Abstract
Preeclampsia (PE) is defined as new onset hypertension after 20 weeks of gestation with evidence of maternal organ or uteroplacental dysfunction or proteinuria. PE is a leading cause of maternal death, with about 55000 deaths worldwide each year. Toxic substances that damage the maternal vascular endothelium induce PE, resulting in liver and kidney malfunction. It is crucial for obstetricians to identify as early as possible the patients who are at risk for PE. Polycystic ovarian disease, sleeping disorders, urinary infections, periodontal disease, smoking, lifestyle and familial history of PE are the major risk factors involved in this disease. VEGF, sFlt1, sENG, PAPP-A, inhibin A and activin A proteins, fetal hemoglobin, heat shock protein and placental protein have been helpful in predicting or diagnosing PE and in understanding its pathogenesis. In addition, a better understanding of PE pathogenesis would aid in identifying the most effective treatments that do not impair the fetus’ prognosis. The aim of our study is a review of the pathophysiology and biomarkers, such as pro- and anti-angiogenic substances, that may be useful in the detection of PE in the future.
Citation
Kalarani I B, Veerabathiran R, Mohammed V. The role of biomarkers in early prediction and molecular mechanisms of preeclampsia. Eur J Transl Clin Med. 2022;5(2):57-66Introduction
Gestational hypertension is a condition that increases blood pressure by > 140 mmHg in the systolic and > 90 mmHg in the diastolic for the first time 20 weeks into the pregnancy without proteinuria. Preeclampsia (PE) is defined as new onset hypertension after 20 weeks of gestation with evidence of maternal organ or uteroplacental dysfunction or proteinuria. Additionally, both the mother and the child may develop diabetes mellitus or cardiovascular illnesses later in life due to PE. There are two types of PE: early onset (symptoms appearing before 33 weeks) and late-onset (symptoms appear after 34 weeks) [1-2]. Although early and late-onset PE is related to some risk factors and overlaps in presenting symptoms, their effects on both mother and child can differ [3]. Placental perfusion problems cause proteinuria, edema, and multiple organ failure. Clinical signs range from mild to severe hypertension. Preeclampsia symptoms include cephalgia, impaired vision, light sensitivity, weariness, emesis, upper right abdomen pain, dyspnea and contusions. Normal pregnancy alters the patient’s immune system, allowing her to withstand illnesses. Because the fetus expresses paternal antigens, a pregnant woman's immune regulatory competence is critical for a safe pregnancy [4]. In PE, genetic and environmental factors contribute to the aetiology and pathophysiology of the disease. The delivery of the fetus and placenta is recognized as the only effective treatment [5].
PE is the leading cause of maternal mortality, accounting for around 55000 fatalities globally each year [6]. It has a complex etiology with early family-based research showing a role for maternal, fetal and paternal genetic factors [7]. We expect the global prevalence of preeclampsia to range from 5% to 14%, with developing countries experiencing a 4% increase. The total prevalence of preeclampsia varies between studies and populations, ranging from 4% to 23% (4% of pregnancies in the United States, 4.74% in Ethiopia, 6.1% in Kenya and 2-14% in Nigeria) [8]. According to the WHO, PE is common in middle-income developing nations such as India, with a 15% incidence rate and more cases in in that country's northern part than in southern. Tripura, the Indian state with the highest PE prevalence, has has demonstrated reduction of risk factors such as smoking and multiple pregnancy terminations. Climate change is also a key risk factor in India, with preeclampsia being more common in the summer and spring. Exposure to sunshine, variations in vitamin D deficiency cigarette consumption, lifestyle and ethnicity are the leading causes of hypertension in India [9-10].
According to the 2019 National Institute for Health and Care Excellence (NICE) guidelines, a pregnant woman with a history of autoimmune disease, diabetes or kidney diseaseI has an increased risk of PE. Various risk factors, including nulliparity, older age, chronic hypertension and gestational diabetes, have been identified as contributing to the high risk of PE. In addition, women with PE have a higher chance of having cardiovascular, kidney or neurological illness later in life, affecting the child's future health [11-12]. Additional clinical factors that enhance the risk of PE include sleep apnea, polycystic ovarian syndrome and infections (including urinary tract infections, periodontal disease and helicobacter pylori). Changing one's lifestyle can reduce PE, e.g. a vegetable and plant-based diet might be linked to a lower incidence of preeclampsia [13]. The aim of this study was to review the pathophysiology and biomarkers, such as pro- and anti-angiogenic substances.
Material and methods
The electronic databases such as PubMed, EMBASE and Cochrane Library were searched for articles published in the years 2010-present. The search terms were: preeclampsia, biomarkers, pathophysiology of PE, risk factors for PE, genetics of PE. We included only the human studies. Papers not written in English were excluded. This is a narrative review, therefore no statistical analysis was performed.
Results
The search retrieved 85 article abstracts. After screening, 26 of them were excluded and we screened the full texts of the remaining articles. A total of 59 articles were included in the review.
Pathophysiology of PE
The pathogenesis of PE is unknown. Currently PE is commonly thought to be a two-stage disease. The first stage is reduced placental perfusion, caused by abnormal implantation and placental vascular development, due to an imbalance in angiogenic factors [14]. The second stage of PE, maternal response, is manifested by widespread inflammation and maternal endothelian cell dysfunction, increased markers of oxidative stress, insulin resistance, reduced immune function and dyslipidemia [15].
Stage 1
Abnormal placentation
During normal placental development, extravillous cytotrophoblast invades the decidual and myometrial spiral arteries [14]. These spiral arteries then lose their endothelial nature and transform themselves from high resistance vessels into large vessels capable of providing adequate placental perfucion to supply nourishment to the developing fetus. This results in impaired spiral artery remodelingand relative placental ischemia. A trophoblast defect can cause shallow placentation, placental ischemia, insufficient spiral artery transformation and the maternal syndrome of preeclampsia [16-17]. The inability of preeclamptic trophoblast cells to change epithelial cell surface integrin expression to an endothelial phenotype restricts their ability to penetrate. In addition, PE may disrupt the activity of decidual natural killer (NK) cells, which play a role in vascular remodeling [18-19].
The NK cells produce angiogenesis-promoting cytokines and proteins but cannot remodel spiral arteries downstream. Furthermore, PE has been shown to activate circulating T and NK cells. In addition, PE may also occur due to the lysis of HLA-G deficient trophoblast cells caused by the uterine NK cells. Additionally, the absence of trophoblast cells, which should infiltrate the developing spiral arteries, would prevent the placenta from receiving enough oxygen and nutrients. According to recent research, there may be at least one common cause of spontaneous abortion or infertility. This cause is virtually identical to the cause of PE in pregnancies that survive 20 weeks. A recent study indicates that spiral arteries develop relatively narrow, thick-walled, and tortuous vessels in preeclampsia due to the failure of endovascular trophoblast invasions to undergo physiological modification.
Moreover, in PE, trophoblasts do not invade the myometrial segment of spiral arteries, in contrast to a normal pregnancy, in which the transformation of the spiral artery is limited to the decidual region of one-third of the myometrium. Consequently, deep placentation is unsuccessful and the placenta's blood flow is constrained, resulting in insufficient perfusion by the uterus. This phenomenon is associated with PE as well as many other pregnancy complications, such as placental abruption, preterm labor, premature rupture of membranes and intrauterine fetal mortality [20].
Hypoxia-inducible factors
Hypoxia-inducible factors (HIF) 1α and 2α, markers of cellular oxygen deficiency, were detected in high quantities in the placentas of PE patients. The production of sFLT1 (soluble fms-like tyrosine kinase 1), a solid anti-angiogenic factor associated with the HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), is inhibited when 2-methoxyestradiol suppresses HIF-1α, an estradiol metabolite that destabilizes HIF-1α. Because many variables besides hypoxia control HIF-1α expression, identifying the dysregulated signal upstream is difficult [21-22]. During placental hypoxia, the transforming growth factor family three (TGF-3) becomes more active, inhibiting the invasion of trophoblasts and the development of cytotrophoblasts. A natural metabolite of estradiol can decrease placental hypoxia; 2-methoxy estradiol, a natural metabolite of estrogen, reduces placental hypoxia by inhibiting HIF-1α expression. 2-methoxy estradiol is elevated throughout the 3rd trimester of normal pregnancy [23].
Oxidative stress
A pro-oxidant-antioxidant imbalance favours oxidation, which results in oxidative stress (OS). However, uncontrolled lipid peroxidation can occur in PE, impairing normal endothelial cell function [24]. According to research, PE contributes to placental oxidative stress by causing hypoperfusion and ischemia through remodeling of spiral arteries and cytotrophoblast invasion. The placenta experiences oxidative stress during a normal pregnancy due to increased mitochondrial activity, resulting in excess reactive oxygen species (ROS). However, improper placentation amplifies this impact during preeclampsia [23]. Preeclamptic trophoblasts cultured ex vivo produce more ROS, inhibiting the Wnt/-catenin signaling pathway and increasing trophoblast invasion. In addition, anti-angiogenic factors, such as sFLT1, may be expressed [25].
Stage 2
Angiogenic growth factors
Numerous molecules govern the processes of angiogenesis and vascular homeostasis. The VEGF (vascular endothelial growth factor ), PlGF (placental growth factor), soluble fms-like tyrosine kinase (sFlt1; fms – feline McDonough sarcoma)and soluble endoglin (sEng) have all been related to the pathophysiology of PE in humans. Endothelial cell activity is required for VEGF, mainly in the liver, glomeruli, and brain, the primary organs affected by preeclampsia. It is a disulfide-linked glycoprotein that is a homodimer involved in angiogenesis and vasculogenesis [26]. The VEGF subfamily functions by interacting with tyrosine kinase receptors, such as EGFR-1, VEGFR-2, and VEGFR-3, that activate cellular responses by phosphorylating the substrate. VEGF and its receptors significantly produced invasive cytotrophoblast in the first trimester of pregnancy for placental vascular development [15]. PlGF is a VEGF family member that plays a role in angiogenesis by binding to VEGFR1/sFLT [27]. It is a powerful angiogenic protein with structural similarity to VEGF that is primarily produced by the placental syncytiotrophoblast. It is also necessary for vasculogenesis during embryonic development in a normal pregnancy. They observed plGF levels to be lower in PE. Women predisposed to PE had lower PlGF levels, whereas salt-1 levels were higher. In PE, sFlt-1 binds to both VEGF and PlGF, inhibiting them from attaching to endogenous receptors. The levels of sFLT1 protein in maternal plasma or serum were high in preeclamptic placentas [28]. Transforming growth factors (TGF) family members, such as TGF-beta 1 and TGF-beta 3 have a cell surface co-receptor called endoglin. These two substances are powerful inhibitors of trophoblast differentiation and migration. The PlGF, TGF-β and sEng are highly expressed in placental tissue in PE [29].
Angiogenic markers
Soluble Endoglin (sEng)
Cellular endoglin is present on the membranes of vascular endothelium and syncytiotrophoblast cells. It functions as a receptor which inhibits the angiogenic factors TGF-β1 and TGF-β3. Its other function is inhibition of nitric oxide (NO) synthase in the endothelial cells, preventing vasodilatory action via the TGF. Several studies show that endoglin has been linked to PE (Figure 1). According to one study, endoglin mRNA and sEng levels were four times greater in preeclamptic women. According to further research, the level of sEng is also used as a prognostic marker. Concentrations of sEng were three-fold higher in patients with mild PE, five fold in severe PE and ten-fold in those with the HELLP syndrome [30]. During PE, placental endoglin is released into the maternal bloodstream, allowing soluble endoglin to become available. A potential mechanism of action is its ability to interfere with TGF-β1 signaling in the vasculature. Using adenoviral vectors, over-expression of sEng in rats resulted in improved vascular permeability and mild hypertension without significant proteinuria. Because of over-expressing sFlt1 and sEng with an adenoviral vector, significant vascular damage, nephrotic range proteinuria, severe hypertension, a condition similar to HELLP syndrome, and fetal growth retardation were observed. It is possible, therefore, that sEng and sFlt1 act in different ways to cause endothelial dysfunction and severe PE [31].
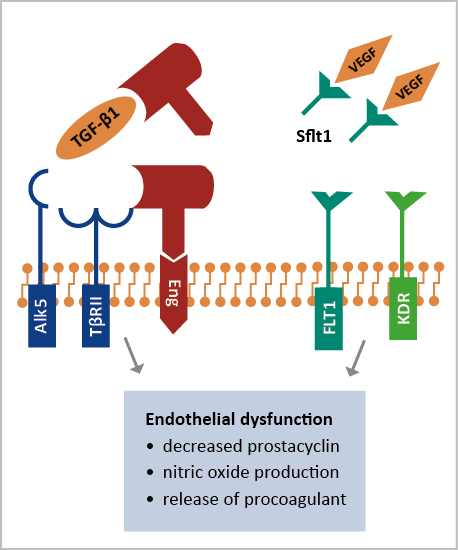
Figure 1. sEng and sFLT-1 cause endothelial dysfunction and leads to preeclampsia
Soluble Fms Like Tyrosine kinase-1 (sFLT-1)
The membrane-bound Flt-1 protein (sFlt-1), a shortened splice variant, circulates freely in the bloodstream and binds to PIGF and VEGF. The link between elevated sFlt-1 and PE has been shown in several studies. Levels of sFlt-1 are strongly linked to illness severity, hypertension, and proteinuria [32-33]. In patients with PE, the concentration of sFlt1 rises in the placenta or blood, while PlGF decreases. During the second trimester, sFlt1 and PlGF have shown significant sensitivity in predicting the development of PE (Figure 1) and a high sFlt1:PlGF ratio may also indicate this condition. However, additional clinical research, particularly randomized trials, will be required to determine their utility [34].
Immunological markers
Pregnancy-associated placental protein A (PAPP-A)
The trophoblast produces PAPP-A, a large glycosylated protein thought to function in the implantation process [35]. It enters the maternal bloodstream and binds to the binding protein of eosinophils, inhibiting their proteolytic activity [36]. The first-trimester serum PAPP-A levels are low before other pregnancy problems (e.g. fetal growth restriction, spontaneous miscarriage, placental abruption, premature delivery, gestational diabetes) occur, limiting its use as a PE biomarker (Figure 2). PAPP-A has been proposed as a better diagnostic for fetal growth restriction than preeclampsia [37].
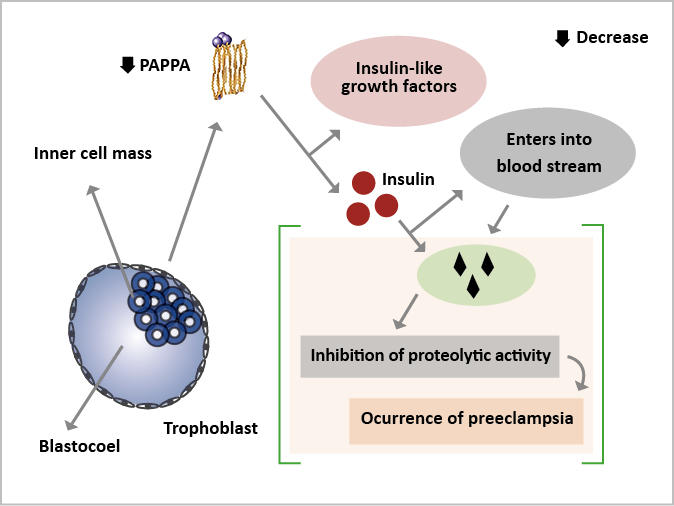
Figure 2. Low levels of the PAPPA protein and the pathogenesis of preeclampsia
Placental protein 13
Placental Protein 13 (PP13), also known as Galectin 13 (Gal-13) is a polypeptide that combines carbohydrates and is associated with swelling, autoimmune response and cell death. Galectins are vital in controlling certain parts of the genital system in some individuals. It has extreme affinity for sugar residues, namely the ABO blood type antigens AB and B. It may stimulate endometrial arterio-venous dilation and preserve maternal vascular integrity by interacting with glycoproteins and glycolipids [38]. According to studies, decreased blood PP13 during premature birth may act as a biomarker to identify PE patients. There are some reports that that blood PP13 levels and uterine artery Doppler examination could help to detect individuals with PE before 34 weeks of gestation [39-41]. PE may also be associated with reduced PP13 expression in the placenta [42]. Although PP13 has some predictive significance for PE, Giguere et al needed more data to allow a more precise assessment [43].
Metabolic Marker
Visfatin
It is known that visfatin, a nicotinamide phosphoribosyl transferase enzyme, is produced by adipose tissue that catalyzes the conversion of 5-phosphoribosyl-1-pyrophosphate into nicotinamide mononucleotide [44]. The levels of maternal plasma visfatin were considerably lower in women with PE and it also linked their levels to the severity of the condition [45] (Figure 3). As a result, larger-scale investigations are needed to assess the relevance of visfatin as a possible PE marker.
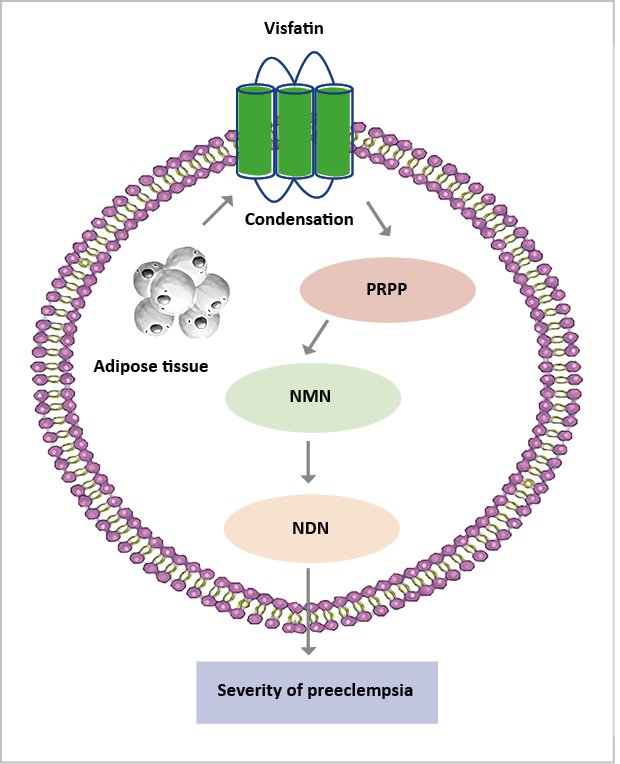
Figure 3. Low-level of visfatin increases the severity of preeclampsia
Endocrine Markers
Inhibin A and activin A
Inhibin A and activin A are glycoprotein hormones that belong to the transforming growth factor-β family. These circulating proteins are primarily obtained from the placenta during pregnancy, and their quantities rise in the third trimester in uncomplicated pregnancies [46]. Inhibin A plays a vital part in the negative feedback of gonadotropins in the endocrine system and Activin A is engaged in various biological functions. Both hormone concentrations rise in the third trimester of a normal pregnancy and their levels rise tenfold with severe PE. In addition, it causes an increase in maternal oxidative stress and systemic inflammation. Oxidative stress stimulates the production and release of activin A in placental explant and endothelial cells. There are conflicting reports on the increase of inhibin A in the second trimester, which was not as high in PE as Activin A (Figure 4) [47].
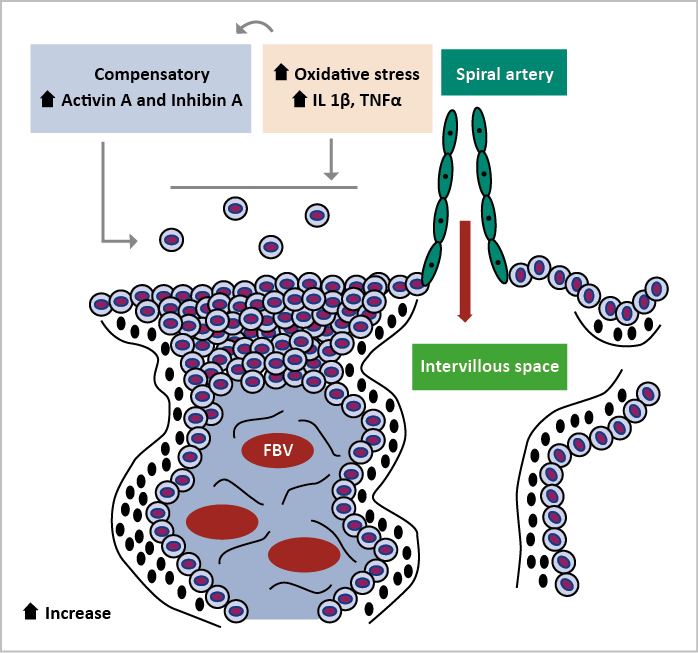
Figure 4. Actin A and Inhibin A are responsible for the manifestation of preeclampsia
Fetal hemoglobin
In PE, fetal hemoglobin (HbF) may provoke oxidative stress. In the placentas and bloodstreams of women with PE, oxidative stress is present. The connection between HbF and PE has prompted researchers to investigate it recently. They have postulated that HbF takes part in PE by causing harm to the placenta, kidneys and other organs, although the actual mechanism remains unknown. Therefore, during PE the placenta has an elevated activity of α1-microglobulin (A1M), showing that oxidative stress responds to its transcription. HbF causes tissue damage that A1M partially alleviates. There may be a possibility of further research on PE using A1M [48]. The fusion of hemoglobin was increased in the placentas of PE patients due to endothelial injury and inflammation [49]. During oxidative stress, patients with PE can have HbF released into their bloodstream. Since the HbF serum of PE patients can be higher during premature gestation, it may be a helpful marker [50].
Heat-shock protein
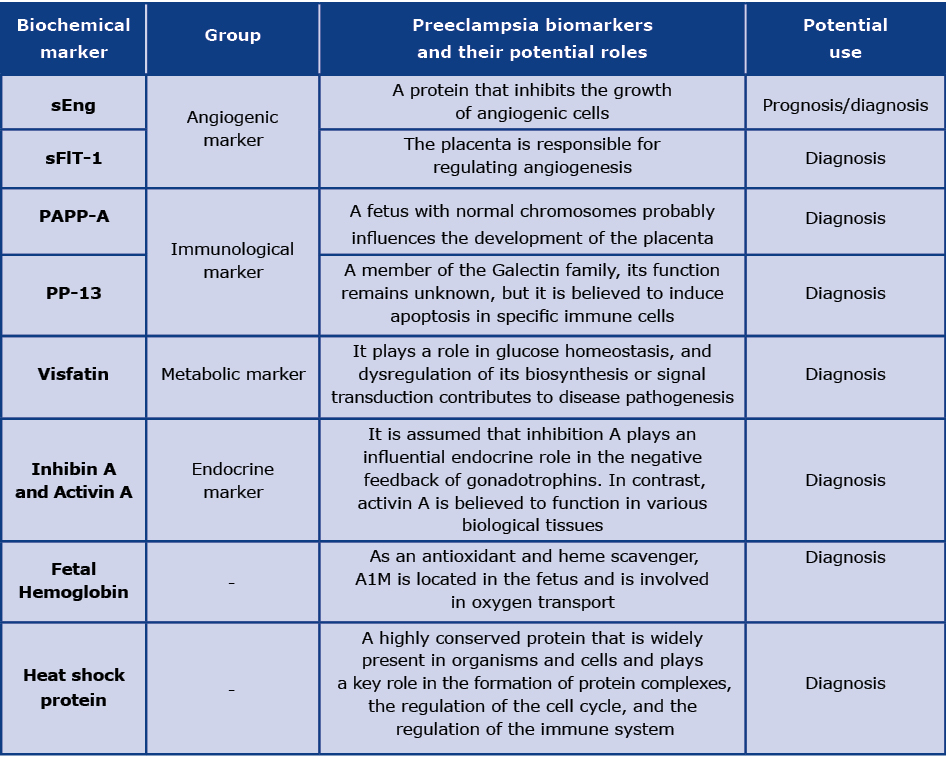
HSP (highly conserved heat shock protein) is typically found in animal/cellular tissues and regulates the cell cycle, immunity and protein synthesis [51]. Concerning the link between HSP and PE, the study focuses mainly on HSP7070, which may may inhibit apoptosis in various biological processes, including protein function. Maternal adverse reactions, placental disease and oxidative stress can all influence HSP70 expression. A study by Peracoli and colleagues has found that HSP70 may be allied with pro-inflammatory cytokines such as TNF-α, interleukin-1β, and interleukin-12 [52]. Salt-induced hypertension is an outcome of HSP70 overexpression [53]. Livingston et al. demonstrated that the serum HSP70 concentration in women with severe PE is not higher than usual [54- 56]. Because of this contradiction, Saghafi et al. performed a meta-analysis and discovered elevated HSP70 serum levels in PE patients compared to women with normal pregnancies [57]. In addition, Hromadnikova et al. found that patients with PE had elevated levels of HSP70 mRNA. It may be possible to examine HSP70 for PE diagnosis, although most data is from case-control studies and prospective studies are rare [58]. The potential biomarkers' functions and their possible use are mentioned in Table 1.
Table 1. Biochemical prediction marker for preeclampsia
Conclusion
Several pregnancy-associated illnesses are associated with PE, which has no known cause. The presence of several protein biomarkers is associated with PE. The following proteins help predict or diagnose PE and aid in the understanding of its pathogenesis: VEGF, sFlt1, sENG, PAPP-A, inhibin A, activin A, fetal hemoglobin, heat shock protein and PP13. Screening for these biomarkers during pregnancy might be clinically helpful. Future research should focus on determining the molecular processes behind the altered angiogenesis and finding new and accurate biomarkers for early disease detection.
Acknowledgements
The authors thank the Chettinad Academy of Research and Education for their ongoing support and encouragement.
Funding
Not applicable.
Conflicts of interest
No relevant financial or non-financial conflicts of interests to report.
References
| 1. |
Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr [Internet]. 2016 May 18;27(2):71-8. Available from: http://cvja.co.za/onlinejournal/vol27/vol27_issue2/#17/z.
|
| 2. |
O’Tierney-Ginn PF, Lash GE. Beyond pregnancy: modulation of trophoblast invasion and its consequences for fetal growth and long-term children’s health. J Reprod Immunol [Internet]. 2014;104-105:37-42. Available from: https://www.sciencedirect.com/science/article/pii/S0165037814000540.
|
| 3. |
Rizwana A, Rajkumar SA, Anuradha CR. Pregnancy and perinatal outcomes in women with polycystic ovarian syndrome. Int J Reprod Contraception, Obstet Gynecol [Internet]. 2021 Nov 30;10:4232+. Available from: https://link.gale.com/apps/doc/A684661087/AONE?u=anon~90263ab6&sid=bookmark-AONE&xid=6cd48756.
|
| 4. |
Varshini GS, Harshini S, Siham MA, Tejaswini GK, Kumar YS, Kulanthaivel L, et al. Investigation of FOXP3 (rs3761548) polymorphism with the risk of preeclampsia and recurrent spontaneous abortion: A systemic review and meta-analysis. Asian Pacific J Reprod [Internet]. 2022;11(3):117. Available from: https://www.apjr.net/article.asp?issn=2305-0500;year=2022;volume=11;issue=3;spage=117;epage=124;aulast=Varshini.
|
| 5. |
Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest [Internet]. 2013 Jul 1;123(7):2775-7. Available from: http://www.jci.org/articles/view/70431.
|
| 6. |
Hypertension in Pregnancy. Obstet Gynecol [Internet]. 2013 Nov;122(5):1122-31. Available from: https://journals.lww.com/greenjournal/Fulltext/2013/11000/Hypertension_in_Pregnancy__Executive_Summary.36.aspx.
|
| 7. |
Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw Open [Internet]. 2021 May 10;4(5):e218401-e218401. Available from: https://doi.org/10.1001/jamanetworkopen.2021.8401.
|
| 8. |
Tessema KF, Gebremeskel F, Getahun F, Chufamo N, Misker D. Individual and Obstetric Risk Factors of Preeclampsia among Singleton Pregnancy in Hospitals of Southern Ethiopia. Katsuya T, editor. Int J Hypertens [Internet]. 2021 Jan 20;2021:1-8. Available from: https://doi.org/10.1155/2021/7430827.
|
| 9. |
Malik A, Jee B, Gupta SK. Preeclampsia: Disease biology and burden, its management strategies with reference to India. Pregnancy Hypertens [Internet]. 2019;15:23-31. Available from: https://www.sciencedirect.com/science/article/pii/S2210778918301764.
|
| 10. |
Agrawal S. Prevalence and Risk Factors for Symptoms Suggestive of Pre-Eclampsia in Indian Women. J Women’s Heal Issues Care [Internet]. 2014;03(06). Available from: http://www.scitechnol.com/prevalence-and-risk-factors-for-symptoms-suggestive-of-pre-eclampsia-in-indian-women-tDg6.php?article_id=2389.
|
| 11. |
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive Disorders of Pregnancy. Hypertension [Internet]. 2018 Jul 1;72(1):24-43. Available from: https://doi.org/10.1161/HYPERTENSIONAHA.117.10803.
|
| 12. |
Geneen LJ, Webster KE, Reeves T, Eadon H, Maresh M, Fishburn S, et al. Protein-creatinine ratio and albumin-creatinine ratio for the diagnosis of significant proteinuria in pregnant women with hypertension: Systematic review and meta-analysis of diagnostic test accuracy. Pregnancy Hypertens [Internet]. 2021;25:196-203. Available from: https://www.sciencedirect.com/science/article/pii/S221077892100074X.
|
| 13. |
Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J Clin Med [Internet]. 2019 Oct 4;8(10):1625. Available from: https://www.mdpi.com/2077-0383/8/10/1625.
|
| 14. |
McDonnold M, Olson G. Preeclampsia: Pathophysiology, Management, and Maternal and Fetal Sequelae. Neoreviews [Internet]. 2013 Jan 1;14(1):e4-12. Available from: https://doi.org/10.1542/neo.14-1-e4.
|
| 15. |
Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal Placentation, Angiogenic Factors, and the Pathogenesis of Preeclampsia. Obstet Gynecol Clin North Am [Internet]. 2010 Jun;37(2):239-53. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889854510000173.
|
| 16. |
Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest [Internet]. 1997 May 1;99(9):2152-64. Available from: http://www.jci.org/articles/view/119388.
|
| 17. |
Brosens I, Renaer M. ON THE PATHOGENESIS OF PLACENTAL INFARCTS IN PRE-ECLAMPSIA. BJOG An Int J Obstet Gynaecol [Internet]. 1972 Sep 1;79(9):794-9. Available from: https://doi.org/10.1111/j.1471-0528.1972.tb12922.x.
|
| 18. |
Lubis MP. Comparison Immunohistochemistry Expression of Desidual Natural Killer (dNK) in Severe Preeclampsia and Normal Pregnancy. Int J Curr Pharm Res [Internet]. 2020;15:58-60. Available from: https://dupakdosen.usu.ac.id/handle/123456789/71546.
|
| 19. |
Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, et al. Changes of NK Cells in Preeclampsia. Am J Reprod Immunol [Internet]. 2012 Apr 1;67(4):278-86. Available from: https://doi.org/10.1111/j.1600-0897.2012.01120.x.
|
| 20. |
Hong K, Kim SH, Cha DH, Park HJ. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int J Mol Sci [Internet]. 2021 Oct 18;22(20):11202. Available from: https://www.mdpi.com/1422-0067/22/20/11202.
|
| 21. |
Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta [Internet]. 2004;25(10):763-9. Available from: https://www.sciencedirect.com/science/article/pii/S0143400404000797.
|
| 22. |
Redman CWG, Sargent IL. Placental Stress and Pre-eclampsia: A Revised View. Placenta [Internet]. 2009;30:38-42. Available from: https://www.sciencedirect.com/science/article/pii/S0143400408003950.
|
| 23. |
Nirupama R, Divyashree S, Janhavi P, Muthukumar SP, Ravindra P V. Preeclampsia: Pathophysiology and management. J Gynecol Obstet Hum Reprod [Internet]. 2021;50(2):101975. Available from: https://www.sciencedirect.com/science/article/pii/S2468784720303457.
|
| 24. |
Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive Cytotrophoblasts Manifest Evidence of Oxidative Stress in Preeclampsia. Am J Pathol [Internet]. 2000;156(1):321-31. Available from: https://www.sciencedirect.com/science/article/pii/S0002944010647335.
|
| 25. |
Huang QT, Wang SS, Zhang M, Huang LP, Tian JW, Yu YH, et al. Advanced oxidation protein products enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts: A possible link between oxidative stress and preeclampsia. Placenta [Internet]. 2013;34(10):949-52. Available from: https://www.sciencedirect.com/science/article/pii/S0143400413005845.
|
| 26. |
Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular Endothelial Growth Factor Induces Endothelial Fenestrations In Vitro . J Cell Biol [Internet]. 1998 Feb 23;140(4):947-59. Available from: https://doi.org/10.1083/jcb.140.4.947.
|
| 27. |
De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med [Internet]. 2012;44(1):1-9. Available from: https://doi.org/10.3858/emm.2012.44.1.025.
|
| 28. |
Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest [Internet]. 2003 Mar 1;111(5):649-58. Available from: http://www.jci.org/articles/view/17189.
|
| 29. |
Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-β superfamily expression and actions in the endometrium and placenta. Reproduction [Internet]. 2006;132(2):217-32. Available from: https://rep.bioscientifica.com/view/journals/rep/132/2/1320217.xml.
|
| 30. |
Staff AC. Circulating predictive biomarkers in preeclampsia. Pregnancy Hypertens An Int J Women’s Cardiovasc Heal [Internet]. 2011;1(1):28-42. Available from: https://www.sciencedirect.com/science/article/pii/S2210778910000139.
|
| 31. |
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N Engl J Med [Internet]. 2006 Sep 7;355(10):992-1005. Available from: https://doi.org/10.1056/NEJMoa055352.
|
| 32. |
Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental Expression of Vascular Endothelial Growth Factor Receptor-1, (Flt-1) and Soluble Flt-1 (sFlt-1), by Peripheral Blood Mononuclear Cells (PBMCs) in Normotensive and Preeclamptic Pregnant Women. Placenta [Internet]. 2005;26(7):563-73. Available from: https://www.sciencedirect.com/science/article/pii/S0143400404002371.
|
| 33. |
Karumanchi SA, Lindheimer MD. Preeclampsia Pathogenesis. Hypertension [Internet]. 2008 Apr 1;51(4):991-2. Available from: https://doi.org/10.1161/HYPERTENSIONAHA.107.100735.
|
| 34. |
Gbadegesin A, Agbara JO, Rabiu KA, Sobande AA, Azeez MA. Placenta Growth Factor and Soluble Fms-Like Tyrosine Kinase 1 in Preeclampsia and Normotensive Pregnant Nigerian Women. Open J Obstet Gynecol [Internet]. 2021;11(06):753-62. Available from: https://www.scirp.org/journal/doi.aspx?doi=10.4236/ojog.2021.116070.
|
| 35. |
Karumanchi SA. Chapter Fourteen - Biomarkers in Preeclampsia. In: Edelstein CLBT-B of KD (Second E, editor. Academic Press; 2017. p. 555-94. Available from: https://www.sciencedirect.com/science/article/pii/B9780128030141000145.
|
| 36. |
Spencer CA, Allen VM, Flowerdew G, Dooley K, Dodds L. Low levels of maternal serum PAPP-A in early pregnancy and the risk of adverse outcomes. Prenat Diagn [Internet]. 2008 Nov 1;28(11):1029–36. Available from: https://doi.org/10.1002/pd.2116.
|
| 37. |
Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem [Internet]. 1957;225(2):759-70. Available from: https://www.researchgate.net/profile/Jack-Preiss/publication/10150760_Enzymatic_synthesis_of_nicotinamide_mononucleotide/links/0deec517ad1fc60e06000000/Enzymatic-synthesis-of-nicotinamide-mononucleotide.pdf.
|
| 38. |
Sammar M, Drobnjak T, Mandala M, Gizurarson S, Huppertz B, Meiri H. Galectin 13 (PP13) Facilitates Remodeling and Structural Stabilization of Maternal Vessels during Pregnancy. Int J Mol Sci [Internet]. 2019 Jun 29;20(13):3192. Available from: https://www.mdpi.com/1422-0067/20/13/3192.
|
| 39. |
Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update [Internet]. 2013 Jul 1;19(4):391-405. Available from: https://doi.org/10.1093/humupd/dmt003.
|
| 40. |
Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, et al. A novel approach to first‐trimester screening for early pre‐eclampsia combining serum PP‐13 and Doppler ultrasound. Ultrasound Obstet Gynecol [Internet]. 2006 Jan 22;27(1):13-7. Available from: https://doi.org/10.1002/uog.2686.
|
| 41. |
Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K. First trimester markers for the prediction of pre-eclampsia in women with a priori high risk. Ultrasound Obstet Gynecol [Internet]. 2010 Jun 1;35(6):n/a-n/a. Available from: https://doi.org/10.1002/uog.7559.
|
| 42. |
Than NG, Balogh A, Romero R, Kárpáti Ã, Erez O, Szilágyi A, et al. Placental Protein 13 (PP13) – A Placental Immunoregulatory Galectin Protecting Pregnancy. Front Immunol [Internet]. 2014 Aug 20;5:348. Available from: http://journal.frontiersin.org/article/10.3389/fimmu.2014.00348/abstract.
|
| 43. |
Giguère Y, Charland M, Bujold E, Bernard N, Grenier S, Rousseau F, et al. Combining Biochemical and Ultrasonographic Markers in Predicting Preeclampsia: A Systematic Review. Clin Chem [Internet]. 2010 Mar 1;56(3):361-75. Available from: https://doi.org/10.1373/clinchem.2009.134080.
|
| 44. |
Fasshauer M, Blüher M, Stumvoll M, Tönessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin Endocrinol (Oxf) [Internet]. 2007 Mar 1;66(3):434-9. Available from: https://doi.org/10.1111/j.1365-2265.2007.02751.x.
|
| 45. |
Hu W, Wang Z, Wang H, Huang H, Dong M. Serum visfatin levels in late pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand [Internet]. 2008 Jan 1;87(4):413-8. Available from: https://www.tandfonline.com/doi/abs/10.1080/00016340801976012.
|
| 46. |
Kar M. Role of biomarkers in early detection of preeclampsia. J Clin Diagn Res [Internet]. 2014 Apr;8(4):BE01-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24959436.
|
| 47. |
Abbas RA, Ghulmiyyah L, Hobeika E, Usta IM, Mirza F, Nassar AH. Preeclampsia: A Review of Early Predictors. Matern Med [Internet]. 2021 Jul 1;3(3):197-202. Available from: https://doi.org/10.1097/FM9.0000000000000088.
|
| 48. |
Hansson SR, Nääv Ã, Erlandsson L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front Physiol [Internet]. 2015 Jan 13;5. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2014.00516/abstract.
|
| 49. |
Centlow M, Carninci P, Nemeth K, Mezey E, Brownstein M, Hansson SR. Placental expression profiling in preeclampsia: local overproduction of hemoglobin may drive pathological changes. Fertil Steril [Internet]. 2008 Nov;90(5):1834-43. Available from: https://www.sciencedirect.com/science/article/pii/S0015028207036539.
|
| 50. |
Henry A, Arnott C, Makris A, Davis G, Hennessy A, Beech A, et al. Blood pressure postpartum (BP2) RCT protocol: Follow-up and lifestyle behaviour change strategies in the first 12 months after hypertensive pregnancy. Pregnancy Hypertens [Internet]. 2020 Oct;22:1-6. Available from: https://www.sciencedirect.com/science/article/pii/S2210778920300908.
|
| 51. |
Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet [Internet]. 1988;22(1):631-77. Available from: https://scholar.archive.org/work/7msluk3e7jel7bb33rnaz57cfe/access/wayback/http://lindquistlab.wi.mit.edu/PDFs/LindquistCraig1988ARG.pdf.
|
| 52. |
Peraçoli JC, Bannwart-Castro CF, Romao M, Weel IC, Ribeiro VR, Borges VTM, et al. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol [Internet]. 2013;100(2):129-34. Available from: https://www.sciencedirect.com/science/article/pii/S0165037813001034.
|
| 53. |
Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol [Internet]. 2014 Jan 19;10(1):56-62. Available from: https://doi.org/10.1038/nrneph.2013.248.
|
| 54. |
Molvarec A, Rigó J, Lázár L, Balogh K, Makó V, Cervenak L, et al. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones [Internet]. 2009 Mar 7;14(2):151. Available from: https://doi.org/10.1007/s12192-008-0067-8.
|
| 55. |
Molvarec A, Prohászka Z, Nagy B, Szalay J, Füst G, Karádi I, et al. Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case–control study. J Hum Hypertens [Internet]. 2006 Oct 1;20(10):780-6. Available from: https://doi.org/10.1038/sj.jhh.1002060.
|
| 56. |
Livingston JC, Ahokas R, Haddad B, Sibai BM, Awaads R. Heat shock protein 70 is not increased in women with severe preeclampsia. Hypertens Pregnancy [Internet]. 2002 Jan 1;21(2):123-6. Available from: https://doi.org/10.1081/PRG-120004767.
|
| 57. |
Saghafi N, Pourali L, Ghavami Ghanbarabadi V, Mirzamarjani F, Mirteimouri M. Serum heat shock protein 70 in preeclampsia and normal pregnancy: A systematic review and meta-analysis. Int J Reprod Biomed [Internet]. 2018 Jan;16(1):1-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29707695.
|
| 58. |
Hromadnikova I, Dvorakova L, Kotlabova K, Kestlerova A, Hympanova L, Novotna V, et al. Circulating heat shock protein mRNA profile in gestational hypertension, pre-eclampsia & foetal growth restriction. Indian J Med Res [Internet]. 2016 Aug;144(2):229-37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27934802.
|











