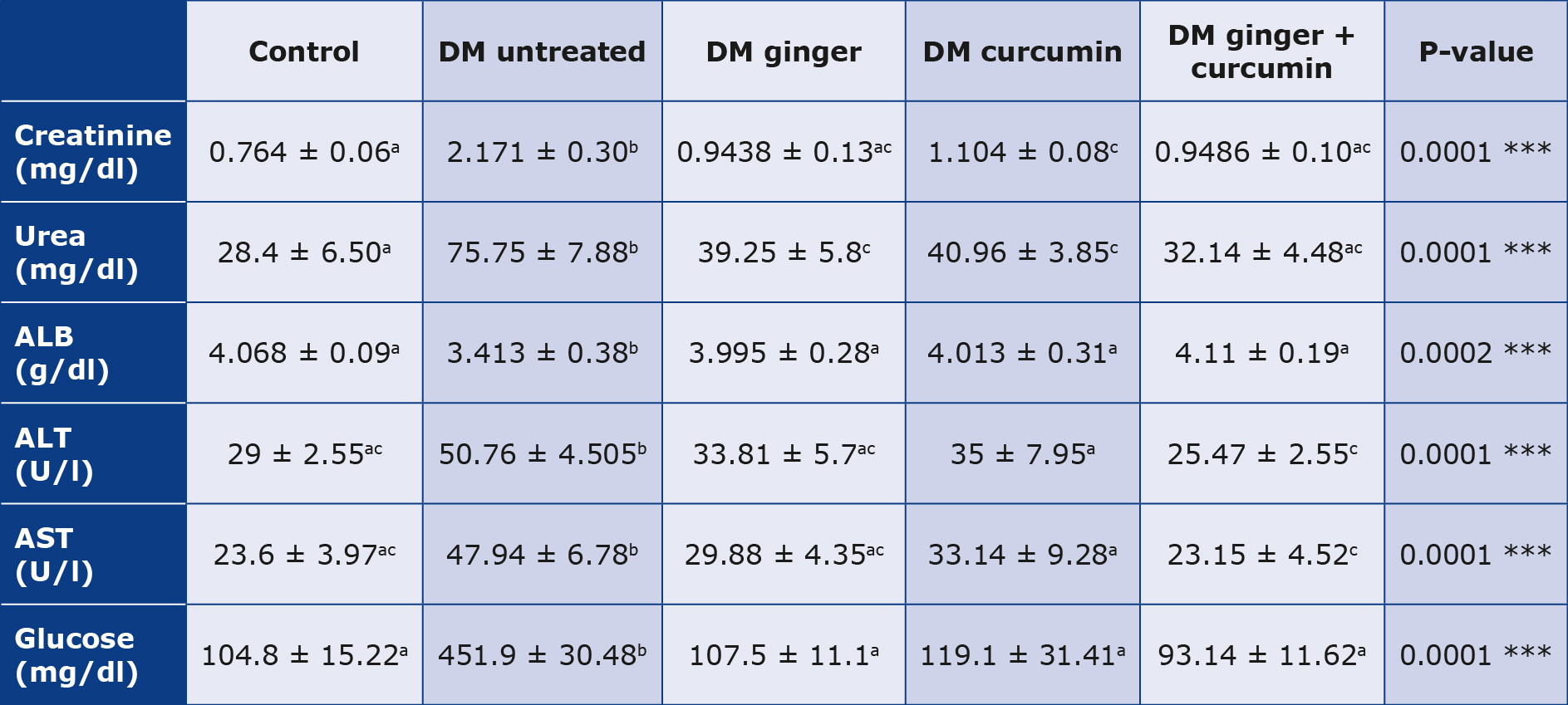
The post-hoc Scheffé test (P < 0.05) was employed after one-way ANOVA was used to analyze the mean and standard deviation of the data. As a result, a < 0.05 p-value indicates that having numerous letters in the same column is significant.
Body weight, kidney weight to body weight, IL-6, OS parameters and AOA
The treated groups’ mean body weight increased after the treatment (p < 0.05). In the treated groups compared to the STZ-diabetic group, the mean kidney weight and the renal weight to body weight ratio decreased (p < 0.05), and OS and inflammation markers decreased (p < 0.05) after extract treatment compared to STZ diabetics that were not treated. Additionally, anti-oxidant levels were significantly higher (p < 0.05) in the treated groups (Table 6). The ratio of kidney weight to body weight significantly decreased following treatment with the extracts (p < 0.05) after treatment with ginger and curcumin extract. It was noted that the treatment using extracts of ginger and curcumin together reduced the kidney weight relative to body weight (p < 0.05) (Table 6).
Table 6. IL-6, OS parameters, AOA, body weight and kidney weight
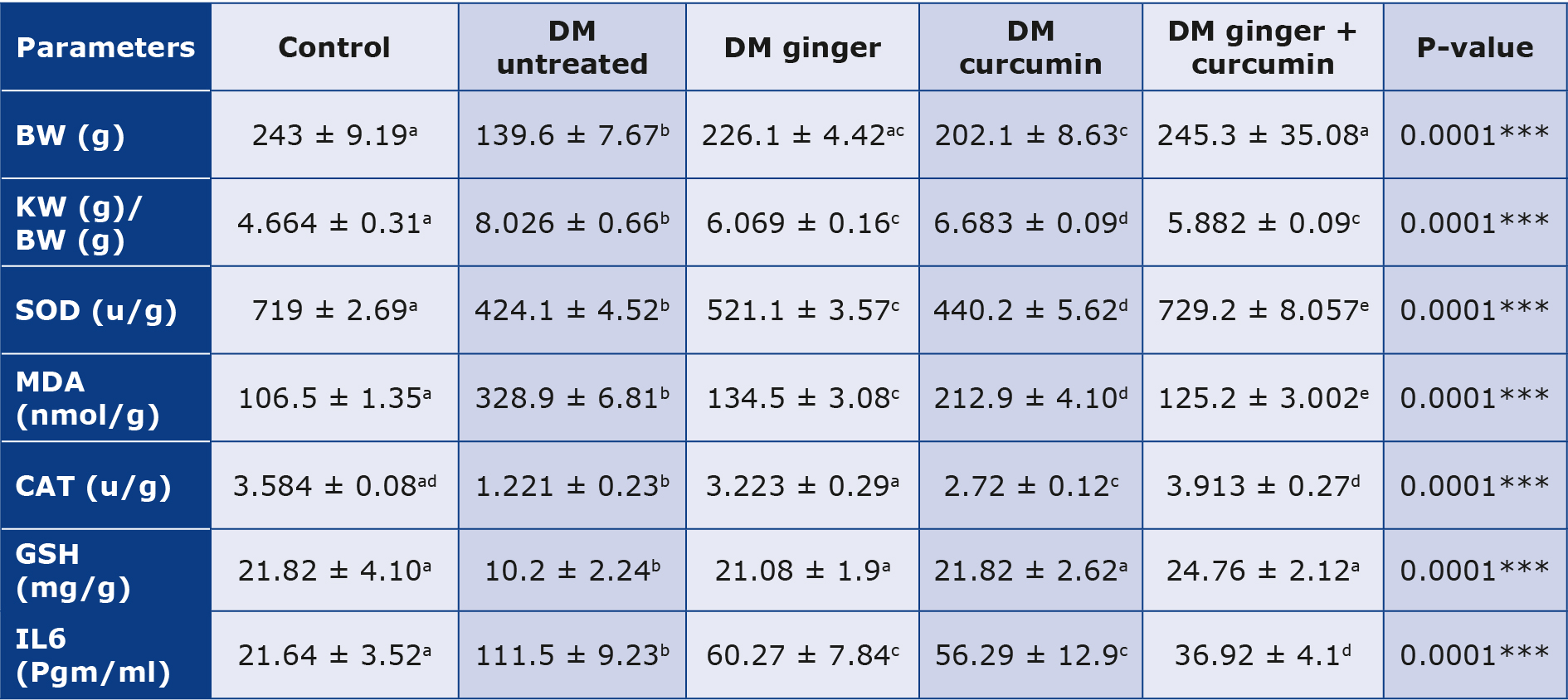
BW - body weight, KW - kidney weight
The mean +SD data were analyzed using one-way ANOVA, and the post-hoc Scheffé test was run (p < 0.05). This means various letters in the same column are significant at p < 0.05.
Following treatment with ginger and curcumin extract, there was a significant (p < 0.05) decrease in IL6 and MDA. The treatment with combined ginger and curcumin extracts was produced a more significant reduction in IL6 and MDA levels (p < 0.05) (Table 6).
There was a significant decrease in glutathione, catalase and SOD levels in the control group compared to the untreated diabetic group (p < 0.05). In contrast, the levels of these antioxidants significantly increased after treatment with the extracts (p < 0.05), with greater increase more following treatment with combined extracts (p < 0.05) (Table 6).
Albuminuria
There were variations in albuminuria (mg/24 h) in all the groups of rats we observed. The rats treated with ginger, curcumin or a combination of the two extracts all had a significant increase in 24-hour urine volume and protein excretion. Starting from the 10th week of treatment, albuminuria decreased in the groups receiving the extracts, with the best effect in rats treated with a combination of ginger and curcumin extracts (p < 0.05) (Table 7).
Table 7. Changes in albuminuria during the study
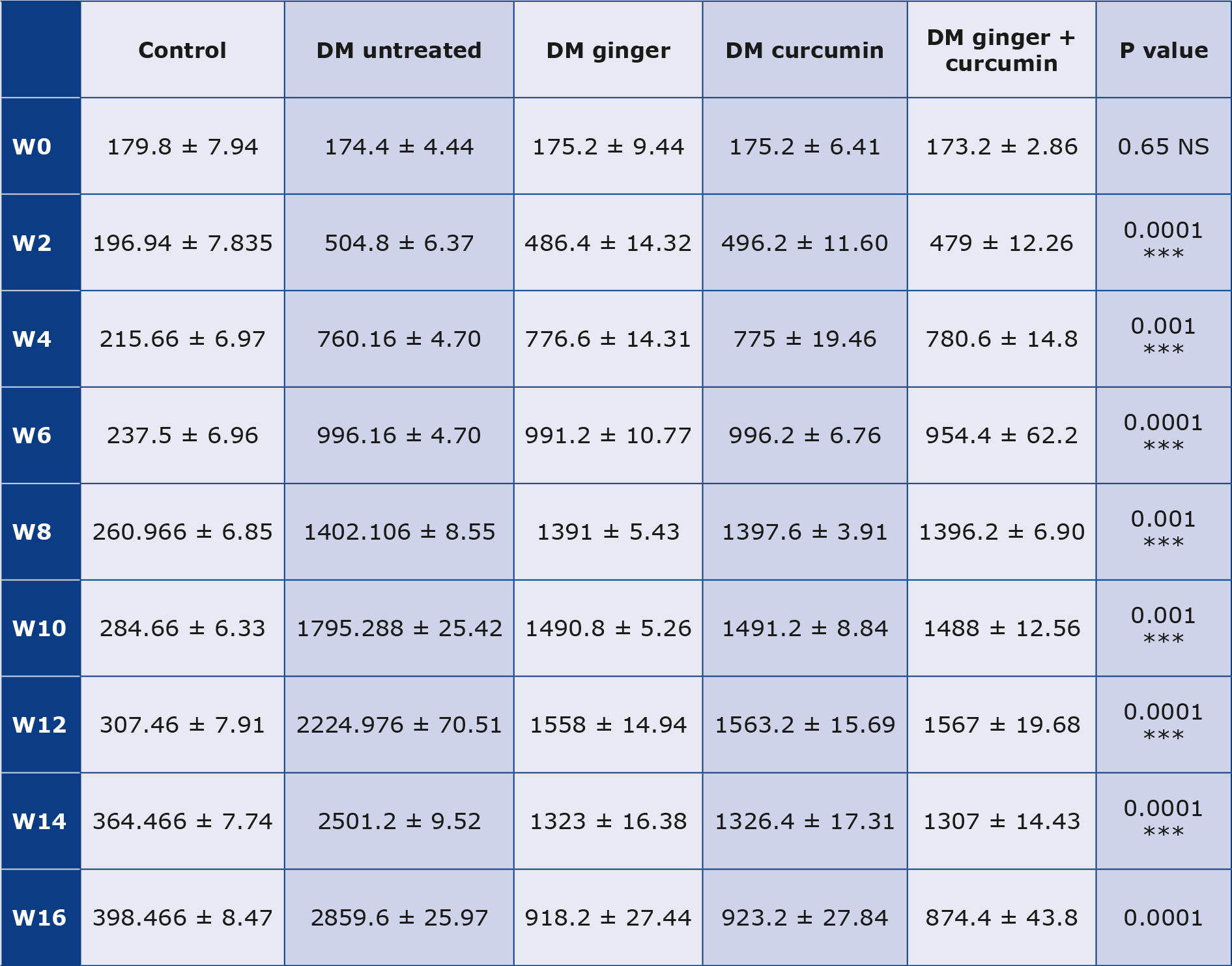
Albuminuria (mg / 24 hours) changes during the 16 weeks (W) of the experiment data are expressed as mean ± SD. An increase in 24-hour urine volume and an increase in urine protein excretion were seen in the diabetic control group. However, when diabetic rats were given the extract, they all decreased (p < 0.05).
Histopathology
The kidney sections obtained from the control group revealed no collagen accumulation, interstitial tissue or abnormal glycogen (Figures 3-B, 3-C, and 3-D). Conversely, sections of kidney samples from DM rats sections displayed significant blue collagen (Figure 3-H) and tubular dilation with glycogen buildup (blue arrows). Kidney sections obtained from rats treated with ginger contained glycogen-free areas, reduced collagen (blue arrows), and milder tubular changes (Figure 3-L, 3-J, and 3-G). Kidney samples from curcumin-treated rats revealed reduced collagen and showed minimal tissue changes (Figure 3-N and 3-O). Finally, sections of kindeys of rats treated with combined ginger and curcumin extracts displayed no collagen, minimal tissue, and no glycogen deposition (Figure 3-R, 3-S, and 3-T).
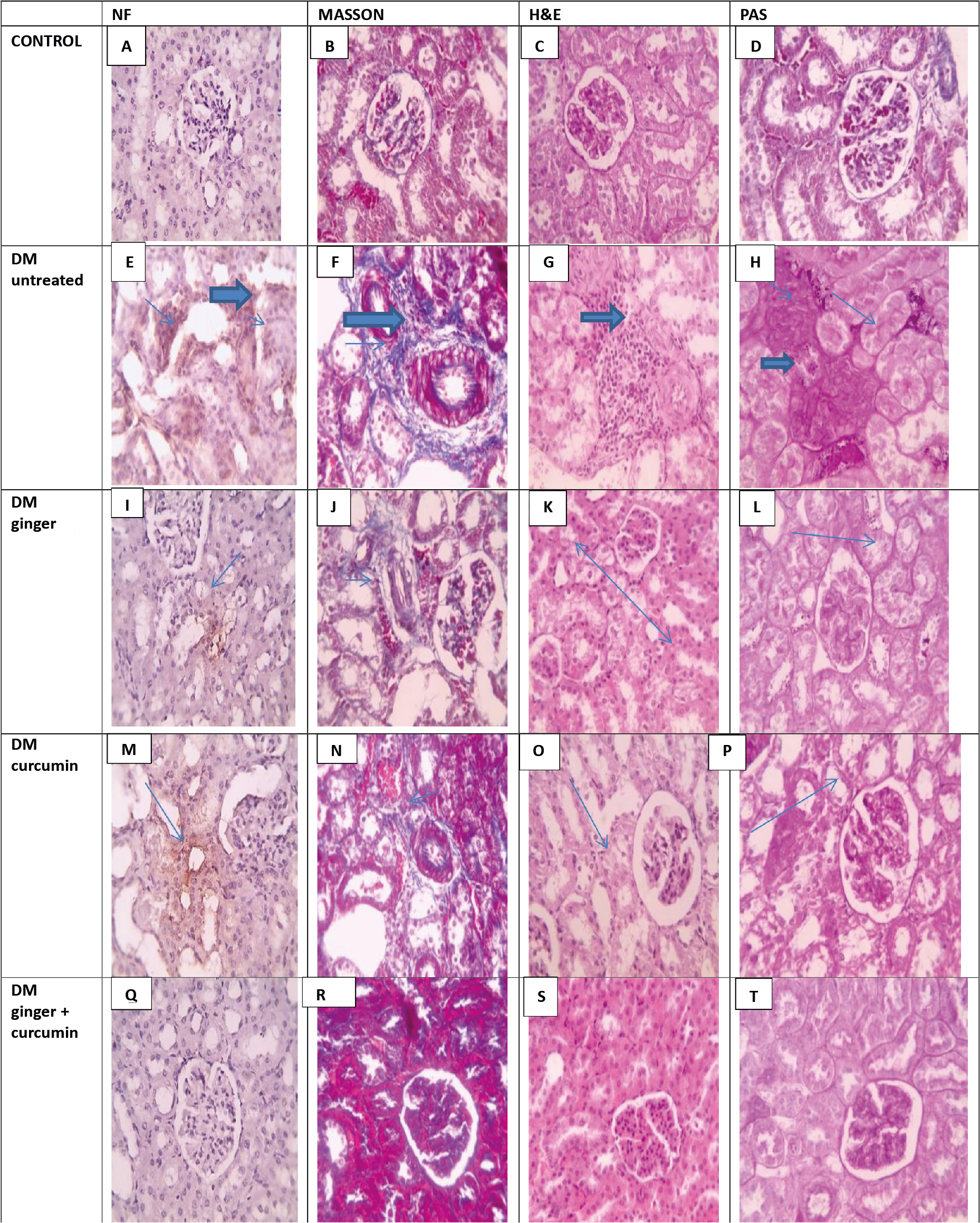
Figure 3. Histopathological and immunohistochemical assessments
Immunohistochemistry
Microscopic views of immunostained renal sections against NF-κB in the control group showed negative expression, according to IHC counterstained with Mayer’s hematoxylin (Figure 3-A). The diabetic group’s brown tubular expression was positive in microscopic images of immunostained renal sections against NF-κB (Figure 3-E): A very slight positive reaction was seen in the diabetic group treated with ginger in comparison to the curcumin-treated diabetic group in microscopic images of immunostained renal sections against NF-κB (Figure 3-M) (Figure 3- I). In the group treated with combined extracts, brown tubular expression (blue arrows) and negative expression are shown (Figure 3-Q).
Discussion
Insulin deficiency leads to persistent hyperglycemia and metabolic imbalances, making diabetes challenging to treat [38]. Diabetic nephropathy (DN) is a major cause of renal failure, affecting 15-25% of T1DM and 30-40% of T2DM patients [39]. STZ-induced diabetes mimics these conditions. In our study, prolonged hyperglycemia led to DN, evident in decreased antioxidant levels, intense ROS production, reduced kidney function and abnormal renal histology [40-41]. These findings emphasize the link between sustained hyperglycemia, oxidative stress and DN progression.
The basis for the DM model in our study was the chemotherapy drug STZ, which damages pancreatic cells and reduces insulin production leading to chronic hyperglycemia [42]. This study demonstrated how persistent hyperglycemia encouraged energy imbalances with strong lipolysis through increased feed intake and weight loss in animals [42-43]. Polydipsia, polyuria and decreased GFR were seen as a result of STZ’s involvement in the hemodynamic alterations brought on by glomerular hyperfiltration [43]. Because systemic hyperglycemia negatively affects glomerular filtration, which leads to an excessive buildup of intracellular glucose, endothelial cells cannot manage glucose transport through the plasma membrane. The high intracellular glucose levels encourage manufacturing cytokines e.g. transforming and vascular endothelial growth factors. Through vasodilating prostanoids and nitric oxide, these substances cause macrovascular endothelial lesions and increase glomerular permeability. Additionally, these macromolecules change the hemodynamics of the glomerulus, intensifying vasodilation of the afferent arteriole compared to the efferent one. Glomerular hyperfiltration results from a final representation of artery imbalance [43-45]. An ongoing rise in hydrostatic pressure (intraglomerular pressure that forces fluid through capillaries while albumin is lost into the ultra-filtrate) is a sign of changes caused by glomerular dysfunction [44, 46]. Proteoglycans and collagen IV, which are in charge of thickening the basal glomerular membrane and altering the negative charge of the podocytes, are two molecules synthesized and catabolized during glomerular dysfunction. Protein loss in the urine and glomerulosclerosis with a reduced GFR are signs of glomerular dysfunction [46-47]. In our study, the characteristics of renal lesions linked to macrovascular changes of diabetic nephropathy were validated in animals that had undergone nephrectomy, demonstrated by the increased kidney and relative weight (kidney weight/body weight). The chronic hyperglycemia affected the glomerular basal membrane’s structure. It promoted extracellular matrix growth in the mesangial area, which resulted in tubular area hyperplasia and hypertrophy [47]. The progression of kidney disease was hastened in diabetic animals that received left nephrectomy in this investigation using the STZ-induced DM model in rats. The signs of DN included increased urine albumin excretion and impaired renal function (elevation in plasma creatinine concentration and reduced GFR).
The main factor thought to contribute most significantly to intracellular ROS generation is chronic hyperglycemia. Significant amounts of glucose are transferred inside the glomerular endothelium, mesangial cells and tubular epithelium cells because some cells cannot maintain intracellular glucose homeostasis, thus speeding up glycolysis and releasing an excessive quantity of ROS [45, 48]. In addition to being mediated by hydrogen peroxide (H2O2), unattached iron, and the creation of additional free radicals such OH- and peroxynitrite, an oxidative lesion cascade is initiated by a chain reaction that breaks down lipids, proteins and nucleic acids by creating the (ONOO-) O2. radical [45, 49]. The elevated levels of urine peroxides and thiobarbituric acid reactive substances (TBARS) in diabetic rats served as additional evidence of the importance of ROS in the experimental model of DN. H2O2 is an extremely effective membrane-crosser and when its level is too high, it emits OH and TBARS are excreted in the urine, a clear sign of lipid peroxidation caused by unbound iron [48-49]. Due to changes in mitochondrial permeability, aberrant ATP synthesis, unbalanced intracellular calcium levels and other ROS-mediated disorders, the mitochondrial area was particularly vulnerable to cell death through necrosis and apoptosis [50]. Excessive ROS triggered the activation of numerous antioxidant mechanisms, including enzymatic systems and free radical scavengers. The anti-oxidant enzymes (e.g. reduced glutathione (GSH), catalase (CAT)) converted H2O2 into water [45, 48]. The unbalanced antioxidant enzymes utilized in hyperglycemia were SOD, GSH and CAT [45]. Thiol levels dropped in diabetic rats after nephrectomy, suggesting that the antioxidant enzyme is active. Much research supported the role of ROS in the diabetic nephropathy lesion mechanism. An in vivo investigation using mesangial cells exposed to high glucose concentrations demonstrated significant ROS production [51], using ginger and curcumin to treat diabetic nephropathy. Ginger and curcumin are two well-known functional foods from the Zingiberaceae family that have anti-inflammatory qualities [52-53]. Ginger is known for its anti-inflammatory properties attributed to its phenolic compounds, which include 6-gingerol (6-g) [54] and 6-shogaol (6-s) [55] as these are the main compounds involved in decreasing the main proinflammatory mediators, e.g., IL-6 and TNF [53]. Short pre-clinical and clinical studies on turmeric’s anti-inflammatory effects were conducted [56-59].
In our study, we found that STZ diabetic rats had higher MDA levels, indicating increased production of free radicals and related lipid peroxidation. However, administering the ginger, curcumin or a combination of both extracts to diabetic rats significantly decreased the MDA level. Accordingly, we hypothesized that administering ginger extract, curcumin extract, or a combination of them for diabetic rats would reduce the generation of free radicals and the peroxidation of lipids, thereby preventing oxidative damage to cellular structures. Our results also showed that those extracts could increase the intracellular activity of SOD, CAT and GSH enzymes. In contrast, the activity of the above-mentioned enzymes all dramatically decreased in the diabetic rats not receiving treatment. This might highlight a weak antioxidant defense against damage caused by free radicals. We hypothesize that treatment with extracts reduced OS by scavenging free radicals and/or enhancing endogenous antioxidant activities, thereby improving diabetic condition, reducing inflammatory state and advancing the treatment of kidney functions and tissue. One treatment method for challenging diseases involving inflammation is combination therapy with a synergistic approach [60]. In particular, the mechanical anti-inflammatory effects of ginger and curcumin share numerous molecular targets and signaling pathways, including Nrf2 activation [53, 61-63]. Despite research into the specific anti-inflammatory properties of ginger and curcumin, their usage is not widespread [53, 57-59]. In our study, the rats treated with combined ginger and curcumin extracts had superior outcome in terms of better glycemia management and less DN-related kidney damage.
Conclusions
Our results demonstrated the anti-inflammatory and the antioxidant effects of ginger and curcumin extracts, administred individually or in combination. Our data have also shown that ginger and curcumin extracts helped manage STZ-induced diabetic nephropathy and oxidative stress via significant suppression of the NF-κB gene expression. These extracts possess anti-inflammatory potential by suppressing inflammatory cytokines and modulators through the suppression of redox-based NF-κB activation.
Acknowledgments
The authors are grateful to the Chemistry Department, Faculty of Science, Suez Canal University, and Mansoura University in Egypt for providing some of the resources required for this study.
Funding
The authors declare that no funds, grants, or other support forms were received during the preparation of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
--------------------------------
(Image source: istockphoto.com)
References
| 1. |
Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives.
|
| 2. |
Eur J Prev Cardiol [Internet]. 2019;26(2_suppl):7-14. Available from: https://doi.org/10.1177/20474873198810212. Schena FP, Gesualdo L. Pathogenetic Mechanisms of Diabetic Nephropathy. J Am Soc Nephrol [Internet]. 2005;16(3_suppl_1). Available from: https://journals.lww.com/jasn/fulltext/2005/03001/pathogenetic_mechanisms_of_diabetic_nephropathy.8.aspx.
|
| 3. |
Tanios BY, Ziyadeh FN. Emerging Therapies for Diabetic Nephropathy Patients: Beyond Blockade of the Renin-Angiotensin System. Nephron Extra [Internet]. 2012;2(1):278-82. Available from: https://doi.org/10.1159/000343312.
|
| 4. |
Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis S, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes. Minerva Med [Internet]. 2018;109(3):218-28. Available from: http://europepmc.org/abstract/MED/29205998.
|
| 5. |
Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic Classification of Diabetic Nephropathy. J Am Soc Nephrol [Internet]. 2010;21(4):556-63. Available from: https://journals.lww.com/jasn/fulltext/2010/04000/pathologic_classification_of_diabetic_nephropathy.7.aspx.
|
| 6. |
Fouda A-M, Ashour RH, El-Banna F, Saad MA, Mostafa FA, Fouda MI. Differential Effects of Low-Dose Erythropoietin in Rat Model of Diabetic Nephropathy. Adv Med Med Res [Internet]. 2018;1(1):25-33. Available from: https://www.researchgate.net/profile/Abdel-Motaal-Fouda/publication/325207039_Differential_Effects_of_Low-Dose_Erythropoietin_in_Rat_Model_of_Diabetic_Nephropathy/links/5e0c45e8a6fdcc28374d4555/Differential-Effects-of-Low-Dose-Erythropoietin-in-Rat-Model-of-Diabetic-Nephropathy.pdf.
|
| 7. |
WHO monographs on selected medicinal plants. World Health Organization; 1999.
|
| 8. |
Zingiber officinale [Internet]. USDA. [cited 2023 Oct 26]. Available from: https://plants.usda.gov/home/plantProfile?symbol=ZIOF.
|
| 9. |
Prasad S, Tyagi AK. Ginger and Its Constituents: Role in Prevention and Treatment of Gastrointestinal Cancer. Shaffer EA, editor. Gastroenterol Res Pract [Internet]. 2015;2015:142979. Available from: https://doi.org/10.1155/2015/142979.
|
| 10. |
Mao Q-Q, Xu X-Y, Cao S-Y, Gan R-Y, Corke H, Beta T, et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods [Internet]. 2019;8(6):185. Available from: https://www.mdpi.com/2304-8158/8/6/185.
|
| 11. |
Zhang S, Kou X, Zhao H, Mak K-K, Balijepalli MK, Pichika MR. Zingiber officinale var. rubrum: Red Ginger’ s Medicinal Uses. Molecules [Internet]. 2022;27(3):775. Available from: https://www.mdpi.com/1420-3049/27/3/775.
|
| 12. |
Butt MS, Sultan MT. Ginger and its Health Claims: Molecular Aspects. Crit Rev Food Sci Nutr [Internet]. 2011;51(5):383-93. Available from: https://doi.org/10.1080/10408391003624848.
|
| 13. |
Aktan F, Henness S, Tran V, Duke C, Roufogalis B, Ammit A. Gingerol Metabolite and a Synthetic Analogue CapsarolTM Inhibit Macrophage NF-κB-Mediated iNOS Gene Expression and Enzyme Activity. Planta Med [Internet]. 2006;72(8):727-34. Available from: http://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2006-931588.
|
| 14. |
Qian Q, Yue W, Wang Y, Yang Z, Liu Z, Chen W. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res [Internet]. 2009;32(4):565-73. Available from: https://doi.org/10.1007/s12272-009-1413-9.
|
| 15. |
Lindstedt I. Ginger and diabetes: A mini-review. Arch Gen Intern Med [Internet]. 2018;2(2):29-33. Available from: https://www.alliedacademies.org/articles/ginger-and-diabetes-a-minireview-9959.html.
|
| 16. |
Ching W-Y, Bin-Yusoff Y, Wan-Amarina W-NB. Extraction of essential oil from Curcuma longa. J Food Chem Nutr [Internet]. 2014;2(1):1-10. Available from: https://esciencepress.net/journals/index.php/JFCN/article/view/310.
|
| 17. |
Mashhadi NS, Ghiasvand R, Askari G, Hariri M, Darvishi L, Mofid MR. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med [Internet]. 2013;4(Suppl 1):S36-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23717767.
|
| 18. |
Nishikawa S, Kato M, Ikehata A, Dochi K, Tani T, Takahashi T, et al. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol Nutr Food Res [Internet]. 2017;61(3):1600471. Available from: https://doi.org/10.1002/mnfr.201600471.
|
| 19. |
Mostafa M, Amer N, Serag M, Khedr AH, Abdel-Mogib M. Phytochemical constituents and antibacterial activity of the medicinal herb Deverra tortuosa (Desf.) DC. Res J Pharm Biol Chem Sci [Internet]. 2020;11(2):108-15. Available from: https://www.researchgate.net/publication/343999329_Phytochemical_Constituents_and_Antibacterial_Activity_of_the_Medicinal_Herb_Deverra_tortuosa_Desf_DC.
|
| 20. |
Khalid S, Shahzad A, Basharat N, Abubakar M, Anwar P. Phytochemical screening and analysis of selected medicinal plants in Gujrat. J Phytochem Biochem [Internet]. 2018;2(1):1-3. Available from: https://www.omicsonline.org/open-access/phytochemical-screening-and-analysis-of-selected-medicinal-plants-in-gujrat-100085.html.
|
| 21. |
Bansode TS, Salalkar BK. Phytochemical analysis of some selected Indian medicinal plants. Int J Pharm Bio Sci [Internet]. 2015;6(1):550-6. Available from: https://www.researchgate.net/publication/281690290_Phytochemical_analysis_of_some_selected_Indian_medicinal_plants.
|
| 22. |
Abdel Wahab F. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of ephedra alata decne [Internet]. 2015 [cited 2023 Oct 26]. Available from: https://repository.najah.edu/items/4bd621a8-3369-46cd-a8b7-7678862c0c1e/full.
|
| 23. |
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci [Internet]. 2011;1(1):98-106. Available from: https://www.researchgate.net/profile/Chukwuebuka-Egbuna/post/Why-does-petroleum-ether-solvent-yield-more-alkaloids-and-polyphenols-and-why-does-aqueous-extraction-yield-more-flavonoids/attachment/59d650a779197b80779a968f/AS%3A504112410497024%401497201172241/download/Phytochemical+screening+and+extraction+-+A+review.pdf.
|
| 24. |
Zohra SF, Meriem B, Samira S, Muneer MA. Phytochemical screening and identification of some compounds from mallow. J Nat Prod Plant Resour [Internet]. 2012;2(4):512-6. Available from: https://www.scholarsresearchlibrary.com/articles/phytochemical-screening-and-identification-of-some-compoundsfrom-mallow.pdf.
|
| 25. |
Fesseha YA. Phytochemical Screening of Some Selected Home Garden Plants in Amhara Region, North Gondar, Gondar [Internet]. Semantic Scholar; 2020 [cited 2023 Oct 26]. Available from: https://api.semanticscholar.org/CorpusID:212552297.
|
| 26. |
Saied S, Begum S. Phytochemical Studies of Berberis vulgaris. Chem Nat Compd [Internet]. 2004;40(2):137-40. Available from: https://doi.org/10.1023/B:CONC.0000033929.60336.bb.
|
| 27. |
Wood LG, Gibson PG, Garg ML. A review of the methodology for assessing in vivo antioxidant capacity. J Sci Food Agric [Internet]. 2006 86(13):2057-66. Available from: https://doi.org/10.1002/jsfa.2604.
|
| 28. |
Moon J-K, Shibamoto T. Antioxidant Assays for Plant and Food Components. J Agric Food Chem [Internet]. 2009;57(5):1655-66. Available from: https://doi.org/10.1021/jf803537k.
|
| 29. |
Albahlol F, Khalil M, Ghoniem G, Aboulnaga E. Evaluation of pan bread fortified with sunflower seeds powder. J Food DairySci [Internet]. 2022;13(10):139-47. Available from: https://jfds.journals.ekb.eg/article_267528.html.
|
| 30. |
Lee M-J, Prabhu S, Meng X, Li C, Yang CS. An Improved Method for the Determination of Green and Black Tea Polyphenols in Biomatrices by High-Performance Liquid Chromatography with Coulometric Array Detection. Anal Biochem [Internet]. 2000;279(2):164-9. Available from: https://doi.org/10.1006/abio.2000.4487.
|
| 31. |
Wahl PR, Hir MLE, Vogetseder A, Arcaro A, Strake A, Waeckerle-Men Y, et al. Mitotic activation of Akt signalling pathway in Han:SPRD rats with polycystic kidney disease. Nephrology [Internet]. 2007;12(4):357-63. Available from: https://doi.org/10.1111/j.1440-1797.2007.00811.x.
|
| 32. |
Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol [Internet]. 2015;70(1):5.47.1-5.47.20. Available from: https://doi.org/10.1002/0471141755.ph0547s70.
|
| 33. |
Shin J-W, Seol I-C, Son C-G. Interpretation of animal dose and human equivalent dose for drug development. J Korean Orient Med. 2010;31(3):1-7. Available from: http://www.jkom.org/upload/31-3%2001%20[01-07].pdf.
|
| 34. |
Trinder P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Ann Clin Biochem Int J Lab Med [Internet]. 1969;6(1):24-7. Available from: http://journals.sagepub.com/doi/10.1177/000456326900600108.
|
| 35. |
Young DS, Friedman RB. Effects of disease on clinical laboratory tests. 2001.
|
| 36. |
Tabacco A, Meiattini F, Moda E, Tarli P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin Chem [Internet]. 1979;25(2):336-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/759035.
|
| 37. |
Schosinsky KH, Vargas M, Luz Esquivel A, Chavarria MA. Simple spectrophotometric determination of urinary albumin by dye-binding with use of bromphenol blue. Clin Chem [Internet]. 1987;33(2):223-6. Available from: https://doi.org/10.1093/clinchem/33.2.223.
|
| 38. |
Bastaki S. Diabetes mellitus and its treatment. Int J Diabetes Metab [Internet]. 2019;13(3):111-34. Available from: https://doi.org/10.1159/000497580.
|
| 39. |
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J nephropharmacology [Internet]. 2016;5(1):49-56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28197499.
|
| 40. |
Fırat U, Kaya S, Çim A, Büyükbayram H, Gökalp O, Dal MS, et al. Increased Caspase-3 Immunoreactivity of Erythrocytes in STZ Diabetic Rats. Galassetti P, editor. Exp Diabetes Res [Internet]. 2012;2012:316384. Available from: https://doi.org/10.1155/2012/316384.
|
| 41. |
Padil VVT, Senan C, Wacſawek S, Ŀerník M. Electrospun fibers based on Arabic, karaya and kondagogu gums. Int J Biol Macromol [Internet]. 2016;91:299-309. Available from: https://doi.org/10.1016/j.ijbiomac.2016.05.064.
|
| 42. |
Badal SS, Danesh FR. New Insights Into Molecular Mechanisms of Diabetic Kidney Disease. Am J Kidney Dis [Internet]. 2014;63(2, Supplement 2):S63-83. Available from: https://doi.org/10.1053/j.ajkd.2013.10.047.
|
| 43. |
Vallon V, Thomson SC. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu Rev Physiol [Internet]. 2012;74(1):351-75. Available from: https://www.annualreviews.org/doi/10.1146/annurev-physiol-020911-153333.
|
| 44. |
Lopes GS, Lemos CCS, Mandarim-de-Lacerda CA, Bregman R. Effect of unilateral nephrectomy on renal function of diabetic rats. Histol Histopathol [Internet]. 2004; Available from: https://digitum.um.es/digitum/handle/10201/21618.
|
| 45. |
Forbes JM, Cooper ME. Mechanisms of Diabetic Complications. Physiol Rev [Internet]. 2013;93(1):137-88. Available from: https://doi.org/10.1152/physrev.00045.2011.
|
| 46. |
O’ Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol [Internet]. 1997;17(2):93-100. Available from: http://europepmc.org/abstract/MED/9148381.
|
| 47. |
Vallon V, Blantz RC, Thomson S. Glomerular Hyperfiltration and the Salt Paradox in Early Type 1 Diabetes Mellitus: A Tubulo-Centric View. J Am Soc Nephrol [Internet]. 2003;14(2):530-37. Available from: https://journals.lww.com/jasn/fulltext/2003/02000/glomerular_hyperfiltration_and_the_salt_paradox_in.30.aspx.
|
| 48. |
Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med [Internet]. 2000;109(8):665-78. Available from: https://doi.org/10.1016/S0002-9343(00)00612-4.
|
| 49. |
Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology [Internet]. 2011;283(2):65-87. Available from: https://doi.org/10.1016/j.tox.2011.03.001.
|
| 50. |
Pingle SC, Mishra S, Marcuzzi A, Bhat SG, Sekino Y, Rybak LP, et al. Osmotic Diuretics Induce Adenosine A1 Receptor Expression and Protect Renal Proximal Tubular Epithelial Cells against Cisplatin-mediated Apoptosis. J Biol Chem [Internet]. 2004;279(41):43157-67. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021925820770717.
|
| 51. |
Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, Cortes P. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest [Internet]. 1995;96(4):1802-14. Available from: http://www.jci.org/articles/view/118226.
|
| 52. |
Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine [Internet]. 2007;14(2):123-8. Available from: https://www.sciencedirect.com/science/article/pii/S0944711306000493.
|
| 53. |
Kota N, Krishna P, Polasa K. Alterations in antioxidant status of rats following intake of ginger through diet. Food Chem [Internet].2008;106(3):991-6. Available from: https://doi.org/10.1016/j.foodchem.2007.07.073.
|
| 54. |
Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem [Internet]. 2006;290(1):87-96. Available from: https://doi.org/10.1007/s11010-006-9170-2.
|
| 55. |
Shishodia S, Sethi G, Aggarwal BB. Curcumin: Getting Back to the Roots. Ann N Y Acad Sci [Internet]. 2005;1056(1):206-17. Available from: https://doi.org/10.1196/annals.1352.010.
|
| 56. |
Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma A V. Curcumin Regulates Expression and Activity of Matrix Metalloproteinases 9 and 2 during Prevention and Healing of Indomethacin-induced Gastric Ulcer. J Biol Chem [Internet]. 2005;280(10):9409-15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021925819629583.
|
| 57. |
Mani H, Sidhu GS, Kumari R, Gaddipati JP, Seth P, Maheshwari RK. Curcumin differentially regulates TGF-β1, its receptors and nitric oxide synthase during impaired wound healing. BioFactors. 2002;16(1-2):29-43. Available from: https://content.iospress.com/articles/biofactors/bio00478.
|
| 58. |
Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci [Internet]. 2004;87(1):44-53. Available from: http://www.jstor.org/stable/24107978.
|
| 59. |
Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, et al. Protective role of vanilloid receptor type 1 in HClinduced gastric mucosal lesions in rats. Scand J Gastroenterol [Internet]. 2004 1;39(4):303-12. Available from: https://doi.org/10.1080/00365520310008647.
|
| 60. |
el Gammal C, Kligman AM. Pretreatment of Photodamaged Forearm Skin with Topical Tretinoin Accelerates Healing of Full-Thickness Wounds BT - Wound Healing and Skin Physiology. In: Altmeyer P, Hoffmann K, el Gammal S, Hutchinson J, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 1995. p. 617-30.
|
| 61. |
Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, et al. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen [Internet]. 1999;7(5):362-74. Available from: https://doi.org/10.1046/j.1524-475X.1999.00362.x.
|
| 62. |
Jagetia GC, Rajanikant GK. Curcumin Treatment Enhances the Repair and Regeneration of Wounds in Mice Exposed to Hemibody γ-Irradiation. Plast Reconstr Surg [Internet]. 2005;115(2):515-28. Available from: https://journals.lww.com/plasreconsurg/fulltext/2005/02000/curcumin_treatment_enhances_the_repair_and.21.aspx.
|
| 63. |
Singer AJ, McClain SA, Romanov A, Rooney J, Zimmerman T. Curcumin Reduces Burn Progression in Rats. Acad Emerg Med[Internet]. 2007;14(12):1125-9. Available from: https://doi.org/10.1197/j.aem.2007.07.012.
|


















