Persistence of long-term insulin independence after islet transplantation and two subsequent pregnancies
Abstract
Pregnancy increases metabolic demand for insulin and may lead to the exhaustion of intraportally transplanted islets and post-gestational hyperglycemia. To prevent these complications, we implemented preemptive insulin supplementation during two subsequent pregnancies in an insulin-independent islet transplant recipient. This strategy resulted in optimal blood glucose control during the pregnancies, the preservation of the optimal islet graft function and the postpartum maintenance of long-term insulin independence.
Citation
Gondek S, Ogledzinski M, Lin W, Milejczyk K, Juengel B, Potter L, Bachul P J, Basto L, Perea L, Wang L, Tibudan M, Witkowska Z, Barth R, Fung J, Witkowski P. Persistence of long-term insulin independence after islet transplantation and two subsequent pregnancies. Eur J Transl Clin Med. 2023;6(1):9-13List of abbreviation
- BG – blood glucose;
- T1DM – Type 1 Diabetes Mellitus;
- IRB – Institutional Review Board;
- ITx – islet transplantation;
- IEQ – islet equivalent; CXCR1/2 – chemokine receptor 1 and 2
Introduction
Blood glucose (BG) in the narrow physiological range is essential for a human body’s homeostasis. BG is controlled by pancreatic islets spread within the pancreatic gland located in the abdominal cavity. Each islet is a micro-organ, having a defined anatomical structure with its vasculature and neural network [1]. Although it is a single anatomical and physiological endocrine unit, the function of each islet is integrated very well with the function of the remaining islets submerged in the exocrine tissue of the pancreas. In normal conditions, only some islets actively regulate BG via secreting an appropriate amount of insulin and glucagon. The remaining islets are dormant and constitute a functional reserve activated only when necessary to respond to higher metabolic demand [2]. Such system allows them to adapt to metabolic demand and protects islets from constant metabolic stress and subsequent failure from exhaustion.
Islet transplantation (ITx) is a minimally-invasive alternative to whole pancreas transplantation for patients with poorly-controlled type 1 diabetes mellitus (T1DM). Islets are retrieved from the deceased donor pancreas in a laboratory and infused intraportally suspended in a transplant media via a small catheter placed percutaneously and transhepatic by an interventional radiologist under local anesthesia. During a single transplant procedure, the patient receives a limited islet mass as current technology allows to retrieve, on average, only 30-60% of the 1 million islets present in the human pancreas. Much less than 75% of those infused islets engraft into the liver and resume metabolic activity [3-5]. Patients with T1DM, on average, require at least two islet transplantations to obtain ~60% of the islet mass physiologically present in a healthy pancreas. Such islet mass is usually able to provide a sufficient amount of insulin for the patient to achieve and maintain insulin independence. However, under such conditions, an islet graft does not provide a functional islet reserve the same way a native pancreas or a whole pancreas transplant does.
Therefore, any extraordinary metabolic demand may lead to islet graft stress. If it persists over an extended period, it may result in islet graft exhaustion, failure and recurrence of diabetes. For this reason, physiologic pregnancy with progressing metabolic demand cause exhaustion of intraportally transplanted islets and recurrent diabetes, as observed previously [6-8]. Importantly, suboptimal blood glucose control, which may occur during pregnancy in islet transplant recipients, increases the chance of complications and birth defects, as presented previously [8]. To prevent these complications, preemptive insulin supplementation during the pregnancy in an islet transplant recipient was implemented and presented as a case report [9]. It resulted in the preservation of islet graft function and optimal blood glucose control without complications to the mother and the baby [9]. In our study, we aimed to verify the efficacy of that approach. We confirmed its utility in the long-term preservation of islet graft function after two subsequent pregnancies in our patient.
The case
The patient was a 29-year-old female with T1DM, hypoglycemia unawareness and frequent severe hypoglycemic episodes, despite advanced diabetic medical treatment. Her body weight was 63.6 kg, and her BMI was 23.7. She developed long-term insulin independence with HbA1c < 5.8% after a single intraportal ITx (islet mass 635,554 islet equivalents (IEQ); and 9,999 IEQ/kg of body weight). Anti-thymocyte globulin (Thymoglobulin, Sanofi, US), along with CXCR1/2 inhibitor (Reparixin, Dompe, Italy), was used for induction and tacrolimus with mycophenolate were used for maintenance immunosuppression. Several months before conception, mycophenolate was replaced with azathioprine 125 mg to eliminate its teratogenic effect while decreasing the tacrolimus target trough level to 4-6 ng/ml. The patient became pregnant twice, 5 years and 7.5 years after her islet transplantation. At the conception of her first pregnancy, she was 34 years old, with a BMI of 25 and 66 kg of body weight. During the first pregnancy, the patient pre-emptively supplemented with insulin doses of 5-10 units per day in the first trimester, 15-45 units per day in the second, and 35-70 units per day in the third trimester. She supplemented with up to 35 units per day during the second pregnancy to target fasting blood glucose < 95 mg/ml.
Postpartum, the patient was successfully weaned off insulin maintaining optimal blood glucose control with HbA1c < 5.7%. Islet graft function before and after the pregnancy remained optimal, as reflected by fasting blood glucose, C-peptide, HbA1c, and BETA-2 index above 17 (Figure 1A)[10]. BETA-2 score is a composite index calculated based on fasting c-peptide, fasting blood glucose, HbA1c, and insulin used. BETA-2 was validated to provide information about islet graft function after allo- and auto-transplantation [10-11]. BETA2 score > 17 is correlated with optimal islet graft function, whereas declining values < 17 correspond to worsening islet graft function. BETA-2 of zero indicates complete graft failure and lack of c-peptide detection in the blood. A mixed meal tolerance test confirmed optimal and stable islet function with comparable C-peptide secretion and peak blood glucose levels < 130mg/ml during the entire 8.5-year follow-up (Figure 1B). Of note, BETA-2 during pregnancy dropped below the insulin independence threshold of 17 due to the pre-emptive insulin supplementation for increased metabolic demand rather than true islet dysfunction.
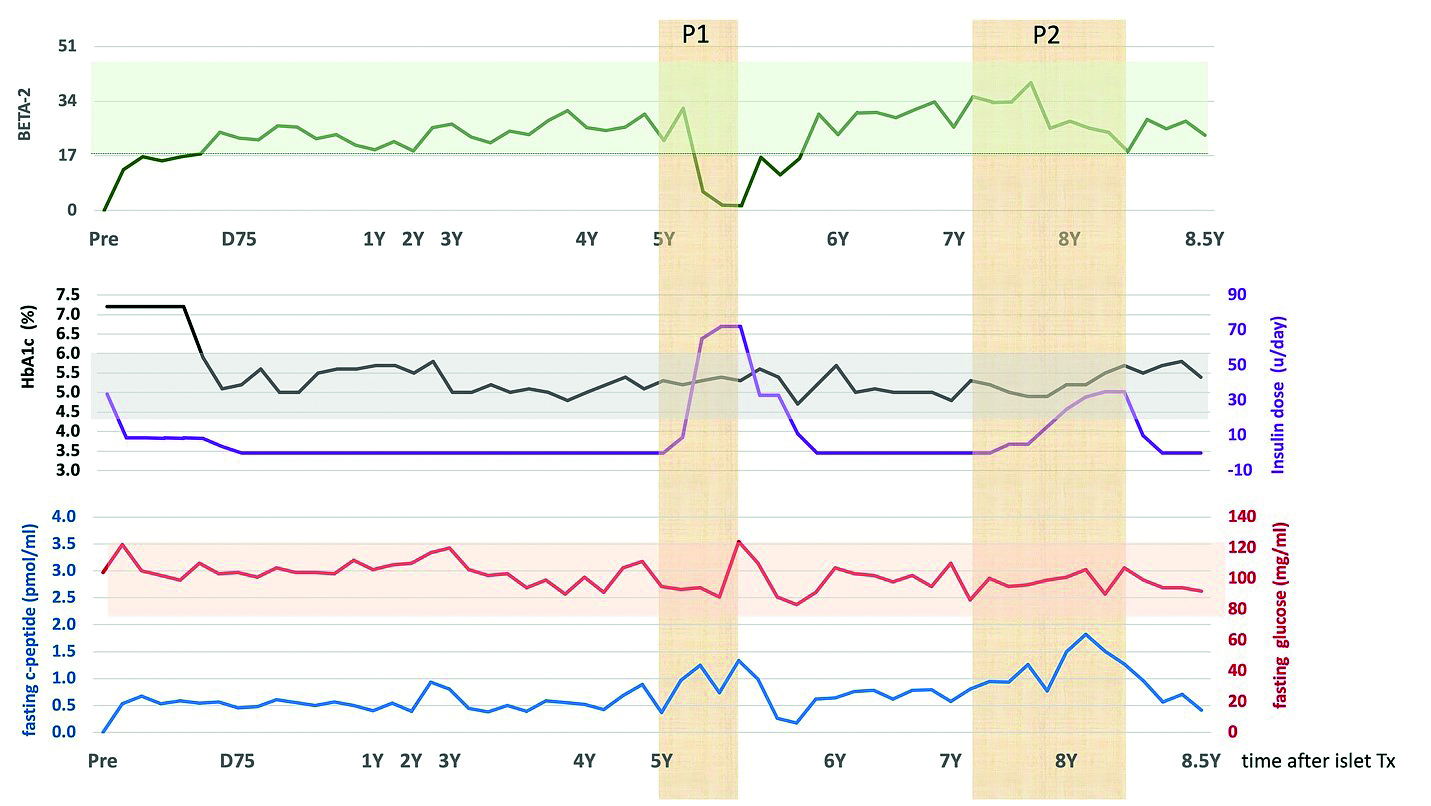
Figure 1A. Islet graft function over time during the follow-up based on BETA-2, HbA1c, fasting C-peptide and blood glucose and insulin requirements. P1 and P2 yellow areas represent period of first and second pregnancy, respectively. Islet graft function remained optimal with a BETA-2 score over the threshold of 17 for insulin independence (green line in the light green zone), besides a period of pre-emptive insulin supplementation during the first pregnancy (P1). HbA1c dropped after the islet transplant and remained below 6.0% during the entire follow-up (black line in gray zone). Purple line represents doses of insulin supplementation. Insulin was given only pre-emptive during both pregnancies. Fasting blood glucose remained in optimal range below 120 mg/ml during the entire follow up (red live in the light red zone) Fasting c-peptide also remained in the physiological range of 0.3- 1.7pmol/ml during the entire follow-up (blue line).
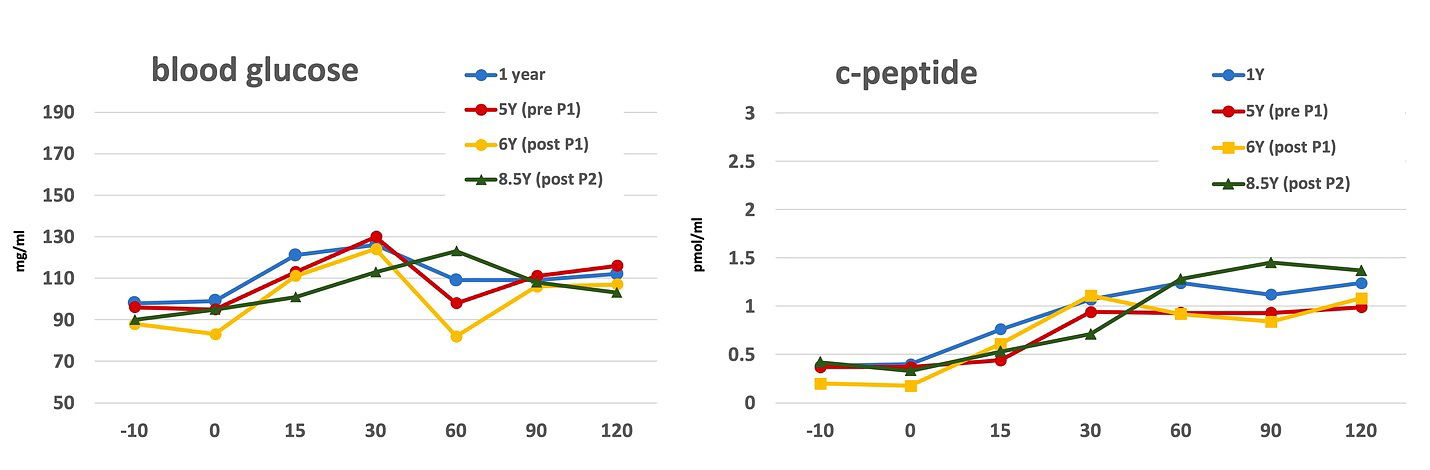
Figure 1B. Islet graft function measured based on the mixed meal tolerance test Islet graft function remained stable and optimal, reflected by fasting blood glucose below 100 mg/ml and peak glucose below 130 mg/ml. Area under the curve for serum c-peptide did not change significantly between 1 year and 8.5 years after the islet transplant: 0.45 min*pmol/ml vs 0.42 min*pmol/ml, respectively (p > 0.05)
Both newborns were premature and delivered at 34 weeks via emergent C-sections due to preeclampsia, even though the recommended prophylactic aspirin dose of 81 mg was administered daily following the 12th week of pregnancy [12]. Unfortunately, the first newborn died due to necrotizing enterocolitis, a known complication after premature delivery (16% mortality). The second child has been nursed and developing appropriately at eight months after their birth, while the patient remains insulin independent with an HbA1c of 5.6%.
Discussion
We found only a single case report presenting a successful pregnancy and the preservation of optimal islet graft function after pre-emptive insulin supplementation during the pregnancy in insulin-independent patients [9]. In contrast to our study, the lack of insulin supplementation in otherwise insulin-independent patients reported in one of the previous studies resulted in suboptimal blood glucose control (elevation of fasting blood glucose) during the pregnancy, birth defect imperforated anus and postpartum gradual islet graft loss and need for insulin therapy [8]. As no signs of immunologic rejection or patient noncompliance to immunosuppression were reported, islet graft dysfunction during and after pregnancy was most likely related to increased metabolic demand during the pregnancy leading to chronic metabolic stress and exhaustion of sub-physiologic islet graft mass [8]. Pre-emptive supplementation of insulin to prevent metabolic stress of islet graft during the pregnancy was reported effective previously, and now we replicated the results confirming its utility in our patient [9].
Despite the absence of known risk factors for preeclampsia (hypertension, elevated creatinine, obesity, and infection), our patient required maintenance immunosuppression, which might have put her at higher risk for preeclampsia. Pre-eclampsia is reported in as 20-30% in organ transplant recipients, compared to 5-6% in the general population [13]. It is possible that immunosuppression medications directly affected maternal microcirculation leading to a mismatch with the fetoplacental demand leading to preeclampsia despite a lack of clear tacrolimus nephrotoxicity as serum creatine remained in the range of 0.9-0.97 mg/ml prior and 8.5 years after the ITx [13].
Our results support the routine implementation of pre-emptive insulin support during pregnancy, however the dose should be carefully monitored and adjusted to prevent hypoglycemic episodes. Our report also underscores the high-risk nature of pregnancy in islet transplant recipients and the need for close clinical monitoring for risk factors and pre-eclampsia.
Conclusions
In light of limited experience and the growing number of patients successfully cured of T1DM with islet transplantation, our results provide important data guiding optimal patient management during pregnancy for the benefit of the mother and the baby and the preservation of the islet graft function.
Acknowledgments
The study was supported by the Dompe Farmaceutici S.p.A. The authors would like to acknowledge the generosity and support of Dr. Martin Jendrisak and the entire team of the Gift of Hope Organ & Tissue Donor Network in Chicago for providing the human pancreas for transplantation. We also acknowledge support from the NIDDK P30 DK020595 and the Kovler Family Fund.
Conflicts of Interest
PW served as a consultant to the Dompe Farmaceutici regarding a liver transplantation study and received a grant/ funding to conduct two studies about islet transplantation. The remaining authors declare no conflict of interest.
Funding
None.
References
| 1. |
Walker JT, Saunders DC, Brissova M, Powers AC. The Human Islet: Mini-Organ With Mega-Impact. Endocr Rev. 2021 Sep 28;42(5):605-57. Available from: https://academic.oup.com/edrv/article/42/5/605/6223906.
|
| 2. |
Weir GC, Bonner-Weir S. Sleeping Islets and the Relationship Between β-Cell Mass and Function. Diabetes 2011;60(8):2018-9. Available from: https://doi.org/10.2337/db10-1808.
|
| 3. |
Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, et al. Positron Emission Tomography in Clinical Islet Transplantation. Am J Transplant. 2009;9(12):2816-24. Available from: https://www.sciencedirect.com/science/article/pii/S1600613522019128.
|
| 4. |
Scharfmann R, Xiao X, Heimberg H, Mallet J, Ravassard P. Beta Cells within Single Human Islets Originate from Multiple Progenitors. Chan-Ling T, editor. PLoS One 2008;3(10):e3559. Available from: https://dx.plos.org/10.1371/journalpone.0003559.
|
| 5. |
Bachul PJ, Golab K, Basto L, Zangan S, Pyda JS, Perez-Gutierrez A, et al. Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation. Cell Transplant. 2021;30:096368972110017. Available from: http://journals.sagepub.com/doi/10.1177/09636897211001774.
|
| 6. |
Wahoff DC, Leone JP, Farney AC, Teuscher AU, Sutherland DE. Pregnancy after total pancreatectomy and autologous islet transplantation. Surgery. 1995;117(3):353-4. Available from: https://pubmed.ncbi.nlm.nih.gov/7878543.
|
| 7. |
Schive SW, Scholz H, Sahraoui A, Kloster-Jensen K, Hafsahl G, Korsgren O, et al. Graft function 1 year after pregnancy in an islet-transplanted patient. Transpl Int. 2015;28(10):1235-9. Available from: https://doi.org/10.1111/tri.12596.
|
| 8. |
Assalino M, Podetta M, Demuylder-Mischler S, Francini K, Pernin N, Randin J-P, et al. Successful pregnancy and delivery after simultaneous islet-kidney transplantation. Am J Transplant. 2018;18(8):2075-8. Available from: https://www.sciencedirect.com/science/article/pii/S160061352209668X.
|
| 9. |
Rickels MR, Markmann E, Naji A. Successful pregnancies after islet transplantation for type 1 diabetes. Am J Transplant. 2019;19(1):298-9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1600613522089249.
|
| 10. |
Bachul PJ, Gołębiewska JE, Basto L, Gołąb K, Anteby R, Wang L-J, et al. BETA-2 score is an early predictor of graft decline and loss of insulin independence after pancreatic islet allotransplantation. Am J Transplant. 2020;20(3):844-51. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1600613522222485.
|
| 11. |
Gołębiewska JE, Bachul PJ, Fillman N, Basto L, Kijek MR, Gołąb K, et al. Assessment of simple indices based on a single fasting blood sample as a tool to estimate beta-cell function after total pancreatectomy with islet autotransplantation – a prospective study. Transpl Int.. 2019;32(3):280-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30353611.
|
| 12. |
Roberts JM, King TL, Barton JR, Beck S, Bernstein IM, Buck TE, et al. Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up. Am J Obstet Gynecol..2023 Apr; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002937823002600.
|
| 13. |
Shah PB, Samra M, Josephson MA. Preeclampsia Risks in Kidney Donors and Recipients. Curr Hypertens Rep. 2018;20(7):59. Available from: https://doi.org/10.1007/s11906-018-0861-3.
|









