Abstract
Giant cell tumor of tendon sheath is a rare and benign soft-tissue tumor, which is even less common in the pediatric population. It may be connected with chromosomal translocations, often the 1p11-13. Symptoms include pain, swelling, palpable mass or limited range of motion. Magnetic resonance is the diagnostic method of choice. Histopathological image obtained from biopsy should be also evaluated. The gold standard of treatment is total surgical resection of the tumor, as incomplete excision may result in recurrence. Physicians should also consider the future function of the affected joint or limb. Our aim was to review the available literature about GCTTS in the pediatric population.
Citation
Kopcik K, Koberling A, Koper J, Bichalska-Lach M, Rudzki M, Waniczek D. Giant cell tumor of tendon sheath in the pediatric population – review. Eur J Transl Clin Med. 2023;6(1):64-69Introduction
Giant cell tumor of tendon sheath (GCTTS), also known as tenosynovial giant cell tumor, is the most frequent form of giant cell tumors and the second most common soft tissue tumor of the hand (after ganglion cyst) [1-3]. GCTTS is most often localized in the hands [4]. This tumor originates from the sheath, bursa or joints [2, 5-6]. Tumor is typically benign, but with aggressive or malignant potential [2, 4]. GCTTS may occur as localized or diffuse form [7-8] and is most common among adults 30 to 50 years of age. [2, 4, 7, 9-10]. The global incidence rate of GCTTS located in the digits, limb and the diffuse-type are respectively 29, 10, and 4 per million person-years [11]. GCTTS is even less common in the pediatric population, with an incidence rate of 2.42 and 1.09 per million person-years in case of localized and diffuse tumors, respectively [12]. The knowledge about pediatric GCTTS is scattered across case reports and case series [7, 13]. Our aim was to review the available literature about GCTTS in the pediatric population.
Material and methods
For the purpose of this narrative review, we searched the PubMed database for articles in English and Polish language regarding GCTTS. The keywords “giant cell tumor of tendon sheath” or “giant cell tumor of tendon sheath pediatric” were used. We focused on articles published in the years 2016 to 2023. Evaluation was based on articles’ titles and abstracts. Main criterium of including the papers to the review was if they concerned pediatric population. After evaluation of abstracts, full-text articles were analyzed, including the references cited.
Results
We found a total of 37 articles, including review articles, case series and case reports from the field of oncology, orthopaedics and radiology. After evaluation, 13 articles were included in our review. The rest of the articles were about adult patients. Cases reports are briefly summarized in the “Pediatric cases of GCTTS” section of this article.
Pathology
GCTTS is mostly located in the hand. Interphalangeal joints are often affected [13]. It may also be found in foot (with tendency to be spotted in forefoot), ankle, knee, hip, elbow or shoulder, although location in large joints or the spine is rare [2, 4]. This tumor can be divided into two types: intra-articular or extra-articular [7]. GCTTS may be described as localized tumor, which tends to be a benign form, and diffuse form, which appears to be more aggressive and more likely to localize in large joins [4]. Local and diffuse forms are morphologically ndistinguishable, however location near large joints or local agressiveness are more characteristic for the diffuse form [13]. The origin of GCTTS is uncertain and the hypothesised pathomechanism involves an inflammatory reaction followed by regenerative hyperplasia or local lipid metabolism abnormality [1, 7]. Recent studies revealed that there may be a connection with giant cell tumors and chromosomal translocations involving short arms of chromosome 1, which are present in most cases of GCTTS [1, 13]. These rearrangements often involve 1p11-13, t(1;2)(p11;q35–36) or translocations of 16q24 [13-14]. It is noteworthy that 1p13 is a location of CSF1 – Colony Stimulating Factor 1. COL6A3 – collagen 6A3 promoter element is located at 2q37 [8, 14]. Regular CFS1 function is stimulating secretion of macrophage colonies. These translocations result in prolonged lifetime of the CSF1 mRNA and its local over-expression, leading to an inflammatory infiltration of mononuclear and multinuclear giant-cell osteoclasts and macrophages [10, 14-15]. This situation, known as „the landscape effect” leads to the fact that only stromal cells present neoplastic mutation, and infiltration of osteoclasts is a reactive element [14]. Therefore GTCCS can be described as mixed disease: neoplasmatic and inflammatory [16].
Macroscopically, GCTTS is usually a 1-3.5 cm tumor with fibrous capsule [17]. In some cases, these tumors may present different colors – grey, yellow-orange, brown-ish – as a result of different composition and amount of hemosiderin, fibrin, collagen and histiocytes inside [16, 18].
Microscopic images of GCTTS show macrophage-like cells, which can be described as spindled or polyhedral, synovial cells and osteoblastic multinucleated giant cells. Synovial cells may appear as proliferating. Cells may contain hemosiderin deposits [4, 17] Some cases may contain xanthoma cells, hyalinization or inflammation factors, ferruginous phagocytes and collagen matrix [7, 16-17]. According to the literature, only 2-16% of cells present neoplastic transformation, and the majority appears as reactive, but not transformed cells [14]. Tumors may appear as lobulated, surrounded by collagen [7]. Malignant forms present tumor necrosis, infiltrative pattern, prominent nucleoli and high nuclear to cytoplasmic ratio. Shape is round to oval. Cells are mostly mononuclear, and may contain up to 5 mitoses per 10 high power fields It is vital to take under consideration the fact that both malignant and benign cells contain apoptotic figures and mitosis [13]. Malignant forms tend to infliltrate local structures, such as muscle, tendon or adipose tissue [13].
Clinical presentation
As the typical patient with GCTTS is 30 to 50 years of age, this is a rare disease in the pediatric population, but its symptoms seem to be similiar in both populations [13, 18]. The clinical presentation may vary, depending on tumor location. Tumor may appear as intra or extra-articular [7, 14]. When located in the hand, the GCTTS is usually an asymptomatic, painless, slow-growing and firm mass, frequently located on the palmar side [4, 16]. Sometimes it may cause swelling or periarticular effusion [4]. Pain and swelling are characteristic for the intra-articular location. When this tumor is located in hip joint, pain is the main symptom, with reduced swelling [14]. Among the significantly less frequent symptoms are: reduced range of motion in the joint, instability or locking of the joint, increased warmth of skin in the are of the affected joint. The extra-articular presentation is usually associated with pain and noticeable tumor mass, while the swelling or joint malfunction appear significatly less often [14]. When located in the digits, the clinical picture may include mobility disfunction or pain [16]. Symptoms are usually present for 10 months to 3 years [14]. GCTTS may appear as a main tumor with satelitary tumors located a few milimeters away from it [4, 16]. GCTTS of the knee may present similarly to meniscal syndrome [4, 14]. Tumor located in the spine is extremely rare, but may cause neck, shoulder or back pain, nerve root compression symptoms and limb disfunction, which are dependent on tumor size. [2].
According to Al-Qattan’s classification, there are two types of GCTTS:
- type 1 – single tumor surrounded by a round or lobulated capsule of various thickness;
- type 2 – multiple separate tumors without clearly marked capsule [1, 16].
Type 2 is described as having higher recurrence rates than type 1 [1, 16].
Differential diagnosis should contain ganglion cysts, lipomas, inclusion cysts, fibromas, rheumatoid nodules, synovial sarcoma, pigmented villonodular synovitis or inflammation of tendon sheath, all of which may be easily diagnosed microscopically [13].
Diagnostic methods
Magnetic resonance imaging (MRI), regarded as diagnostic method of choice, allows to recognize precisely, how the tumor is affecting its surroundings (e.g. nerves, joints or veins). It is also helpful when segregating tumors to types 1 and 2 in Al-Qattan classification. MRI can be also used as a tool to plan surgical treatment. Tumors appear as structures connected with tendon and its sheath, which is pathognomonic for GCTTS [1, 16, 19-21]. MRI scans of intra-articular tumors can also reveal joint effusion, synovial hypertrophy or pressure erosions [22]. Localized forms are well-defined, while diffuse forms usually appear as located in joints and associated with its effusion. Hemosiderin deposits present in some tumors are connected with weak to intermediate T1 and T2 signals, with enhancement after gadolinium injection [2, 19]. Nguyen et al. reported, that in MRI scans of 24 children, most of them presented joint effusion signs, and it was significantly more frequent in patients with diffuse type of tumor [22].
In X-ray images it is possible to notice the impression of a growing tumor on bone, particularly in the phalanges. Erosion of cortical bone, degenerative lesions, cystic erosions or soft-tissue masses may be also described [1, 2, 10, 16].
Doppler ultrasonography allows assessment of the tumor’s vascularization. In most cases GCTTS is not vascularized [16, 19-20]. Ultrasound-guided fine needle aspiration and cytology may also be performed as a part of the diagnostic process because in most cases its results correspond with histopathology findings [19, 21].
Treatment options
The gold standard of GCTTS treatment is total resection surgery with a careful preservation of tendons, arteries, veins and nerves in the local area of the lesion [1, 14]. In localized tumors a single surgical procedure is usually sufficient. If applicable, arthroscopy should be the method of choice, as it reduces morbidity and recurrence rate [14]. Diffuse GCTTS usually requires surgical excision. It may be problematic to obtain total resection of affected tissue, so recurrences may appear more frequently [14]. The most important issues regarding surgical management are joint damage, loss of function and recurrence [1, 7]. Risk factors of GCTTS recurrence are presented in Table 1 [1, 4, 16, 23].
Table 1. Risk factors of GTCCS recurrence
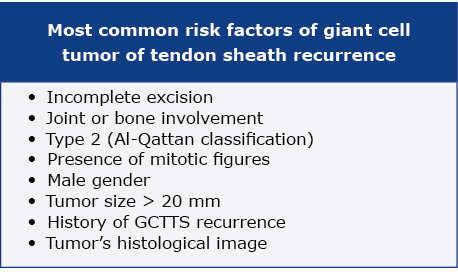
Cases of incomplete resection may need post-operative radiotherapy, to avoid recurrence lesions, according to Gouin et al. [19]. Radiotherapy may be applied as an external beam or intra-articular injection of radioactive isotopes [14].
The ENLIVEN study published in 2016 demonstrated that oral doses of pexidartinib (inhibitor of the colony stimulating factor 1 receptor, CSF1R) is effective in treating GCTTS. It is noteworthy that this study included only patients above 18 years of age and at this moment there are no published articles regarding its use in the pediatric population [12, 14, 23-24].
Pediatric cases of GCTTS
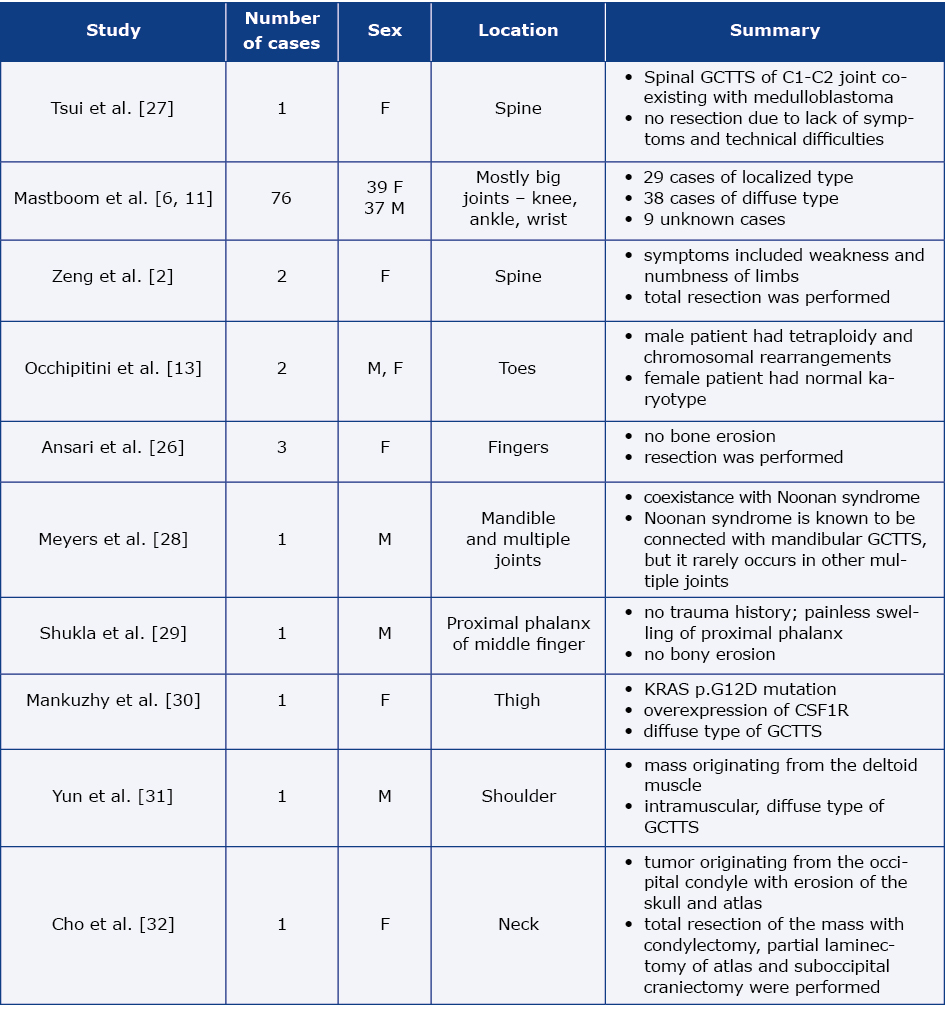
As noted earlier, GCTTS is indeed a rare disease in the pediatric population. In Table 2 we summarised selected case reports published in the literature [2, 13, 25-32].
Table 2. Selected pediatric cases of GCTTS summarized
In their study of 26 patients with GCTTS of foot and ankle, Cevik et al. included 6 children (see the summary in Table 3) [23].
Table 3. Clinical details of pediatric patients with GCTTS located in foot and ankle according to Cevik et al.
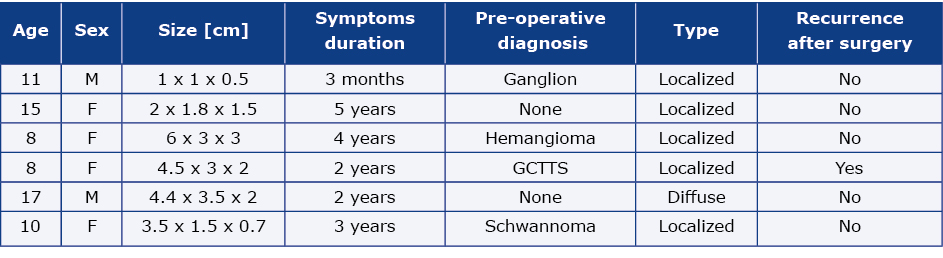
Their analysis revealed that most of the children presented symptoms for a few years before being diagnosed and surgically treated and pre-operative diagnoses were mostly wrong, only one patient was diagnosed correctly. Five out of 6 children with GCTTS had a localized type of tumor, and one had a diffuse type. Five of them did not present signs of recurrence after a follow up, and one female patient with a localized type of tumor manifested recurrence symptoms after 24 months, due to incomplete resection. There was no recurrence after re-operation. [23].
There are case reports describing GCTTS mimicking septic arthritis. This clinical situation is uncommon, but should be taken into consideration, as it may lead to unnecessary or inadequate surgical intervention [33-34]. According to the literature, such cases may be applicable in pediatric population, especially in patients with locomotory disfunctions and elevated inflammatory markers.
Conclusions
Giant cell tumor of tendon sheath is relatively rare disease among adults, and even more rare in pediatric population. Pexidartinib appears promising in the treatment of GCTTS, however the ENLIVEN study did not include the assessment of its effect on children. It is worth remembering, that the knowlegde about GCTTS is still limited and there is a need for diagnostic and therapeutic recommendations.
Funding
None.
Conflicts of interests
None.
References
| 1. |
Ozben H, Coskun T. Giant cell tumor of tendon sheath in the hand: analysis of risk factors for recurrence in 50 cases. BMC Musculoskelet Disord [Internet]. 2019;20(1):457. Available from: https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-019-2866-8.
|
| 2. |
Zeng P, Zhang A, Song L, Liu J, Yuan H, Zhang W. Giant cell tumour of the tendon sheath of the spine: clinical features and imaging findings. Insights Imaging [Internet]. 2021;12(1):98. Available from: https://insightsimaging.springeropen.com/articles/10.1186/s13244-021-01025-2.
|
| 3. |
Kant KS, Manav AK, Kumar R, Abhinav, Sinha VK, Sharma A. Giant cell tumour of tendon sheath and synovial membrane: A review of 26 cases. J Clin Orthop trauma. 2017;8(Suppl 2):S96-9.
|
| 4. |
Zendeoui A, Gharbi MA, Nafiss M, Ezzine MH, Bouzidi R, Tborbi A. Giant cell tumor of the tendon sheath of the toe: case report of an unusual localization. Int J Surg Case Rep [Internet]. 2023;102:107797. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2210261222010434.
|
| 5. |
Ehrenstein V, Andersen SL, Qazi I, Sankar N, Pedersen AB, Sikorski R, et al. Tenosynovial Giant Cell Tumor: Incidence, Prevalence, Patient Characteristics, and Recurrence. A Registry-based Cohort Study in Denmark. J Rheumatol [Internet]. 2017;44(10):1476-83. Available from: http://www.jrheum.org/lookup/doi/10.3899/jrheum.160816.
|
| 6. |
Mastboom MJL, Palmerini E, Verspoor FGM, Rueten-Budde AJ, Stacchiotti S, Staals EL, et al. Surgical outcomes of patients with diffuse-type tenosynovial giant-cell tumours: an international, retrospective, cohort study. Lancet Oncol [Internet]. 2019;20(6):877-86. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204519301007.
|
| 7. |
Lv Z, Liu J. Giant cell tumor of tendon sheath at the hand: A case report and literature review. Ann Med Surg [Internet]. 2020;58:143-6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2049080120303150.
|
| 8. |
Nakayama R, Jagannathan JP, Ramaiya N, Ferrone ML, Raut CP, Ready JE, et al. Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: initial experience of molecularly targeted therapy. BMC Cancer [Internet]. 2018;18(1):1296. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-018-5188-6.
|
| 9. |
Dhaniwala MN, Dhaniwala NS, Ahmed A. A Case Report of Giant Cell Tumor of the Flexor Tendon Sheath in Index Finger. J Orthop Case Reports [Internet]. 2020;9(6):78–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32548035.
|
| 10. |
AbuMoussa S, Roshan MP, Souza FF, Daley D, Rosenberg A, Pretell J, et al. Soft Tissue Masses of the Hand: A Review of Clinical Presentation and Imaging Features. Curr Oncol [Internet]. 2023;30(2):2032–48. Available from: https://www.mdpi.com/1718-7729/30/2/158.
|
| 11. |
Mastboom MJL, Verspoor FGM, Verschoor AJ, Uittenbogaard D, Nemeth B, Mastboom WJB, et al. Higher incidence rates than previously known in tenosynovial giant cell tumors. Acta Orthop [Internet]. 2017;88(6):688-94. Available from: https://actaorthop.org/actao/article/view/9750.
|
| 12. |
Dudzisz-Śledź M, Rogala P. Advances in systemic treatment of advanced soft tissue sarcomas. Oncol Clin Pract [Internet]. 2019;14(6):377-91. Available from: https://journals.viamedica.pl/oncology_in_clinical_practice/article/view/61809.
|
| 13. |
Occhipinti E, Heinrich SD, Craver R. Giant cell tumor of tendon sheath arising in the toe. Fetal Pediatr Pathol. 2004;23(2-3):171-9.
|
| 14. |
Kager M, Kager R, Fałek P, Fałek A, Szczypiór G, Niemunis-Sawicka J, et al. Tenosynovial giant cell tumor. Folia Med Cracov [Internet]. 2022;62(2):93-107. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36256897.
|
| 15. |
Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular Biology of Osteosarcoma. Cancers (Basel) [Internet]. 2020;12(8):2130. Available from: https://www.mdpi.com/2072-6694/12/8/2130.
|
| 16. |
Żyluk A, Owczarska A. Outcomes of surgery for schwannomas of the upper extremity. Pol Przegl Chir [Internet]. 2021;94(2):49-53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35485319.
|
| 17. |
Shweta D, George DL, K DKAV, Sukesh D, Rao DAC, Philip DNR. Giant cell tumor of tendon sheath: An institutional experience. Int J Clin Diagnostic Pathol [Internet]. 2020;3(1):141-3. Available from: http://www.patholjournal.com/archives/2020/vol3issue1/C/3-1-18.
|
| 18. |
Asaad SK, Bapir R, Salh AM, Abdullah AM, Tahir SH, Mikael TM, et al. Giant cell tumor of the tendon sheath in a 5-year-old child; A case report. Ann Med Surg [Internet]. 2021;69:102599. Available from: https://journals.lww.com/10.1016/j.amsu.2021.102599.
|
| 19. |
Gouin F, Noailles T. Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis). Orthop Traumatol Surg Res [Internet]. 2017;103(1):S91-7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1877056816301876.
|
| 20. |
Dębek A, Czyrny Z, Nowicki P. Zmiany patologiczne ręki w badaniu ultrasonograficznym. J Ultrason [Internet]. 2014;14(56):74-88. Available from: http://www.jultrason.pl/index.php/wydawnictwa/volume-14-no-56/sonography-of-pathological-changes-in-the-hand?aid=230.
|
| 21. |
Kitagawa Y, Takai S. Optimal Treatment for Tenosynovial Giant Cell Tumor of the Hand. J Nippon Med Sch [Internet]. 2020;87(4):184-90. Available from: https://www.jstage.jst.go.jp/article/jnms/87/4/87_JNMS.2020_87-408/_article.
|
| 22. |
Nguyen JC, Biko DM, Nguyen MK, Othman S, Weber KL, Ganley TJ, et al. Magnetic resonance imaging features of intra-articular tenosynovial giant cell tumor in children. Pediatr Radiol [Internet]. 2021;51(3):441-9. Available from: http://link.springer.com/10.1007/s00247-020-04861-4.
|
| 23. |
Çevik HB, Kayahan S, Eceviz E, Gümüştaş SA. Tenosynovial giant cell tumor in the foot and ankle. Foot ankle Surg Off J Eur Soc Foot Ankle Surg. 2020;26(6):712-6. Available from: https://doi.org/10.1016/j.fas.2019.08.014.
|
| 24. |
Tap W. ENLIVEN study: Pexidartinib for tenosynovial giant cell tumor (TGCT). Futur Oncol [Internet]. 2020;16(25):1875-8. Available from: https://www.futuremedicine.com/doi/10.2217/fon-2020-0307.
|
| 25. |
Mastboom MJL, Verspoor FGM, Uittenbogaard D, Schaap GR, Jutte PC, Schreuder HWB, et al. Tenosynovial Giant Cell Tumors in Children: A Similar Entity Compared With Adults. Clin Orthop Relat Res [Internet]. 2018;476(9):1803-12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29494352.
|
| 26. |
Ansari SM, Tambe S, Nayak C. Giant cell tumor of tendon sheath in children. Indian J Dermatol Venereol Leprol [Internet]. 2019;85(5):496. Available from: https://ijdvl.com/giant-cell-tumor-of-tendon-sheath-in-children/.
|
| 27. |
Tsui WWC, Fung KFK, Chan PKJ, Yuen MKE, Kan YLE. Cervical spine tenosynovial giant cell tumor involving the atlantoaxial joint in a pediatric patient with medulloblastoma. Skeletal Radiol [Internet]. 2022;51(6):1317-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34773486.
|
| 28. |
Meyers AB, Awomolo AO, Szabo S. Multifocal tenosynovial giant cell tumors in a child with Noonan syndrome. Pediatr Radiol [Internet]. 2017;47(3):361-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27878339.
|
| 29. |
Shukla M, Arora R. Giant Cell Tumor of the Tendon Sheath in a Male Pediatric Patient. J Pediatr Health Care [Internet]. 2021;35(4):430-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33386197.
|
| 30. |
Mankuzhy NP, Anderson B, Heider A, Michniacki TF, Kumar-Sinha C, Mody R. KRAS mutant tenosynovial giant cell tumor in a pediatric patient: a case report. Transl Pediatr [Internet]. 2019;8(5):449-54. Available from: http://tp.amegroups.com/article/view/33394/27271.
|
| 31. |
Yun SJ, Hwang SY, Jin W, Lim SJ, Park SY. Intramuscular diffuse-type tenosynovial giant cell tumor of the deltoid muscle in a child. Skeletal Radiol [Internet]. 2014;43(8):1179-83. Available from: http://link.springer.com/10.1007/s00256-014-1854-1.
|
| 32. |
Cho JM, Chang JH, Kim SH, Lee KS. Pediatric giant cell tumor of the tendon sheath of the craniocervical junction involving the occipital condyle. Child’s Nerv Syst [Internet]. 2016;32(1):175-9. Available from: http://link.springer.com/10.1007/s00381-015-2820-5.
|
| 33. |
Honig E, Harris A, Sabharwal S, Levin A, Honcharuk E. Tenosynovial Giant Cell Tumor Mimicking Acute Septic Arthritis of the Hip: A Case Report. JAAOS Glob Res Rev [Internet]. 2022;6(6). Available from: https://journals.lww.com/10.5435/JAAOSGlobal-D-22-00011.
|
| 34. |
Söylemez MS, Shattat KMI, Çelik A, Söylemez UPO, Tosun I, Yıldırım AT. Intra-Articular Tenosynovial Giant Cell Tumor Mimicking Septic Arthritis: A Report of Two Cases. J Orthop case reports [Internet]. 2022;12(5):1-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36660144.
|









