A quantitative analytical investigation on the oxycodone side effects listed in recent clinical trials
Abstract
Background: Oxycodone is a semi-synthetic narcotic analgesic medication with numerous side effects. Patients who use oxycodone may have one or more side effects depending on their clinical status and/or other factors. Our aim was to analyze clinical trials for any potential pattern related to the side effects of oxycodone.
Methods: We searched the ClinicalTrials.gov database for clinical studies conducted around the world regarding oxycodone. A number of general data analytic and text manipulation techniques were used in the Python programming language.
Results: We analyzed 842 clinical trials involving oxycodone. Our results suggest that the researchers focused on oxycodone’s typical side effects (e.g. vomiting), as opposed to the less evident and/or long-term side effects e.g. depression, confusion and constipation.
Conclusions: The adverse effects of oxycodone most commonly reported in the clinical trials are nausea, vomiting and depression. Despite the clinical use of oxycodone in all the age groups (including infants), very few of the publicly-available clinical trials included participants < 18 years of age.
Citation
Toussi A G, Toosi F G. A quantitative analytical investigation on the oxycodone side effects listed in recent clinical trials. Eur J Transl Clin Med. 2023;6(1):58-63Introduction
Strong analgesics are generally divided into two groups known as opiates and opioids. The first group, opiates, include opium, morphine, codeine, and heroin which are derived from naturally-occurring plants [1]. In contrast, opioids such as dextromethorphan, methadone, dextropropoxyphene, oxycodone and tramadol are fully synthesized in the laboratory. One of the main reasons for using oxycodone is to tranquilize moderate to severe pain such as diabetic foot, cancer-related pains, osteoarthritis, low back pain, gastrointestinal pain, etc [2].
Oxycodone has several pharmaceutical forms such as elixir, vials, and capsules. Oxycodone acts directly on the opioid receptors, namely µ (mu), κ (kappa) and δ (delta), in the central nervous system and provides pain relief by interrupting the pain signal transduction between the brain and the body [3-4]. Although this opioid often can relieve moderate to severe pain, it also has some hazardous outcomes, therefore it should be prescribed only when non-opioids are not effective.
While the mass media raise alarm about an “opioid epidemic” in the United States, the data confirms that number of prescriptions for opioids in the United States increased by 35.2% from 2000 to 2009 [5]. The same article also reported that the dosage of opioids (in milligrams) increased by at least 100% in US pharmacies during the period of 2000 to 2010. This huge increase in opioid use, including oxycodone, also means that the adverse effects of these medications are more frequent. Some of the common adverse effects of oxycodone include dizziness, confusion, constipation, diarrhea, vomiting, and nausea. Oxycodone toxicity is increasing nowadays because of deliberate and/or accidental use. Therefore, oxycodone should be prescribed carefully by a physician who knows how to manage its adverse effects. To this end, there is a need to design and run new experiments to study opioid side effects. The aim of this study was to assess recent clinical trials for any potential pattern related to the side effects of oxycodone.
Materials and Methods
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home) is a database of privately and publicly funded clinical studies conducted around the world. It collects data about several features of these studies, including inclusion and exclusion criteria, age range, background history, patient status, etc. Details of the studies (clinical trials) are entered manually. Some of the data in this database is labelled/categorized data and some (e.g. study title or outcome measures) is open-format data (e.g. text) without control label on terminology and structure.
We searched this database for all clinical trials that contained the term ‘oxycodone’ and retrieved a total of 842 records. The status of these clinical trials ranged from ‘recruiting’ or ‘active’ to ‘completed.’ The retrieved number of trials was not large enough to use more advanced tools such as artificial intelligence (i.e. machine learning), we relied on general analytic techniques (such as data cleansing, data categorization and textual manipulation) in the Python 3.7 programming language (Python Software Foundation, Delaware, USA) and its modules e.g. Numpy, Pandas and Matplotlib.
Results
The results of each analysis are presented separately in this section.
Inclusion Criteria/Age
As shown in Figure 1, the most common age group among the participants of the oxycodone-related clinical trials is 20 to 45 years. Hardly any trials included participants who were < 20 years of age. We noted 2 trials involving infants. Morse et al. indicated that oxycodone is prescribed for patients as young as premature neonates as well as adults of any age, therefore, it is important to investigate the side effects of such medication for a wider age-range i.e., neonates to adolescent [6].
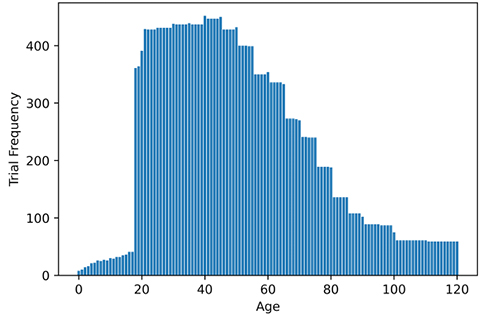
Figure 1. Age of oxycodone clinical trial participants
Adverse Effects of Oxycodone
Next, we analyzed oxycodone’s adverse effects described in these clinical trials. We focused on the 6 most common adverse effects of oxycodone as described by Gallego et al. (see Table 1), itching and depression [7-8]. Although itching and pruritus are synonymous, authors used either term in their studies, therefore we used both terms in order to find all studies with this particular adverse effect.
Table 1. The common side effects of oxycodone reported by Gallego et al.
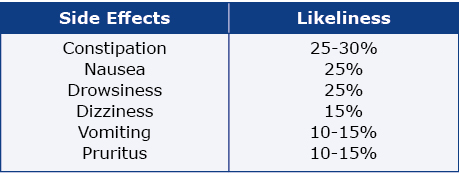
These data were manually entered into the ClinicalTrials. gov database, thus there might be inconsistent terminology describing the same condition, e.g. vomit and vomiting. Therefore, text analytical functions were applied to extract every variation.
We looked at the rate at which the study title and, more importantly, the outcome measures included either of these adverse effects described above. As shown in Figure 2, nausea, vomiting and depression were most often mentioned in the trials’ outcome measures. Whereas in the study titles adverse effects were much less frequently mentioned and constipation leads the list. Study titles are much more exposed online, outside the ClinicalTrials.gov database as well, therefore one can conclude that constipation has been one of the main concerns of the researchers [9].
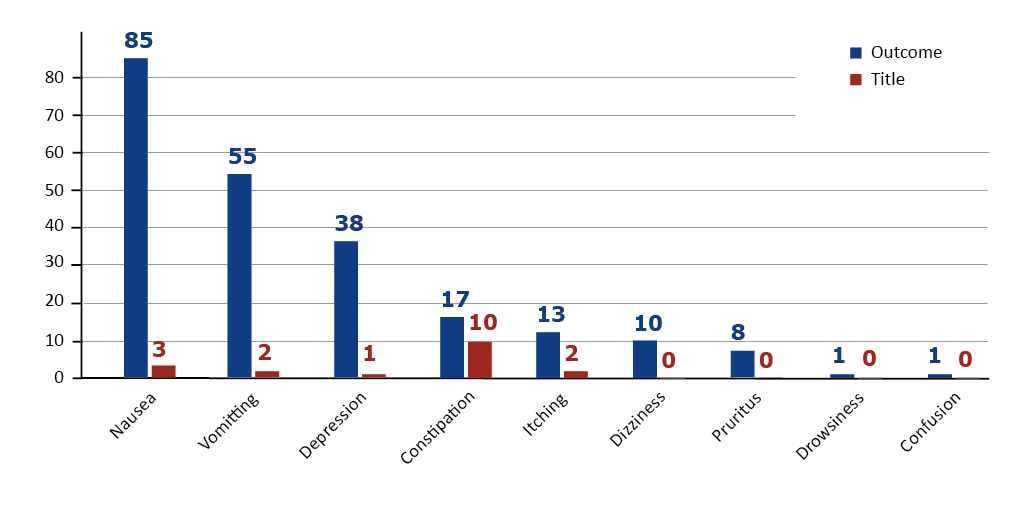
Figure 2. Number of trials that contained the particular adverse effect in the study title or outcomes
Next, we assessed the age distribution of patients reporting each of these adverse effects. Majority of the patients reporting the three adverse effects of oxycodone most commonly noted in the clinical trial outcome measures (nausea, vomiting, depression) all have a very similar age distribution: 20-60 years of age, with a decreasing number of studies involving an older age group (Fig. 3 A-C). Constipation is another adverse effect that is prominent in the 40−60 years of age group, with no participants younger than 20 (Fig. 3 D). This lack of attention to the younger age group in the clinical trials surprised us given how common is constipation secondary to oxycodone [10]. Pruritus and itching (see Fig. 3 E and G) have a similar distribution, albeit slightly younger. Confusion was mentioned in only one trial with participants > 20 years of age (Fig. 3 D). Dizziness has been the focus of around 25 studies for the age range of 20-40 and 1 or 2 studies for the older age group (Fig. 3 F). Finally, drowsiness was discussed in only 2 studies with the participants’ age range of 20-50.
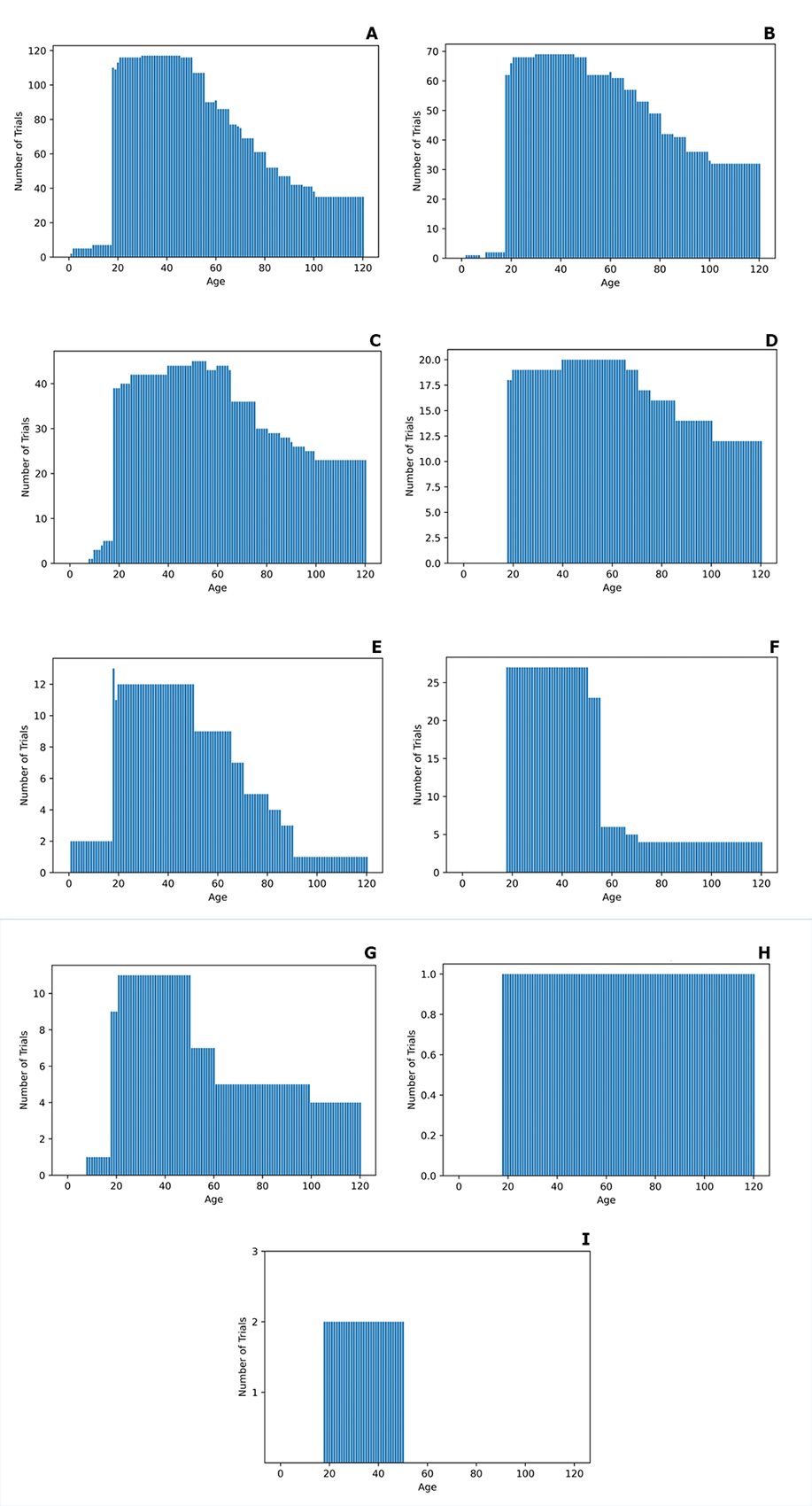
Figure 3. The details of the age distribution of clinical trail patients reporting the following adverse effects: A – nausea, B – vomiting, C – depression, D – constipation, E – itching, F – dizziness, G – pruritus, H – confusion, I – drowsiness
Discussion
As mentioned in the Results section, most of the analyzed trials focused on three adverse effects: nausea, vomiting and depression. However, since nausea and vomiting may be referring to similar medical state, we combined them into one category and reproduced the figure.
As shown in Figure 4, nausea and vomiting have the highest attention of researchers, while other side effects that might not be immediately evident received less attention. This might be due to the difficulty of keeping patients on track for a long time which requires further investigation in future works. On the other hand, since several studies focused on vomiting and nausea, one can argue that these side effects are more frequent than others, therefore, solutions to reduce these side effects may be worth investigating. Basic solutions include reducing physical movement immediately after eating and consuming enough fiber for the proper movements of the digestive system after oxycodone use. It is also worth comparing the nausea effects of oxycodone with other opioid-related medications such as methadone and oxycodone first as a pilot study in animals such as guinea pigs [11-12] and then in humans if the preliminary results are promising.
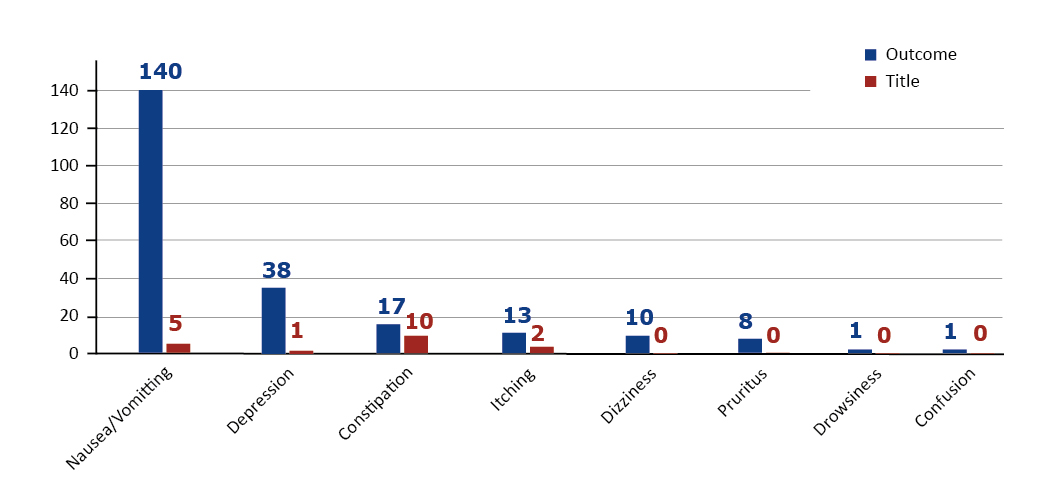
Figure 4. Number of Trials that includes each side-effect separated by Outcome and Title details and ordering by outcome details
Another highlight of this study is the lack of attention to the younger age range. Studies [13-15] show that the use of oxycodone is also common in the younger age group (< 20 years of age) while our findings show that this age range was neglected (or less focused) in recent clinical trials. The lack of such important details is a limitation in treating youngsters with opioids.
Limitations
The main limitation of our analysis is related to the dataset we obtained. Although researchers usually publish their results and methodologies, this is not mandatory therefore there might be relevant trials that were not included in this database. In addition, this database includes only those studies whose results have been made to some extent public.
Conclusions
The adverse effects of oxycodone most commonly reported in the clinical trials are nausea, vomiting and depression. Despite the clinical use of oxycodone in all the age groups (including infants), very few of the publicly-available clinical trials included participants < 18 years of age.
Data Availability Statement
The data that support the findings of this study are openly available at https://clinicaltrials.gov/ct2/home.
Funding
None.
Conflicts of interests
None.
References
| 1. |
Alcohol and Drug Policy Commission: Opiates or Opioids — What’s the difference?: State of Oregon [Internet]. oregon.gov. [cited 2023 May 4]. Available from: https://www.oregon.gov/adpc/pages/opiate-opioid.aspx.
|
| 2. |
Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS, Page AJ, et al. Oxycodone for cancer‐related pain. Cochrane Database Syst Rev [Internet]. 2022;(6). Available from: https://doi.org//10.1002/14651858.CD003870.pub7.
|
| 3. |
Leppert W. Role of oxycodone and oxycodone/naloxone in cancer pain management. Pharmacol Reports [Internet]. 2010;62(4):578–91. Available from: https://www.sciencedirect.com/science/article/pii/S1734114010703169.
|
| 4. |
Quirion B, Bergeron F, Blais V, Gendron L. The Delta-Opioid Receptor; a Target for the Treatment of Pain. Front Mol Neurosci [Internet]. 2020;13. Available from: https://www.frontiersin.org/article/10.3389/fnmol.2020.00052/full.
|
| 5. |
Kenan K, Mack K, Paulozzi L. Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med [Internet]. 2012;6(2):e41. Available from: https://www.scienceopen.com/document_file/5da741e6-b2ee-4fda-af17-210a65519091/PubMedCentral/5da741e6-b2ee-4fda-af17-210a65519091.pdf.
|
| 6. |
Morse JD, Hannam JA, Anderson BJ, Kokki H, Kokki M. Oxycodone target concentration dosing for acute pain in children. Pediatr Anesth [Internet]. 2021;31(12):1325-31. Available from: https://doi.org/10.1111/pan.14282.
|
| 7. |
Ordóñez Gallego A, González Barón M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol [Internet]. 2007;9(5):298-307. Available from: https://doi.org/10.1007/s12094-007-0057-9.
|
| 8. |
Burness CB, Keating GM. Oxycodone/Naloxone Prolonged-Release: A Review of Its Use in the Management of Chronic Pain While Counteracting Opioid-Induced Constipation. Drugs [Internet]. 2014;74(3):353-75. Available from: https://doi.org/10.1007/s40265-014-0177-9.
|
| 9. |
Andreassen TN, Klepstad P, Davies A, Bjordal K, Lundström S, Kaasa S, et al. Is Oxycodone Efficacy Reflected in Serum Concentrations? A Multicenter, Cross-Sectional Study in 456 Adult Cancer Patients. J Pain Symptom Manage [Internet]. 2012;43(4):694-705. Available from: https://www.sciencedirect.com/science/article/pii/S0885392411005148.
|
| 10. |
Czarnecki ML, Jandrisevits MD, Theiler SC, Huth MM, Weisman SJ. Controlled-release oxycodone for the management of pediatric postoperative pain. J Pain Symptom Manage [Internet]. 2004;27(4):379-86. Available from: https://www.sciencedirect.com/science/article/pii/S0885392404000028.
|
| 11. |
Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept [Internet]. 2009;155(1):11-7. Available from: https://www.sciencedirect.com/science/article/pii/S0167011509000731.
|
| 12. |
Kanemasa T, Koike K, Takase K, Arai T, Nakamura A, Morioka Y, et al. Pharmacological Profile of Naldemedine, a Peripherally Acting μ-Opioid Receptor Antagonist: Comparison with Naloxone and Naloxegol. J Pharmacol Exp Ther [Internet]. 2020;373(3):438 LP – 444. Available from: http://jpet.aspetjournals.org/content/373/3/438.abstract.
|
| 13. |
Wilens TE, Hammerness PG, Biederman J. The Changing Face of Teenage Drug Abuse —The Trend toward Prescription Drugs. Psychiatry [Internet]. 2005;66:253-9. Available from: http://www.lynneshealth.com/resources/Kids/Teenage drug abuse.pdf.
|
| 14. |
Jones JD, Vosburg SK, Manubay JM, Comer SD. Oxycodone Abuse in New York City: Characteristics of Intravenous and Intranasal Users. Am J Addict [Internet]. 2011;20(3):190-5. Available from: https://doi.org/10.1111/j.1521-0391.2011.00120.x.
|
| 15. |
Kemppainen V, Mentula M, Palkama V, Heikinheimo O. Pain during medical abortion in early pregnancy in teenage and adult women. Acta Obstet Gynecol Scand [Internet]. 2020;99(12):1603-10. Available from: https://doi.org/10.1111/aogs.13920.
|











