Clinical and demographic features of acutely hospitalised schizophrenia patients according to Toxoplasma gondii serostatus
Abstract
Background: Few data exist concerning the clinical correlates of Toxoplasma gondii infection in persons with schizophrenia. The aim of this study was to investigate the correlation between toxoplasmosis and schizophrenia regarding the quality of life, symptoms and course of hospitalisation in patients with schizophrenia.
Methods: Acutely hospitalised patients (n = 67) were examined twice during their hospital stay. Schizophrenia psychopathology, quality of life, extrapyramidal symptoms and T. gondii antibody titres were assessed upon admission and at discharge.
Results: Toxo-IgG (+) patients (59.7%) were older, less educated, more obese and less eager to undertake psychotherapy. Female gender and higher fertility were dominant in this group with abnormal involuntary movements more commonly observed. Lower antipsychotic drug doses and monotherapy were used more frequently for Toxo-IgG (+) patients. Lower education (OR 2.41, 95% CI 1.21-4.79) was the most important factor associated with higher likelihood of IgG seropositivity. High levels of Toxo-IgM antibodies correlated with lower quality of life (r = -0.37; p = 0.02) and more severe positive (r = 0.40; p = 0.01) and focal (r = 0.32; p = 0.04) schizophrenia symptoms.
Conclusions: Toxoplasmosis is more common in older, obese women with lower education. Recent infection is linked to more severe schizophrenia symptoms. Patients with toxoplasmosis history were given less medication.
Citation
Grabowski J, Waszak P M, Przybylak M, Bidzan L. Clinical and demographic features of acutely hospitalised schizophrenia patients according to Toxoplasma gondii serostatus. Eur J Transl Clin Med. 2023;6(1):14-24Introduction
A possible link between schizophrenia and toxoplasmosis has been the subject of study for the last 70 years [1-3]. It was suggested that infection by the protozoa Toxoplasma gondii may have an etiological role in the development of schizophrenia [4]. Epidemiological observations showed that the rates of T. gondii antibodies in people with schizophrenia are 2.7 higher than in healthy controls [5], with an odds ratio exceeding that for genetic or any other environmental factors identified so far.
In this study we aimed to provide a different approach and methodology than studies performed hitherto about the correlation between toxoplasmosis and schizophrenia. Our first aim was to measure the quality of life and extrapyramidal symptoms (EPS) in T. gondii seropositive patients with schizophrenia. The second aim was to measure the symptomatology of schizophrenia in patients over time with relation to toxoplasma IgG and IgM antibody titres.
Material and methods
This is a case series study. Only schizophrenia patients (cases) made up the sample in this study and the exposure to T. gondii was evaluated retrospectively.
Subjects in the study were inpatients of Regional Psychiatric Hospital in Gdańsk (Poland) hospitalised due to exacerbation of schizophrenia. With 111 patients enrolled, final analysis was possible for a study sample that consisted of 67 patients. The study protocol and the informed consent form were approved by the Regional Ethics Committee. All of participants in this study voluntarily gave their informed consent. Main inclusion criteria were age 18-65 and a hospitalisation due to exacerbation of schizophrenia diagnosed according to the DSM-V (Diagnostic and Statistical Manual of Mental Disorders). The following were the exclusion criteria: lack of consent or refusal to participate in the study at any time during the observation, involuntary hospitalisation, co-existence of another axis I disorder according to the DSM-V, co-existing serious somatic illness (uncontrolled cardio-vascular disorders, uncontrolled respiratory disease, kidney or liver failure, recent stroke or heart attack etc.), features or a history of organic lesion of the central nervous system (CNS), except those clearly related to T. gondii infection. Subjects were enrolled upon hospital admission with procedures performed twice during the hospitalisation: within three days of admission and up to 7 days prior to discharge from hospital.
T. gondii antibodies’ level was measured by enzyme-linked immune-sorbent assay (ELISA) test (positivity index > 1.0 for IgM and > 0.130 for IgG). We used the electrochemiluminescence immunoassay (ECLIA) on the Cobas e analyzer (Roche, Switzerland). Samples for IgM antibodies were taken upon admission and at discharge (to exclude a possible window period), while samples for IgG evaluation only on admission to the hospital. Patients were divided into two main groups based on IgG seropositivity with additional calculations made for IgM (+) and IgM (-) subjects.
PANSS (Positive and Negative Syndrome Scale) [6] results compared between the groups and subgroups were analysed as a whole, with classical distinction to positive (P1-P7), negative (N1-N7) and general (G1-G16) symptoms, PANSS focal symptoms [7], as well as according to the PANSS five-factor model (PANSS-FCTcr) [8]. Assessment of psychopathological improvement was made according to Leucht’s 25% and 50% PANSS reduction criteria [9]. The schizophrenia remission rate was compared with the use of three different remission concepts [7-8, 10].
Extrapyramidal symptoms were assessed using the modified Simpson-Angus Scale (mSAS) [11] and the Abnormal Involuntary Movement Scale (AIMS) [12]. Additionally, the presence of extrapyramidal symptoms (EPS) during the hospitalisation was acknowledged by reaching one of mSAS thresholds (≥ 0.3 and ≥ 0.65) [13] or by the necessity to administer any drug to alleviate the EPS.
Quality of life was assessed using the Personal and Social Performance Scale (PSP) [14]. Doses of medication at discharge were recalculated into chlorpromazine equivalents (CPZE) [15-16] to enable comparison between different drugs. All researchers were certified to use the study scales. The researchers did not participate in the direct treatment process of the participants, therefore they were not aware of the subjects՚ serostatus.
Data from the structured interview including the socio-demographic status, course of illness to date, history of pharmacotherapy side-effects and compliance was gathered as a source for comparison of study groups. Smoking status was determined to exclude its potential impact on negative and extrapyramidal symptoms according to the self-medication hypothesis, as well as on medication doses by inducing the CYP4501A2 metabolism pathway. Patients՚ characteristics are shown in Table 1.
Table 1. Baseline patient characteristics
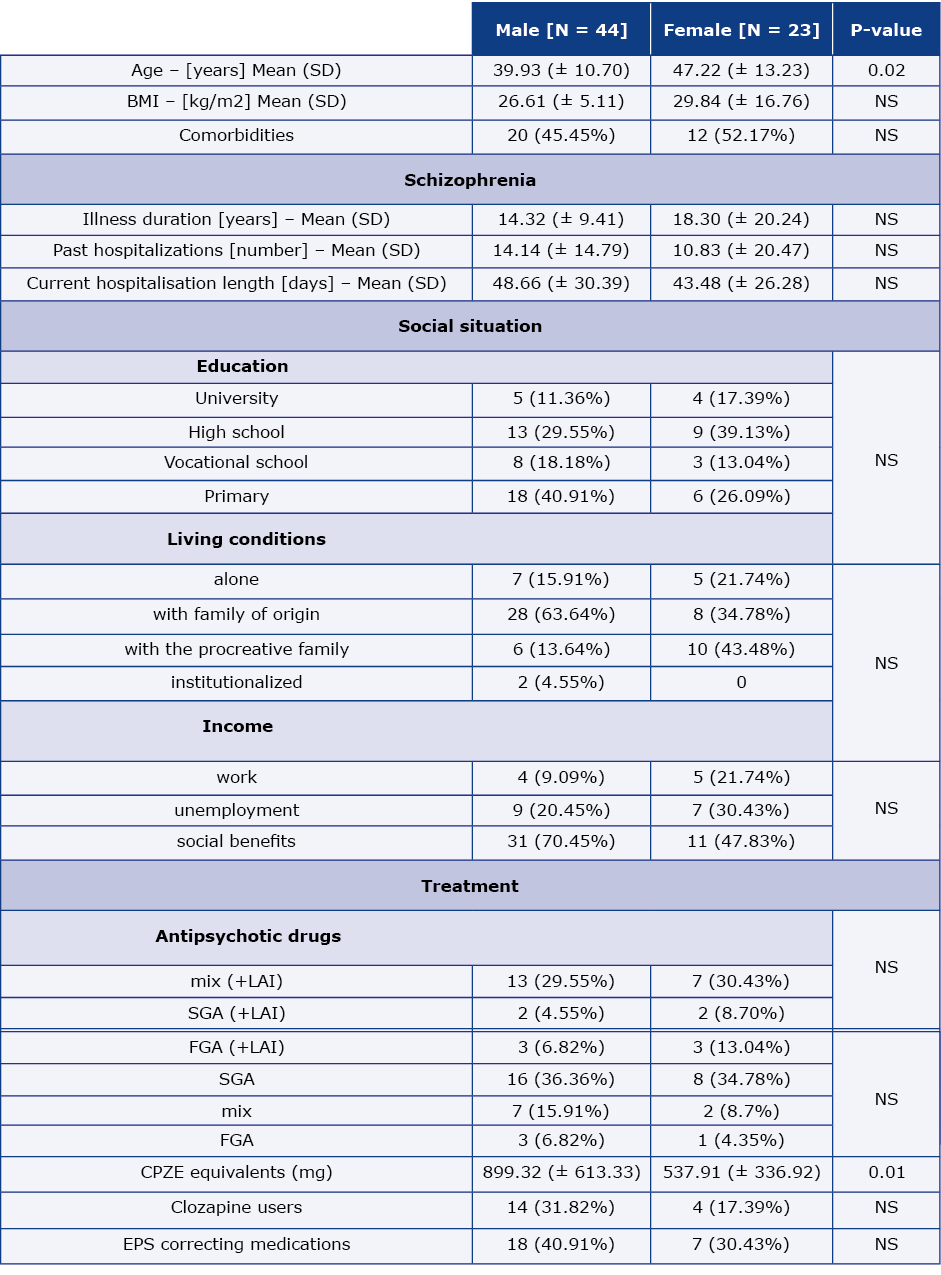
BMI – body mass index, CPZE – chlorpromazine equivalent doses, EPS – extrapyramidal symptoms, FGS – first generation antipsychotics, LAI – long-acting injectable antipsychotic drugs, NS – not significant, SD – standard deviation, SGA – second generation antipsychotics
Relative proportions were calculated with a confidence interval of 95%. Possible associations were identified using the Chi-Square and Fisher’s exact statistical tests, as well as the t-Student (or Mann-Whitney U test). Verification of whether the sample came from a normally distributed population was made using the Shapiro-Wilk test while Levene’s (Brown-Forsythe) test was used to assess the equality of variances for a variable calculated for two or more groups. Analysis of the differences among group means was made with the help of ANOVA (Analysis of variance) or Kruskal-Wallis test. The Spearman correlation was used to analyse the correlation between continuous variables. Stepwise multivariate logistic regression analysis was performed with Somers՚ D and Kolmogorov–Smirnov statistics. Odds ratios were also calculated. Significance level was p = 0.05. Statystical analysis was performed using the Statistica software (StatSoft Co, Tulsa, OK; USA), with the exception of power analysis for which we used ClinCalc (ClinCalc LLC; available at https://clincalc.com/).
Results
44 men and 23 women were assessed. Women were slightly older than men (47.22 vs. 39.93; p = 0.02) and differed from them in lower doses of antipsychotic drugs (537.91 vs. 899.32 CPZE equivalents; p = 0.01).
Characteristics of subjects with a history of latent toxoplasmosis infection (59.7% with positive Toxo-IgG antibodies) differ from the Toxo-IgG (-) group in some aspects (Table 2). No significant differences were found between Toxo-IgG (+) and Toxo-IgG (-) subjects in terms of current course of the disease, level of cooperation in the treatment or used drugs of abuse. The course of current hospitalisation did not differ regarding the drug groups (classical/atypical/long acting injectables) used or the percentage of patients who responded to treatment or achieved remission of schizophrenia symptoms.
Table 2. Sample characteristics based on Toxo-Ig status

AIMS TS – Abnormal Involuntary Movement Scale Total Severity, CPZE equivalents – Chlorpromazine equivalent doses, EPS – extrapyramidal symptoms, mSAS – modified Simpson–Angus Scale, PANSS – Positive and Negative Syndrome Scale, PSP – Personal and Social Performance Scale, SD – standard deviation
However, Toxo-IgG (+) patients received mean lower doses of antipsychotics (CPZE mg 671.2) and were more often treated with monotherapy than the Toxo-IgG (-) patients (37.5% vs. 14.8%).
We observed a significantly higher intensity of involuntary movements in the Toxo-IgG (+) group regarding both AIMS-Total Severity (AIMS-TS) and AIMS-Global Severity (AIMS-GS) subscales. Tardive dyskinesia threshold of AIMS scale [17] was reached more frequently by patients with Toxo-IgG (+) than those with Toxo-IgG (-) but the results were not statistically significant (12.5% vs. 0.0%; p = 0.0562). Significant reduction in the extrapyramidal symptoms measured with the mSAS scale was more frequent in the anti-toxoplasmosis IgG (+) group (p < 0.05). The results of other scales and subscales were not significantly different between the groups studied.
We noted a correlation between acute toxoplasmosis infection (based on the level of IgM class antibodies) and the course of schizophrenic psychosis. The Toxo-IgM (+) patients had higher values of PANSS positive factor and focal symptoms subscales upon admission compared to Toxo-IgM (-) patients with a significant correlation of symptoms and antibody titres (Table 3). Additionally, at discharge the PANSS excitement factor increased together with an increase in the Toxo-IgM titre for both Toxo-IgM (+) (R = 0.41; p < 0.01) and Toxo-IgM (-) patients (R = 0.52; p < 0.01). No statistically significant differences were observed in the scope of other subscales of the PANSS scale including factors of PANSS-FCTcr on admission, at discharge or comparing the admission period with the discharge period.
Table 3. Correlations of clinical scales with antibodies level
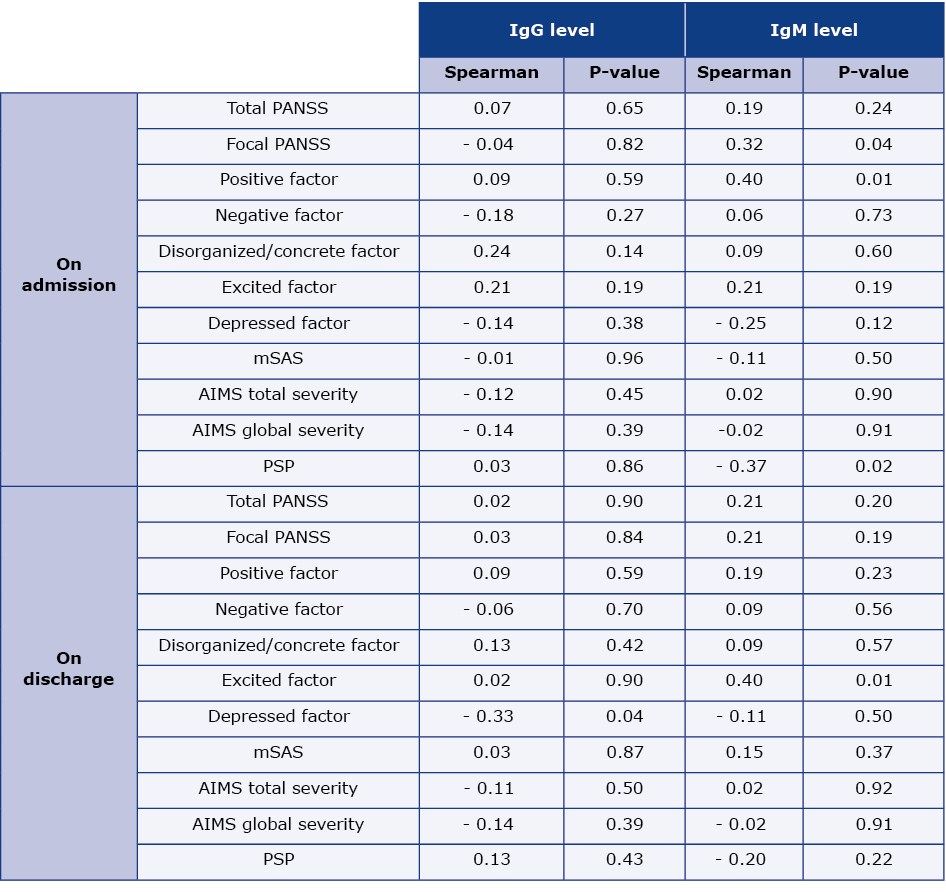
AIMS – Abnormal Involuntary Movement Scale, mSAS – modified Simpson-Angus Scale, PANSS – Positive and Negative Syndrome Scale, PSP – Personal and Social Performance Scale
Toxo-IgM (+) patients had lower quality of life as measured by the PSP scale when admitted compared to Toxo-IgM (-) patients, and these results correlated with the antibody concentration (Table 2 and 3). There were no differences in this scale at discharge and there were no differences in the distribution of the PSP subscales.
Toxo-IgM (+) patients had a longer duration of illness, involuntary hospitalisation, lower Toxo-IgM (-) patients (Table 2). There were no patients taking drugs for the T. gondii infection in the analysed period.
The main limitation of our study is the small number of patients with positive (6%) or doubtful (3%) Toxo-IgM index. The post-hoc analysis comparing Toxo-IgM (+) groups showed only 43.7% statistical power of the IgM calculations. To reach statistical significance threshold (Alpha = 0.05 Bet a = 0.2 and Power = 0.8), future studies should involve a sample size of at least 188 (94 females + 94 males).
For Toxo-Ig, an independent variable, the stepwise multivariate binary logistic regression model was built using the significant results from the univariate analysis (Table 2). After eliminating statistically insignificant results, finally 4 variables were included in the multivariate model (Somers՚ D = 0.69; Kolmogorov–Smirnov statistic = 0.55; p = 0.0001). The analysis revealed that the factors reducing the likelihood of ToxoIgG (+) were: < 40 years of age (OR 0.54, 95% CI 0.29-1.00; p = 0.04), no obesity (OR 0.36, 95% CI 0.15-0.89; p = 0.03) and male gender (OR 0.35, 95%CI 0.16-0.76; p = 0.008). The only factor significantly associated with an increased likelihood of Toxo-IgG (+) was lower education (primary or vocational school) OR 2.41, 95% CI 1.21-4.79; p = 0.01 (Figure 1).
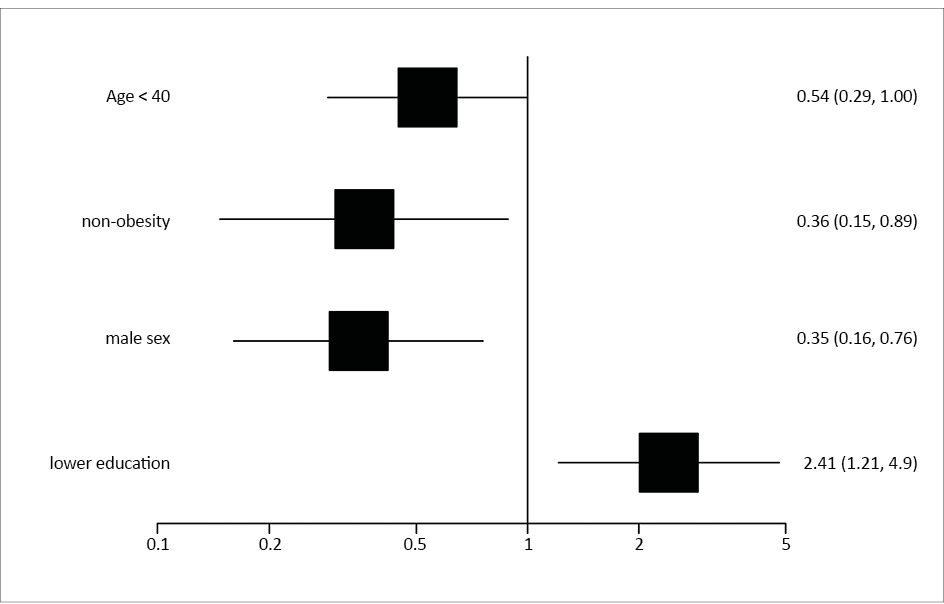.jpg)
Figure 1. Multivariate logistic regression
Discussion
We present the toxoplasmosis immune status in a case series study of patients acutely hospitalised due to an exacerbation of schizophrenia. Many significant differences were observed, both in the demographic characteristics and in the scales assessing the severity of disease symptoms. Seropositive patients significantly differ from the seronegative patients mainly in terms of sex, age, BMI and education level. Acute toxoplasmosis (assessed as IgM (+)) seems to be significantly associated with higher rates of positive symptoms (as measured by the PANSS scale). It is important to note there was limited sample size of IgM-positive subjects in our study.
The occurrence of latent toxoplasmosis is estimated at up to 30% of the world՚s population. Various studies have shown that it can influence the host՚s behaviour by influencing neurotransmitters [18]. Many studies have suggested the influence of T. gondii infection on the development of schizophrenia [18-20].
The exact molecular and cellular mechanisms underpinning the association between toxoplasmosis and schizophrenia remain poorly understood [21-22]. Various mechanisms explaining the influence of infection on human behaviour have been proposed, e.g. alterations in neurotransmitter release, cyst location, and neuroinflammation [23-24]. The causality of the link between T. gondii and schizophrenia is also unclear. This infection may spread by eating undercooked meat or unwashed fruits and vegetables containing protozoan oocysts [25-26]. This may be a result of negligence accompanying the predominant negative and disorganisation symptoms of schizophrenia, so in such case a T. gondii infection would be secondary to schizophrenia. An indirect proof of this hypothesis may be found in a meta-analysis which demonstrated a significant increase in prevalence of positive T. gondii IgM antibodies in patients with acute psychosis compared with controls, while the observed association was stronger for patients with chronic schizophrenia than those with a first episode of psychosis [27]. Similarly, increased T. gondii seropositivity was also observed in patients with treatment-resistant schizophrenia [28].
However, the relationship between T. gondii infection and schizophrenia seems even more complicated. A large survey of military members showed significant associations between increased levels of scaled T. gondii IgG antibodies and schizophrenia (measured prior to and after diagnosis) [29].
Few data exist concerning the clinical correlates of toxoplasmosis in persons with schizophrenia [5, 18, 30-31]. Patients with a latent T. gondii infection were proven to have 15 times higher probability of experiencing continuous course of disease than Toxoplasma-free subjects [32]. T. gondii seropositive patients with schizophrenia in general were found to have a worse course of schizophrenia and more severe positive symptoms [33]. The same study showed that higher Positive and Negative Syndrome Scale (PANSS) scores were associated with lower titres of anti-Toxoplasma antibodies suggesting that psychopathology may deteriorate with duration of that parasitic infection. Another study suggested that seropositive subjects present poor impulse control, personality aberrations or neurocognitive impairment, as well as a higher risk of suicide [34]. There are also studies suggesting there is no correlation between latent toxoplasmosis and schizophrenia symptomatology at all [23, 35].
According to the authors of a 2020 meta-analysis, T. gondii infection has a negligible impact on the intensity of positive and total symptoms of schizophrenia in people who are in the early stages of that disease [18]. Although further research is still required, this is consistent with the theory that T. gondii infection is directly associated with schizophrenia. [18].
Our results show a correlation between the course and symptomatology of schizophrenia and acute or latent infection with Toxoplasma gondii. Higher PANSS positive and excitement factor scores in patients with positive anti-Toxoplasma IgM class antibodies may point towards a sometimes postulated CNS metabolism change induced by T. gondii and leading to dopamine overproduction with increased dopamine concentration in the mesolimbic pathway being associated with positive symptoms of schizophrenia [36]. Higher severity of these symptoms may, by disorganising behaviour, also lead to lower quality of life in patients with higher Toxo-IgM antibodies that was observed in this study. Perhaps latent toxoplasmosis in the course of schizophrenia may lead to a more severe positive psychopathology and perhaps less favourable course of schizophrenia. Holub et al found that T.gondii-infected patients with schizophrenia scored higher in the positive subscale of the PANSS and had higher scores in Total PANSS [33]. The authors also found that infected patients remained in the hospital for about 33 days longer during their most recent admission than the uninfected ones [33].
The age effect seems to be interesting as well. Some studies suggest that the percentage of T. gondii seropositive people increases with age [37]. In our study, we found that age below 40 was reducing the likelihood of Toxo-IgG (+). On the contrary, according to Kezai et al, T. gondii seroprevalence was found to significantly increase with age in controls, but this association was not seen in patients with schizophrenia [30]. That study sample included a high percentage of seropositive subjects who were under the age of 38 [30]. Based on this, the authors claimed that T. gondii infection may contribute to the development of schizophrenia. In a recent case-control study, Ademe et al observed more T. gondii infections among schizophrenia patients who were male and > 35 years of age [38]. This contrasts with our results, which suggest that men are less likely to be seropositive. Single studies have examined the role of education level on T. gondii serostatus, finding no statistically significant associations [37].
The data from a German study were significant for cross-sectional studies in the general population [39]. Seroprevalence increased from 20% in the 18-29 age group to 77% in the 70-79 age group, according to the authors՚ analysis of a representative sample of 6,663 individuals. Younger males and older females had higher seroprevalences, researchers found. Being male, overweight or obese was positively correlated with seropositivity. Having a high socioeconomic status was a protective factor for T. gondii infection [39].
Our results highlighted that compared to Toxo-IgG (-) patients, Toxo-IgG (+) patients received mean lower doses of antipsychotic drugs and were more frequently treated with monotherapy. In another study it was also found that patients with a latent toxoplasmosis required lower doses of antipsychotics than seronegative patients (at least in terms of assessed hospitalisation), yet involuntary movements and tardive dyskinesia were more frequent, which is another argument for the possible influence of T. gondii infection on neurotransmission [40]. Lower doses of CPZE may also indicate a self-restricting character of psychosis in Toxo-IgM (+) patients due to alleviation of acute toxoplasmosis.
On the other hand, patients with positive Toxo-IgG antibodies have more prevalent parameters that may indirectly indicate lower self-care: more frequent obesity, higher glucose levels, lower education and less frequent psychotherapy. This may suggest that those initially less aware of or less willing to apply healthy behaviours are predisposed to toxoplasmosis infection (for instance by eating undercooked meat, unwashed vegetables), which could partly explain the high prevalence of toxoplasmosis in patients with schizophrenia where neglect is often a result of negative symptoms of the disease [41]. This argument is also supported by a higher mean age and longer duration of the disease in patients with a history of toxoplasmosis, and thus a longer period of risky behaviour [18]. The connection between Toxo-IgG (+) and the female gender remains unclear, requiring more detailed analyses.
Potential explanation of these contradictory hypotheses could be that schizophrenia predisposes to toxoplasmosis, which in turn has its role in a relapse of psychosis by altering neurophysiological mechanisms in CNS. Another view may be that increased IgM antibodies are not a marker of recent infection in these patients, but rather a sign of reinfection of latent toxoplasmosis associated with a relapse of schizophrenia [5].
Future studies on both Toxo-IgM (+) and Toxo-IgG (+) subjects with schizophrenia should include neuroimaging, particularly assessing dopaminergic neurotransmission, neuroinflammatory changes or lesions within the CNS. There is some evidence that antipsychotic drugs may inhibit tachyzoite replication in vitro and reduce T. gondii antibody titres, which could be investigated closely [22].
Presumed differences between patients with and without toxoplasmosis could suggest a different basis for similar schizophrenia symptoms, which would entail further steps such as assessing the validity of toxoplasmosis screening in the context of expected different treatment efficacy and a possible consideration of including anti-protozoan drugs in the treatment of patients with schizophrenia.
Several of the limitations associated with the descriptive nature of our study must be addressed. Based on our results, we were not able to find out how T. gondii infection could be related to schizophrenia, particularly because there we did not have a comparison group. The presented study groups were given to offer a possible explanation for how T. gondii infection may play a part in the pathogenesis of schizophrenia. However, the main limitation in this regard was inability to make many because there was no reference group.
Our study was conducted at a single site during a single patient hospitalisation. This makes it impossible to draw conclusions about cause-effect relationships. The number of patients recruited for the study was too small, especially for Toxo IgM (+) patients. As power analysis demonstrated, the sample size too small. In addition, there is the lack of verification of enzyme-linked immunosorbent assay (ELISA) tests by polymerase chain reaction (PCR).
One of the most important results in logistic regression turned out to be age and gender, but it should be noted that the groups of women and men differed statistically in age and the dose of the antipsychotic drug received (Table 1). Therefore, these results should be interpreted with caution.
In addition to the discussion of the relationship between T. gondii infection and schizophrenia, there is a need for additional evidence beyond the correlation of these two factors, especially using the Bradford Hill criteria for assessing causal relationships in observational data [11].
Overall, the study findings may indicate a particular role for T. gondii infection in schizophrenia patients. We believe that this study offers a significant starting point for additional research. Our findings imply that monitoring T.gondii infection in schizophrenia patients may have clinical implications for issues like symptom severity and antipsychotic medication doses.
Conclusions
Seropositive toxoplasma patients were mainly older females with obesity and lower level of education. Acute toxoplasmosis seems to be significantly associated with higher rates of positive symptoms of schizophrenia. Patients with Toxo IgG (+) received lower mean doses of antipsychotics and were more frequently treated with monotherapy. However, the small number of patients and descriptive nature of this study suggests that its results should be interpreted with caution.
Acknowledgements
We used the original version of the PSP by Morosini P. (Copyright © 2000 Wiley) which was reproduced with permission of John Wiley & Sons Ltd.
Data Availability Statement
The data that support the findings of this study are not openly available (due to containing sensitive human data) and are available from the corresponding author upon reasonable request.
Ethical considerations
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Funding
This study was supported by grant MN – 01-0246/08/222 funded by the Polish Ministry of Science and Higher Education.
Conflicts of interests
None.
References
| 1. |
Chorlton SD. Toxoplasma gondii and schizophrenia: a review of published RCTs. Parasitol Res [Internet]. 2017;116(7):1793-9. Available from: http://link.springer.com/10.1007/s00436-017-5478-y.
|
| 2. |
Galuszka J, Skorczynski M, Szaflarski J. Problematyka toksoplazmozy w pismiennictwie polskim do roku 1960 [in Polish]. Wiadomości Parazytol [Internet]. 1960;6(2–3):173-84. Available from: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-70cfc08b-c8ee-4df9-964e-afe6dd6b7aa2.
|
| 3. |
Kozar Z. Studies on toxoplasmosis in mental diseases. Biul Panstw Inst Med Morskiej i Trop w Gdansku Biulleten’Gosudarstvennogo Instituta morskoi i Trop meditsiny v Gdan’ske Bull State Inst Mar Trop Med Gdansk, Pola. 1953;5:134.
|
| 4. |
Torrey EF, Yolken RH. Toxoplasma gondii and Schizophrenia. Emerg Infect Dis [Internet]. 2003;9(11):1375-80. Available from: http://wwwnc.cdc.gov/eid/article/9/11/03-0143_article.htm.
|
| 5. |
Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and Other Risk Factors for Schizophrenia: An Update. Schizophr Bull [Internet]. 2012;38(3):642-7. Available from: https://doi.org/10.1093/schbul/sbs043.
|
| 6. |
Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull [Internet]. 1987;13(2):261-76. Available from: https://doi.org/10.1093/schbul/13.2.261.
|
| 7. |
van Os J, Burns T, Cavallaro R, Leucht S, Peuskens J, Helldin L, et al. Standardized remission criteria in schizophrenia. Acta Psychiatr Scand [Internet]. 2006;113(2):91-5. Available from: https://doi.org/10.1111/j.1600-0447.2005.00659.x.
|
| 8. |
Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res [Internet]. 2012;137(1):246-50. Available from: https://www.sciencedirect.com/science/article/pii/S0920996412000564.
|
| 9. |
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res [Internet]. 2005;79(2):231-8. Available from: https://www.sciencedirect.com/science/article/pii/S0920996405001611.
|
| 10. |
Andreasen NC, Carpenter WT, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in Schizophrenia: Proposed Criteria and Rationale for Consensus. Am J Psychiatry [Internet]. 2005;162(3):441-9. Available from: http://psychiatryonline.org/doi/abs/10.1176/appi.ajp.162.3.441.
|
| 11. |
Cox LA. Modernizing the Bradford Hill criteria for assessing causal relationships in observational data. Crit Rev Toxicol [Internet]. 2018;48(8):682-712. Available from: https://www.tandfonline.com/doi/full/10.1080/10408444.2018.1518404.
|
| 12. |
Guy W, Programs NI of MH (U.S). PRBD of ER. ECDEU assessment manual for psychopharmacology [Internet]. U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. (DHEW publication). Available from: https://books.google.pl/books?id=B4mZEZQRZUEC.
|
| 13. |
Janno S, Holi MM, Tuisku K, Wahlbeck K. Validity of Simpson-Angus Scale (SAS) in a naturalistic schizophrenia population. BMC Neurol [Internet]. 2005;5(1):5. Available from: https://doi.org/10.1186/1471-2377-5-5.
|
| 14. |
Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatr Scand [Internet]. 2000;101(4):323-9. Available from: https://doi.org/10.1034/j.1600-0447.2000.101004323.x.
|
| 15. |
Davis JM. Dose equivalence of the antipsychotic drugs. MATTHYSSE SW, KETY SSBT-C and S, editors. J Psychiatr Res [Internet]. 1975;11:65-73. Available from: https://www.sciencedirect.com/science/article/pii/B9780080182421500155.
|
| 16. |
Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry [Internet]. 2003;64(6):663-7. Available from: https://www.psychiatrist.com/jcp/neurologic/neurology/chlorpromazine-equivalent-doses-newer-atypical-antipsychotics/.
|
| 17. |
Schooler NR, Kane JM. Research Diagnoses for Tardive Dyskinesia. Arch Gen Psychiatry [Internet]. 1982;39(4):486-7. Available from: https://doi.org/10.1001/archpsyc.1982.04290040080014.
|
| 18. |
Sutterland AL, Mounir DA, Ribbens JJ, Kuiper B, van Gool T, de Haan L. Toxoplasma gondii Infection and Clinical Characteristics of Patients With Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull Open [Internet]. 2020;1(1):sgaa042. Available from: https://doi.org/10.1093/schizbullopen/sgaa042.
|
| 19. |
Xiao J, Prandovszky E, Kannan G, Pletnikov M V, Dickerson F, Severance EG, et al. Toxoplasma gondii: Biological Parameters of the Connection to Schizophrenia. Schizophr Bull [Internet]. 2018;44(5):983-92. Available from: https://doi.org/10.1093/schbul/sby082.
|
| 20. |
Ebrahimzadeh A, Shahraki MK, Mohammadi A. Serological and molecular diagnosis of Toxoplasma gondii in patients with schizophrenia. J Parasit Dis [Internet]. 2018;42(2):177-81. Available from: https://doi.org/10.1007/s12639-018-0979-x.
|
| 21. |
Elsheikha HM, Büsselberg D, Zhu X-Q. The known and missing links between Toxoplasma gondii and schizophrenia. Metab Brain Dis [Internet]. 2016;31(4):749-59. Available from: https://doi.org/10.1007/s11011-016-9822-1.
|
| 22. |
Webster JP, Kaushik M, Bristow GC, McConkey GA. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J Exp Biol [Internet]. 2013 Jan 1;216(1):99-112. Available from: https://doi.org/10.1242/jeb.074716.
|
| 23. |
Johnson HJ, Koshy AA. Latent Toxoplasmosis Effects on Rodents and Humans: How Much is Real and How Much is Media Hype? Garsin DA, editor. MBio [Internet]. 2020;11(2):e02164-19. Available from: https://doi.org/10.1128/mBio.02164-19.
|
| 24. |
Milne G, Webster JP, Walker M. Toxoplasma gondii: An Underestimated Threat? Trends Parasitol [Internet]. 2020;36(12):959-69. Available from: https://www.sciencedirect.com/science/article/pii/S147149222030221X.
|
| 25. |
Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol [Internet]. 2000;30(12–13):1217-58. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0020751900001247.
|
| 26. |
Lass A, Pietkiewicz H, Szostakowska B, Myjak P. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur J Clin Microbiol Infect Dis [Internet]. 2012;31(6):1101-8. Available from: https://doi.org/10.1007/s10096-011-1414-8.
|
| 27. |
Monroe JM, Buckley PF, Miller BJ. Meta-Analysis of Anti- Toxoplasma gondii IgM Antibodies in Acute Psychosis . Schizophr Bull [Internet]. 2015;41(4):989-98. Available from: https://doi.org/10.1093/schbul/sbu159.
|
| 28. |
Vlatkovic S, Sagud M, Svob Strac D, Sviben M, Zivkovic M, Vilibic M, et al. Increased prevalence of Toxoplasma gondii seropositivity in patients with treatment-resistant schizophrenia. Schizophr Res [Internet]. 2018;193:480-1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0920996417304723.
|
| 29. |
Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected Infectious Agents and Risk of Schizophrenia Among U.S. Military Personnel. Am J Psychiatry [Internet]. 2008;165(1):99-106. Available from: https://doi.org/10.1176/appi.ajp.2007.06081254.
|
| 30. |
Kezai AM, Lecoeur C, Hot D, Bounechada M, Alouani ML, Marion S. Association between schizophrenia and Toxoplasma gondii infection in Algeria. Psychiatry Res [Internet]. 2020;291:113293. Available from: https://www.sciencedirect.com/science/article/pii/S0165178120312221.
|
| 31. |
Dogruman-Al F, Aslan S, Yalcin S, Kustimur S, Turk S. A possible relationship between Toxoplasma gondii and schizophrenia: A seroprevalence study. Int J Psychiatry Clin Pract [Internet]. 2009;13(1):82-7. Available from: http://www.tandfonline.com/doi/full/10.1080/13651500802624738.
|
| 32. |
Celik T, Kartalci S, Aytas O, Akarsu GA, Gozukara H, Unal S. Association between latent toxoplasmosis and clinical course of schizophrenia – continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha) [Internet]. 2015;62. Available from: http://folia.paru.cas.cz/doi/10.14411/fp.2015.015.html.
|
| 33. |
Holub D, Flegr J, Dragomirecká E, Rodriguez M, Preiss M, Novák T, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand [Internet]. 2013;127(3):227-38. Available from: https://doi.org/10.1111/acps.12031.
|
| 34. |
Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma Gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort. Tanowitz HB, editor. PLoS One [Internet]. 2016;11(2):e0148435. Available from: https://dx.plos.org/10.1371/journal.pone.0148435.
|
| 35. |
Adolescent mental health [Internet]. [cited 2022 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health.
|
| 36. |
McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour – location, location, location? J Exp Biol [Internet]. 2013;216(1):113-9. Available from: https://doi.org/10.1242/jeb.074153.
|
| 37. |
Ansari-Lari M, Farashbandi H, Mohammadi F. Association of Toxoplasma gondii infection with schizophrenia and its relationship with suicide attempts in these patients. Trop Med Int Heal [Internet]. 2017;22(10):1322-7. Available from: https://doi.org/10.1111/tmi.12933.
|
| 38. |
Ademe M, Kebede T, Teferra S, Alemayehu M, Girma F, Abebe T. Is latent Toxoplasma gondii infection associated with the occurrence of schizophrenia? A case-control study. Foroutan M, editor. PLoS One [Internet]. 2022;17(6):e0270377. Available from: https://dx.plos.org/10.1371/journal.pone.0270377.
|
| 39. |
Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep [Internet]. 2016;6(1):22551. Available from: https://doi.org/10.1038/srep22551.
|
| 40. |
Tedford E, McConkey G. Neurophysiological Changes Induced by Chronic Toxoplasma gondii Infection. Vol. 6, Pathogens. 2017.
|
| 41. |
Flegr J, Horáček J. Negative Effects of Latent Toxoplasmosis on Mental Health. Front Psychiatry [Internet]. 2020;10. Available from: https://www.frontiersin.org/article/10.3389/fpsyt.2019.01012/full.
|







.jpg)


