Periprocedural decrease in tumor necrosis factor alpha is a risk factor for atrial fibrillation recurrence after ablation
Abstract
Background: Concentration of tumor necrosis factor alpha (TNF-alpha) might be useful in selecting patients with paroxysmal atrial fibrillation (PAF) who will benefit the most from pulmonary vein isolation.
Material and methods: This is a prospective cohort study among patients with PAF who had sinus rhythm prior to undergoing either radiofrequency ablation or cryoablation procedure. Blood samples were collected at the start of the procedure and 16-24 h after. TNF-alpha concentrations were measured. Follow-up data was obtained during a structured telephone interview and 24-hour ECG Holter monitoring 12 months after the ablation procedure.
Results: Thirty seven patients were enrolled. After 12-month follow-up 27 patients maintained sinus rhythm, 8 had recurrence of AF and 2 were lost to follow-up. There was no significant correlation between TNF-alpha concentrations in any of the samples and the recurrence of arrhythmia (for pre-procedural samples: 1.75 pg/ ml vs. 1.74 pg/ml; p = 0.72; for post-procedural samples: 1.49 pg/ml vs. 1.79 pg/ml; p = 0.16). In patients who had a recurrence of AF, we observed a decrease in the periprocedural TNF-alpha concentration (-0.12 pg/ml vs 0.05 pg/ml; p = 0.05).
Conclusions: Neither pre- nor post-procedural TNF-alpha concentrations are predictive of ablation outcome in patients with PAF. We observed a decrease in the periprocedural TNF-alpha concentration in patients who had AF recurrence.
Citation
Szczerba E, Koźluk E, Januszkiewicz Ł, Lisicka M, Nowak J, Kondracka A, Majstrak J, Rodkiewicz D, Piątkowska A, Kiliszek M, Opolski G. Periprocedural decrease in tumor necrosis factor alpha is a risk factor for atrial fibrillation recurrence after ablation. Eur J Transl Clin Med. 2022;5(2):16-23Background
It is estimated that up to 2% of the population suffers from atrial fibrillation (AF) [1]. Mechanisms contributing to the development of AF remain to be fully discovered. Currently, it is believed that electrical and structural remodeling, changes in atrial cardiomyocytes and inflammatory processes play a pivotal role in initiation and perpetuation of AF [1-2]. The role of inflammatory cytokines deserves special attention, among which TNF-alpha needs further investigation. This molecule is synthesized mainly by monocytes and macrophages which infiltrate tissues during inflammatory processes, e.g. as a response to injury [3]. Immunologically active monocytes/macrophages infiltrate the atrial midmyocardium more extensively in patients with AF compared to those with sinus rhythm [4]. Animal models of TNF-alpha overexpression in the heart reveal a predisposition to atrial arrhythmias such as premature atrial beats, atrial flutter and AF, as well as increased collagen deposition [5]. Injection of TNF-alpha into Swiss albino mice leads to atrial fibrosis and altered connexin-40 expression [6]. In vitro studies have shown that TNF-alpha can increase the arrythmogenicity of pulmonary vein cardiomyocytes and influence cellular calcium homeostasis [7].
We hypothesized that the inflammatory reaction to atrial tissue damage measured by the change in TNF-alpha concentration could predict pulmonary vein isolation (PVI) outcome. The aim of our study was to compare TNF-alpha concentrations pre- and post-procedurally in patients who underwent pulmonary vein isolation due to paroxysmal AF (PAF) with and without recurrence of arrhythmia.
Material and Methods
We performed a prospective cohort study among patients with PAF undergoing radiofrequency (RF) ablation or cryoablation. PAF was defined according to the current ESC guidelines [1]. Patients who underwent the ablation procedure in the past were disqualified from the study. The protocol of the study was approved by the local ethics committee (ID: KB 46/2011). Informed written consent was obtained from each patient.
Exclusion criteria included any disease that is known to influence the TNF-alpha concentration, including heart failure (Table 1). Patients were also excluded from the analysis if AF was the initial rhythm observed at the beginning of the ablation procedure. Blood samples from a peripheral vein were obtained from each participant twice: before insertion of the ablation catheter into the vessel at the beginning of the ablation procedure and 16-24 h after. The blood was then centrifuged, serum was stored in -70°C. TNF-alpha levels were measured by high-sensitivity ELISA kit (R&D, Human TNF-alpha Quantikine HS ELISA Kit). Concentration > 30 pg/ ml was recognized as an extreme value and excluded from the analysis according to the Tukey principles. Analyzed values included pre-procedural, post-procedural TNF-alpha concentration and the assessment of its periprocedural dynamics. Periprocedural TNF-alpha serum variations were calculated as a subtraction between post-procedural and pre-procedural TNF-alpha concentrations. The inflammatory response is dynamic only in the early post-ablation period, therefore we did not analyze TNF-alpha concentration during hospitalization.
Table 1. Exclusion criteria
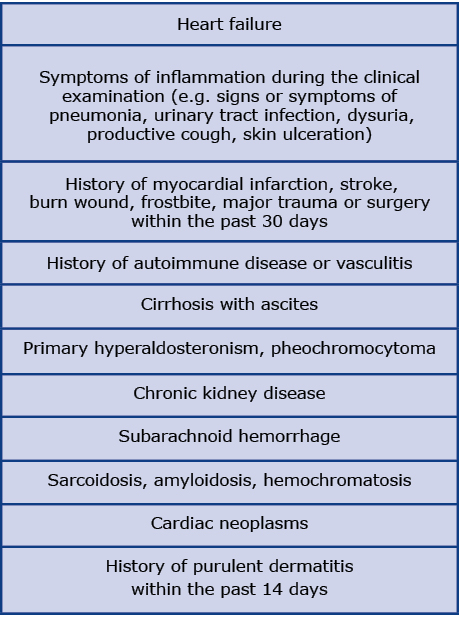
All the patients had 24-hour Holter electrocardiograph (ECG) monitoring performed at least 4 times per year and were instructed to perform an ECG in case of palpitations. AF recurrence was defined as AF episode lasting > 30 seconds registered by the 3-lead 24-hour Holter ECG. The guidelines recognize 24-hour Holter ECG monitoring as a follow-up method. We are aware that the longer the tachycardia is monitored, the more often silent (asymptomatic) AF is detected. However, at present the goal of ablation is to improve the quality of life, not to eliminate atrial arrhythmia. In this approach, the main focus was on symptomatic AF. Follow-up data was obtained during a structured telephone interview 12 months after the ablation procedure, focused on identifying episodes of AF recurrence.
Statystical analysis
SAS 9.3 (SAS Institute, Cary, USA) was used for statistical analysis. All variables were tested for a normal distribution with the Shapiro-Wilk test. Normally distributed continuous variables are represented as mean ± standard deviation (SD) and nonnormally distributed continuous variables are represented as median [25th-75th percentile (IQR)]. Categorical variables are presented as number (percentage). Statistical comparisons for normally distributed continuous variables were performed with the Student t-test. TNF-alpha concentrations were not normally distributed, therefore Wilcoxon test was performed to compare the concentrations between patients with recurrence of AF and successful ablation as well as between the pre- and post-procedural samples. Statistical comparisons for categorical variables were performed with the χ2 test. Spearman's rank correlation coefficient was used to calculate the correlations.
Results
Study population
Out of 82 patients with PAF qualified for their first-time ablation procedure, 37 patients met the inclusion criteria and were enrolled in the study [22 male (59.5%),15 female; mean age 55.7 ± 7.85 years]. The median time since diagnosis of AF was 36 months (IQR 19-108 months), the median duration of AF episodes was 8 hours (IQR 3-24 hours) with the median AF episode frequency of 1 per month (IQR 0.33-5 per month). Baseline characteristics of the study population are shown in the Table 2. The most common concomitant diseases were hypertension (54%) and dyslipidemia (65%).
Table 2. Baseline characteristics of the study population

Twenty one patients underwent RF ablation whereas 16 patients underwent balloon cryoablation with the median duration of the ablation procedure of 2.5 hours. Detailed periprocedural data are shown in Table 3. A successful PVI was achieved in all patients. At the time of discharge from the hospital, 34 patients had sinus rhythm (SR). During 12-month follow-up period 27 patients maintained stable SR, whereas 8 had recurrence of AF. Two patients were lost to follow-up.
Table 3. Periprocedural characteristics of study cohort
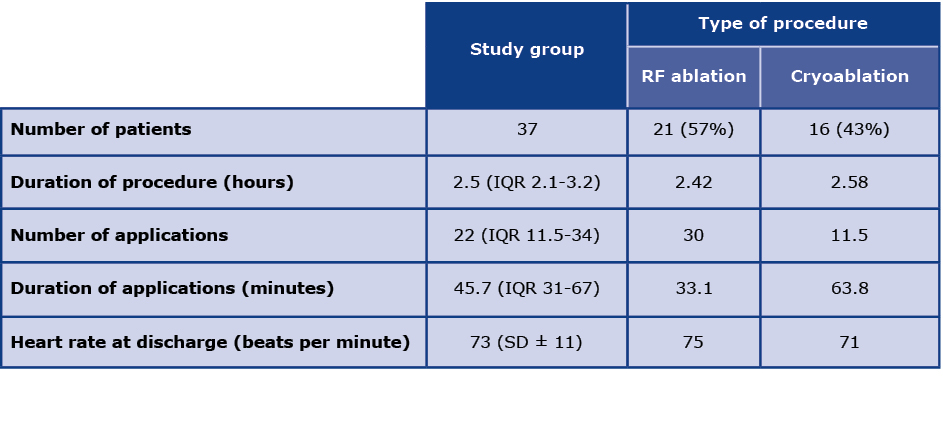
RF - radiofrequency
Serum samples analysis
The median TNF-alpha serum concentration was 1.75 pg/ml (IQR 1.39-2.19 pg/ml) in the pre-procedural sample and 1.69 pg/ml (IQR 1.41-2.29 pg/ml) in the post-procedural. No significant difference in the TNF-alpha concentrations between the samples in the whole studied group was observed (p = 0.91).
TNF-alpha levels and recurrence of arrhythmia
There was no significant difference between TNF-alpha serum concentrations in any of the samples when comparing the recurrence and the non-recurrence groups (for pre-procedural samples: 1.75 pg/ml vs. 1.74 pg/ml; p = 0.72; for post-procedural samples: 1.49 pg/ml vs. 1.79 pg/ml; p = 0.16). No correlation was observed between TNF-alpha serum concentration and the recurrence of AF after both types of ablation procedure (Figures 1 and 2). However, the analysis revealed a significant decrease in the periprocedural TNF-alpha serum levels in the recurrence group when the entire study group was analyzed (TNF-alpha variation -0.12 pg/ml vs. 0.05 pg/ml; p = 0.05).

Figure 1. TNF alpha concentrations (pg/ml) and recurrence of arrhythmia in RF ablation group
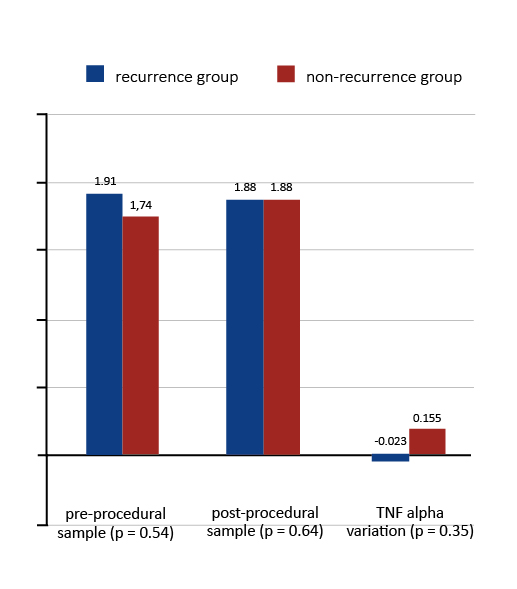
Figure 2. TNF alpha concentrations (pg/ml) and recurrence of arrhythmia in cryoablation group
Subanalysis of TNF-alpha concentrations in RF ablation and cryoablation groups
There was no significant difference in the TNF-alpha serum concentrations of the patients from the RF ablation group and cryoablation group in any of the samples (for pre-procedural samples: 1.75 pg/ml vs. 1.78 pg/ml p = 0.86; for post-procedural samples 1.61 pg/ml vs. 1.88 pg/ ml p = 0.22).
TNF-alpha levels and other variables
A positive correlation between TNF-alpha levels in the pre-procedural and post-procedural sample was observed (r = 0.59; p < 0.001). Baseline TNF-alpha serum concentration were not influenced by any of the following variables: age, sex, BMI, median time from diagnosis of arrhythmia, median duration of arrhythmia episode and median atrial fibrillation episodes frequency, renal function, left atrial diameter, time of procedure and summarized time of applications.
Discussion
Although neither pre-procedural nor post-procedural TNF-alpha concentration were associated with ablation outcome, the periprocedural decrease of this parameter was more common in the AF recurrence group. To the best of our knowledge this is the first study that evaluated periprocedural dynamics of TNF-alpha concentrations as a predictive factor for ablation outcome in this selected group of patients. Inclusion of patients with PAF with sinus rhythm and without heart failure prior to the procedure is one of the advantages of presented results.
Our results are opposite to those in the literature, which demonstrate the involvement of TNF-alpha in the pathogenesis of AF and suggest that AF recurrence would be associated with an increased concentration of TNF-alpha or its rise in the periprocedural period [4-7]. However, all our patients had a history of AF. TNF-alpha is one of the basic cytokines which are present in the early stage of the inflammation. This 185-aminoacid glycoprotein peptide hormone is produced mainly by macrophages and monocytes. TNF-alpha stimulates secretion of other proinflammatory factors such as IL-6 and IL-8 initiating the inflammatory reaction [8]. In an animal model of AF it was shown that TNF-alpha induces sustained atrial fibrosis [6] which plays a central role in the pathophysiology of AF [9]. In post-operative AF, TNF-alpha was identified as one of the mediators involved in the alternative pathway of local inflammation caused by surgical incision [10].
Wu et al. showed that an increase in circulating inflammatory factors such as CRP and IL-6 is associated with a higher risk of AF in the general population and patients undergoing CABG and with recurrence of AF after EC or ablation [18].
Frustaci et al. demonstrated that endomyocardial biopsy of the septal region of the right atrium in patients with lone AF showed histological abnormalities (e.g. lymphomononuclear infiltrates, necrosis of the adjacent myocytes, severe hypertrophy, vacuolar degeneration of the atrial myocytes, patchy fibrosis) in contrast to the normal histology results in the control group. Over 60% of patients fulfilled the histological diagnosis of atrial myocarditis, 16.5% of non-inflammatory cardiomyopathy and remaining 16.5% of patchy fibrosis [11]. These discoveries support hypothesis that inflammatory processes that occur in the atria may be a reaction to atrial tissue injury. AF could be therefore one of the manifestations of atrial myocarditis secondary to injury.
On the other hand, it is possible that a decrease in TNF-alpha concentration within the first 24 hours after ablation is an indicator of impaired immunological reaction to myocardial injury resulting in prolongation of the inflammatory process. Moreover, 24 hours might be too short to observe changes in the TNF-alpha concentration in the post- -ablation period. Sata et al. showed that TNF- alpha, interleukin 6 and C-reactive protein levels were higher in patients who underwent pharmacological cardioversion because of first AF episode compared with healthy controls. However, no difference was found between TNF-alpha sample taken before cardioversion, 24 hours later and 2 weeks later after conversion to sinus rhythm based on a group of 15 male patients, aged between 41 and 69 years (mean, 58 years) and had no history of organic heart diseases (e.g. valvular or coronary artery diseases and cardiomyopathy). In the control group there were 11 male patients with normal SR (mean age, 57 years; age range, 47 to 67 years) [12].
This may suggest that inflammatory processes related to AF are more profound and systemic. Some epidemiological data show that inflammation is one of the underlying process in AF. In a long-term observation of 2863 participants of the Framingham Offspring cohort, an increase in the inflammatory biomarkers was associated with more frequent incidents of AF [13]. Similar conclusions were drawn by the researchers from the Cardiovascular Health Study [14] and from the paper by Sata et al. [12]. In patients with AF, the TNF-alpha concentration, similarly to other inflammatory biomarkers, was elevated in comparison to the control group. The biomarker profile was different depending on the specific type of AF. TNF-alpha was lower in patients with paroxysmal AF and higher in patients with permanent AF [15].
Rafaqat et al. described the role of metabolic syndrome biomarkers in the pathogenesis of AF. TNF-alpha has been shown to contribute to the pathogenesis of chronic AF. Patients with valvular AF also showed high levels of TNF-α, more severe leukocyte infiltration and greater fibrosis. The level of IL-6 associated with increased left atrial size is a well-known risk factor for AF. Interleukin-10 and TNF-α levels influenced AF recurrence after ablation [17].
One of the reasons for the lack of differences between pre- and post-procedural concentrations of TNF-alpha might be connected to the fact that ablation using different sources of energy cause specific damage to cardiomyocytes. Although statistically insignificant, we observed that TNF-alpha levels fall after RF ablation, contrary to the concentration in the cryoablation group. In an animal model, Aupperle et al. demonstrated that endocardial cryoablation induces different type of endocardial, transmural and epicardial injury in comparison to RF ablation [16].
Kimura et al. showed that high levels of MMP-2 (Matrix metalloproteinase-2) accompanied by high levels of TNF-α were an independent predictor of AF recurrence [19]. MMP-2 and TNF-alpha levels may be useful in predicting the initial response to the ablation of AF.
Neuromodulation is a new treatment for AF. A study by Stavrakis et al. is the first to investigate the anti-arrhythmic and anti-inflammatory effects of LLTS (low-level tragus stimulation) in humans. 40 patients with paroxysmal AF qualified for ablation were randomly assigned to the 1 h LLTS. Patients were divided into 2 groups of 20 in the LLTS group and the sham control group. There were no statistically significant differences between the groups in the baseline clinical and echocardiographic characteristics. The study consisted of placing a flat metal clip on the tragus causing LLTS (20 Hz) in the right ear (50% lower than the voltage that slows the sinus rhythm). Under general anesthesia, atrial fibrillation was induced by pulsatile atrial stimulation at the beginning and 1 hour of LLTS or sham treatment. Subsequently, blood samples were taken from the patients from the coronary sinus and the femoral vein at the above-mentioned time points. Blood was analyzed for inflammatory cytokines such as TNF alpha and CRP. The study demonstrated for the first time in humans that the duration and inducibility of atrial fibrillation and the levels of inflammatory cytokines were non-invasively suppressed by the low-level transcutaneous electrical stimulation of the tragus [20].
Çetin et al. showed that rosuvastatin reduces the levels of inflammatory cytokines, including TNF-alpha [21]. It can be concluded that rosuvastatin has a protective effect against inflammation. In our study, 13 patients were treated with statins and received their doses of those drugs before and after ablation. The dosing of the drugs was not changed during the periprocedural period. It can be concluded that in our cohort the statins did not have an effect on the dynamic of TNF-alpha levels. This is confirmed by the fact that in 7 patients treated with statins we achieved reductions in TNF-alpha.
Limitations
We did not analyze whether the level of myocardial injury assessed by concentration of myocardial necrosis protein during ablation correlate with TNF-alpha. We did not control other indicators of inflammatory reaction such as C-reactive protein. No correlation between left atrium parameters (LAVi) and AF recurrence was analyzed. We didn’t perform comparison analysis or subanalysis in subgroups with hypertension or dyslipidemia. The group of patients included in the study was not large although sufficient to perform reliable statistical analysis. After ablation, antiarrhythmic drugs were discontinued. In the event of AF recurrence, the antiarrhythmic drugs were ordered by the family physician. In our opinion ordering antiarrhythmic drugs did not affect the number of recurrences. Our group was too small for multivariate analysis. This is a preliminary study to a larger study which will take these factors into account.
Clinical implications and future research
Our results show that the periprocedural dynamics of TNF-alpha are involved in the recurrence of AF. Future studies should concentrate on whether influencing the post-ablation healing processes in the atria might result in better scar formation and as a result more effective ablation results. Another question is if the fibrotic processes might be altered in patients with a decrease in TNF-alpha. Obtained data also suggest that injury to atrial tissue caused by RF ablation and cryoablation results in different secretion profile of TNF-alpha. This observation deserves further research.
Conclusion
Neither the pre-procedural nor post-procedural TNF-alpha concentrations are predictive of ablation outcome in patients with PAF. However its periprocedural decrease is associated with AF recurrence. TNF-alpha is a stable biomarker during the periprocedural period in patients with PAF patients undergoing their first ablation procedure.
Acknowledgments
The study was supported by a grant for young scientists of the Medical University of Warsaw.
Funding
None.
Conflicts of interests
None.
References
| 1. |
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the Europea. Eur Heart J [Internet]. 2021 Feb 1;42(5):373-498. Available from: https://doi.org/10.1093/eurheartj/ehaa612.
|
| 2. |
Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J [Internet]. 2009;157(2):243-52. Available from: https://www.sciencedirect.com/science/article/pii/S0002870308008909.
|
| 3. |
Guo Y, Lip GYH, Apostolakis S. Inflammation in Atrial Fibrillation. J Am Coll Cardiol [Internet]. 2012;60(22):2263-70. Available from: https://www.sciencedirect.com/science/article/pii/S0735109712044907.
|
| 4. |
Yamashita T, Sekiguchi A, Iwasaki Y, Date T, Sagara K, Tanabe H, et al. Recruitment of Immune Cells Across Atrial Endocardium in Human Atrial Fibrillation. Circ J [Internet]. 2010;74(2):262-70. Available from: http://www.jstage.jst.go.jp/article/circj/74/2/74_CJ-09-0644/_article.
|
| 5. |
Saba S, Janczewski AM, Baker LC, Shusterman V, Gursoy EC, Feldman AM, et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-α. Am J Physiol Circ Physiol [Internet]. 2005 Oct 1;289(4):H1456-67. Available from: https://doi.org/10.1152/ajpheart.00733.2004.
|
| 6. |
Liew R, Khairunnisa K, Gu Y, Tee N, Yin NO, Naylynn TM, et al. Role of Tumor Necrosis Factor-α in the Pathogenesis of Atrial Fibrosis and Development of an Arrhythmogenic Substrate. Circ J [Internet]. 2013;77(5):1171-9. Available from: https://www.jstage.jst.go.jp/article/circj/77/5/77_CJ-12-1155/_article.
|
| 7. |
Lee S-H, Chen Y-C, Chen Y-J, Chang S-L, Tai C-T, Wongcharoen W, et al. Tumor necrosis factor-α alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci [Internet]. 2007;80(19):1806-15. Available from: https://www.sciencedirect.com/science/article/pii/S0024320507001956.
|
| 8. |
Wan S, Yim APC. Cytokines in myocardial injury: impact on cardiac surgical approach. Eur J Cardio-Thoracic Surg [Internet]. 1999 Sep 1;16(Supplement_1):S107-11. Available from: https://doi.org/10.1016/S1010-7940(99)00200-6.
|
| 9. |
Burstein B, Nattel S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J Am Coll Cardiol [Internet]. 2008;51(8):802-9. Available from: https://www.sciencedirect.com/science/article/pii/S0735109707037862.
|
| 10. |
Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. EP Eur [Internet]. 2012 Feb 1;14(2):159-74. Available from: https://doi.org/10.1093/europace/eur208.
|
| 11. |
Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological Substrate of Atrial Biopsies in Patients With Lone Atrial Fibrillation. Circulation [Internet]. 1997 Aug 19;96(4):1180-4. Available from: https://doi.org/10.1161/01.CIR.96.4.1180.
|
| 12. |
Sata N, Hamada N, Horinouchi T, Amitani S, Yamashita T, Moriyama Y, et al. C-reactive Protein and Atrial Fibrillation Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J [Internet]. 2004;45(3):441-5. Available from: http://www.jstage.jst.go.jp/article/jhj/45/3/45_3_441/_article.
|
| 13. |
Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, et al. Relation of Multiple Inflammatory Biomarkers to Incident Atrial Fibrillation. Am J Cardiol [Internet]. 2009 Jul;104(1):92-6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914909006808.
|
| 14. |
Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation [Internet]. 2003 Dec 16;108(24):3006-10. Available from: https://doi.org/10.1161/01.CIR.0000103131.70301.4F.
|
| 15. |
Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Hear Rhythm [Internet]. 2010 Apr;7(4):438-44. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1547527109013800.
|
| 16. |
Aupperle H, Doll N, Walther T, Ullmann C, Schoon H-A, Wilhelm Mohr F. Histological findings induced by different energy sources in experimental atrial ablation in sheep. Interact Cardiovasc Thorac Surg [Internet]. 2005 Oct 1;4(5):450-5. Available from: https://doi.org/10.1510/icvts.2005.109413.
|
| 17. |
Rafaqat S. Biomarkers of Metabolic Syndrome: Role in Pathogenesis and Pathophysiology of Atrial Fibrillation. J Atr Fibrillation [Internet]. 2021 Aug 31;14(2). Available from: http://jafib.com/published.php?type=full&id=20200495.
|
| 18. |
Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int J Cardiol [Internet]. 2013 Oct;169(1):62-72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167527313016616.
|
| 19. |
Kimura T, Takatsuki S, Inagawa K, Katsumata Y, Nishiyama T, Nishiyama N, et al. Serum Inflammation Markers Predicting Successful Initial Catheter Ablation for Atrial Fibrillation. Hear Lung Circ [Internet]. 2014 Jul;23(7):636-43. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1443950614000730.
|
| 20. |
Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, et al. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation. J Am Coll Cardiol [Internet]. 2015;65(9):867-75. Available from: https://www.sciencedirect.com/science/article/pii/S0735109714075810.
|
| 21. |
Çetin A, Çetin İ, Yılmaz S, Şen A, Savaş G, Çimen B, et al. Oxydative stress markers and cytokine levels in rosuvastatin-medicated hypercholesterolemia patients. 2019;44(4):530-8. Available from: https://doi.org/10.1515/tjb-2018-0267.
|











