The importance of obesity and carbohydrate metabolism disorders on the course of gastroesophageal reflux disease – a pilot study
Abstract
Introduction
Carbohydrate metabolism disorders, obesity and a severe course of gastroesophageal reflux correlate with more frequent development of esophageal complications. The aim of this study was to assess the influence of obesity and carbohydrate disorders on the characteristics of gastroesophageal reflux disease (GERD).
Methods
The study included 58 patients with excess weight. Anthropometric parameters (including the body mass index, BMI), data regarding GERD (severity of symptoms, gastroscopy and esophageal pH monitoring results) were included in the study. Correlations between obesity and GERD parameters were analyzed. Subjects were divided into a diabetic and a control group and the severity of GERD was compared.
Results
GERD was diagnosed in 40 patients and occurred more frequently in the obese group (73%) than in the overweight group (57%). Increased GERD severity was associated with increased BMI only for postprandial parameters. GERD was diagnosed in most of the group with carbohydrate disorders (78% vs 63% in the non-diabetic group). No differences in the severity of GERD were observed between groups depending on carbohydrate disorders.
Conclusions
In our study, GERD was common in obesity and in diabetic disorders. Increased severity of postprandial reflux was associated with an increased BMI. Diabetic disorders were not associated with more severe GERD.
Citation
Pardak P, Filip R, Krzaczek M. The importance of obesity and carbohydrate metabolism disorders on the course of gastroesophageal reflux disease – a pilot study. Eur J Transl Clin Med. 2022;5(1):17-26Introduction
Factors linked to modern lifestyle, e.g. physical inactivity, overnutrition and poor sleep quality have led to the widespread incidence of obesity, type 2 diabetes (T2DM) and gastroesophageal reflux disease (GERD) [1-5]. In many countries obesity is an important public health problem and its prevalence results in an increase in the incidence of GERD and type 2 diabetes [6-8]. Gastroesophageal reflux disease is a common health problem which still requires research. Depending on the region, the prevalence of GERD is estimated at 10-20% of the population [2, 5]. Overweight, especially abdominal obesity, correlates with the severity of GERD. The main causes of GERD are impaired function of the lower esophageal sphincter, hiatal hernia, impaired esophageal motility and increased intra-abdominal pressure [9-18]. Gastroesophageal reflux disease leads to the development of Barrett's esophagus (BE) or metaplasia, which is a precancerous condition leading to the development of dysplasia and subsequently of esophageal adenocarcinoma (EAC) [4, 19- 20]. Numerous studies have noted that not only the severity of GERD, but the presence of abdominal obesity and T2DM, increase the risk of developing BE and EAC [21-25].
In abdominal obesity, the development of BE and EAC plays a major role in the increased amount of adipokines and inflammatory cytokines produced by visceral adipose tissue, which leads to a chronic inflammatory process, and thus, promotes cancerous transformation [26-28]. Patients with T2DM are often obese, making them more prone to GERD. In T2DM, as in high-grade obesity, the clinical course of GERD is more often atypical. The differences between patients with T2DM result from additional factors contributing to GERD, including esophageal peristalsis disorders, gastroparesis, hyperglycemia, neuropathy and usage of diabetic medications (e.g. GLP-1 receptor agonists and metformin) [29]. The atypical, often mild or asymptomatic course of GERD in T2DM may delay the decision to perform diagnostics, leading to delayed diagnosis of complications, particularly BE and EAC. Therefore, it is important to study the natural course of GERD in obese and T2DM patients in order to reduce the risk of complications through early diagnosis and treatment.
Aim
The aim of our study was to characterize GERD in overweight patients and to assess the correlation between obesity parameters and the severity of GERD. The second aim of this study was to assess the differences in the clinical characteristics of GERD, depending on the diagnosis of carbohydrate disorders, which could explain the increased risk of complications in this group.
Materials and Methods
Study population
Our analysis covers data collected from 58 patients who were diagnosed for sleep breathing disorders at the Department of Internal Diseases of the Institute of Rural Medicine in Lublin. The exclusion criteria were: chronic use of drugs which may interfere with the assessment of GERD parameters (proton pump inhibitors, H2 blockers, alkali, nitrates) or previous significant gastrointestinal surgery (gastrectomy, bowel resection). This patient group also served as study participants in our previous work [30]. We collected anthropometric measurements and the responses from GERD-complaint questionnaires. If GERD was suspected, patients were referred for additional tests (gastroscopy and esophageal pHmeasurement). The results from these additional tests were included in the study.
Criteria for the diagnosis of GERD
The diagnosis of GERD was established according to the definitions of the Lyon consensus [4]. Therefore, the diagnosis was based on the combined assessment of clinical symptoms, endoscopic evaluation of the esophageal mucosa, esophageal pH monitoring and response to therapeutic intervention. Clinical diagnosis was made when the patient had persistent symptoms characteristic of GERD which include heartburn and acid regurgitation. Persistence of symptoms was recognized when symptoms of mild intensity occurred at least 2 days a week or when they were more severe and caused deterioration in general well-being and occurred at least 1 day a week. Moreover, the diagnosis of GERD confirmed the presence of inflammatory changes in the esophagus (LA grade C) and the result of pH-measurement with esophageal acid exposure time > 6%, DeMeester Score > 14.72 or > 80 reflux episodes per 24 hours. Additionally, the diagnosis of GERD was confirmed by the reduction of symptoms after starting treatment with a proton pump inhibitor [4].
Anthropometric data
All participants underwent a physical examination. The body mass index (BMI) was calculated as the body weight in kilograms divided by the height in meters squared (kg / m2). The waist circumference (the circumference at midpoint between the lower border of the rib cage and the iliac crest) was measured in the standing position. Overweight was diagnosed if BMI was in the range of 25-29.9 kg / m2 ; class I obesity when BMI was 30-34.9 kg / m2, class II obesity when BMI was 35-39.9 kg / m2; and class III obesity when BMI was at ≥ 40 kg / m2 [8].
Survey data regarding the severity of GERD complaints
The severity of GERD clinical symptoms was assesed using a questionnaire about the overall intensity of complaints (within a score range of 0-10) and the intensity and frequency of symptoms considered typical for GERD (within a score range of 0-52). GERD symptoms included the feeling of heartburn and presence of regurgitation typical for GERD situations, acid reflux and dysphagia. In addition, the presence of GERD symptoms was assessed at night, during sleep and after an overnight sleeping period. Patients were instructed to describe the symptoms occurring in the month prior to completing the questionnaire. Survey data from all patients were collected by the primary investigator. The full version of the questionnaire is available in the Supplementary Materials [in Polish]: https://ejtcm.gumed.edu.pl/files/67.
Gastroscopy and esophageal pH monitoring
All procedures were undertaken by experienced physicians before the introduction of GERD treatment. All gastroscopic examinations were done using the Fujifilm (Japan or Pentax) (Japan). Whereas the esophageal pH monitoring was done using the ComforTEC Plus PHNS single-channel probe and a recorder made by Sandhill Scientific (USA, REF: Z07-2000-A, SN: H109007C). The degree of esophagitis was determined according to the Los Angeles classification, and numerical values with a range of 1 to 4 were given for subsequent grades (grade A-D); 0 was designated as no inflammatory lesions [4, 10].
Diagnosis of pre-diabetes and type 2 diabetes
In order to assess the importance of carbohydrate disorders on the course of GERD, the subjects were divided into a group with carbohydrate disorders (23 patients) and a control group (35 patients). The group with carbohydrate disorders included patients diagnosed with T2DM, impaired glycemic tolerance (IGT) or impaired fasting glycemia (IFG). In all participants with T2DM, diabetes was well-controlled. Diagnoses of carbohydrate disorders were established before inclusion in the study and were based on the Polish Diabetes Association guidelines [31].
Data Analyses and Statistical Methods
All statistical analyses were carried out with the Statistica software package (version 13, TIBCO Software Inc., USA). After confirming that all variables meet the criteria of a normal distribution, Spearman's rank correlation coefficient was used for the analysis. Correlations between variables were calculated using Spearman’s rank correlation coefficient, while comparisons between two independent groups were performed using the Mann–Whitney U test. P values < 0.05 were considered statistically significant. Because the distribution of GERD measurements was characterized by a large asymmetry, the median should be taken as the key measurement (possibly including the range of variability in the form of IQR) while assessing its severity depending on the presence of diabetic disorders.
Results
Baseline data
The study population consisted of 58 patients (48 males and 10 females) aged 34-75 years (mean = 54.5 years; Me = 56 years; s = 11.2 years). In the study group, obesity was diagnosed in 44 subjects (75.9%). Of these, class I obesity was seen in 17 subjects, class II obesity was seen in 12 subjects, and class III obesity was seen in 15 subjects. Fourteen (24.1%) patients were overweight. The average patient weight was 104 kg (range 77-161 kg), while average waist circumference was 115.5 cm (range 96-147 cm). The mean BMI was 34.8 kg / m2 (range 25.1-49.7 kg / m2). Carbohydrate disorders were reported by 23 subjects (~ 40%), of which 14 subjects (24.1%) had T2DM and 9 subjects (15.5%) had pre-diabetes. Table 1 presents the basic characteristics of the study population.
Table 1. Basic characteristics of the study population, severity of GERD symptoms and distribution of esophageal pH monitoring results
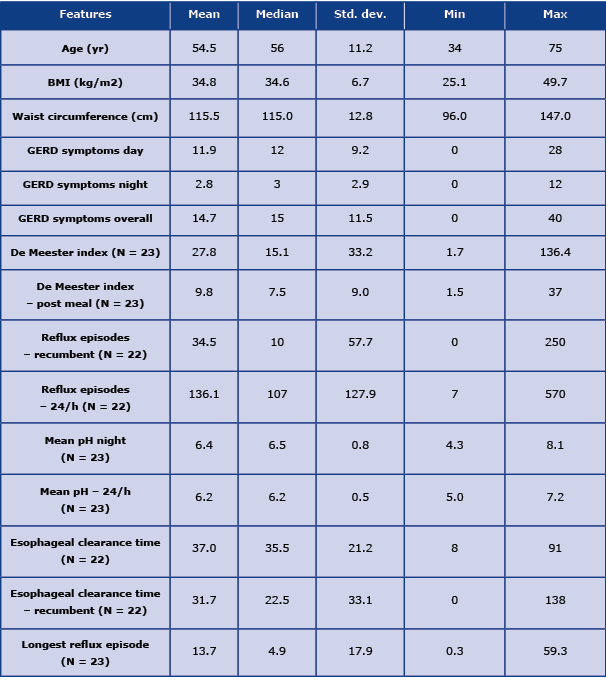
In our study, GERD was diagnosed in 40 patients (~ 69%). In the obese group, GERD was diagnosed in 32 subjects (73%), while the in overweight group, GERD was diagnosed in 8 subjects (57%). Gastroscopy was performed in 32 patients. In 2 cases, no esophageal inflammatory changes were observed. Most of the gastroscopic examinations revealed esophageal inflammatory changes with grade A, B and C of esophagitis recognized in 22, 7, and 1 subject, respectively. Grade D lesions or peptic stricture were not observed in any of the patients. Moreover, neither BE nor EAC were diagnosed. In 23 patients, pH-metry was performed. Table 1 presents the distribution of the variables.
Apart from obesity, carbohydrate disorders and GERD, the majority participants had comorbidities mainly related to the circulatory system. The most common were: arterial hypertension (47 patients, 81%); dyslipidemia (34 patients, 59%), coronary artery disease (16 patients, 28%); hyperuricemia (10 patients, 17%); chronic obstructive pulmonary disease (5 patients, 9%) and heart failure (4 patients; 7%).
Gastroesophageal reflux in obesity and in diabetic disorders
We investigated the relationship between obesity parameter values (body weight, abdominal circumference, and BMI) and GERD severity parameters. Since all considered features were consistent with a normal distribution, Spearman's rank correlation coefficient was used for the analysis. Table 2 presents correlation coefficient values between individual features along with the assessment of their statistical significance. In the studied group, no statistically significant relationships were found between the obesity parameters and the assessed reflux parameters.
Table 2. Distribution of correlations between obesity parameters and the severity of GERD complaints

A tendency toward greater values with increasing obesity parameters was seen only for the “feeling of heartburn after meals” parameter.
The relationships between GERD parameters from gastroscopy, pH measurement and obesity parameters were calculated. Only the correlation between the number of postprandial reflux episodes and BMI was near statistical significance (test probability values p = 0.0084), however its strength was rather small rS = 0.36. In addition, there was a trend toward greater values for the postprandial De Meester index and for the duration of gastric acid exposure with increasing BMI (Table 3).
Table 3. Correlations between GERD parameters from gastroscopy, pH measurement and obesity parameters

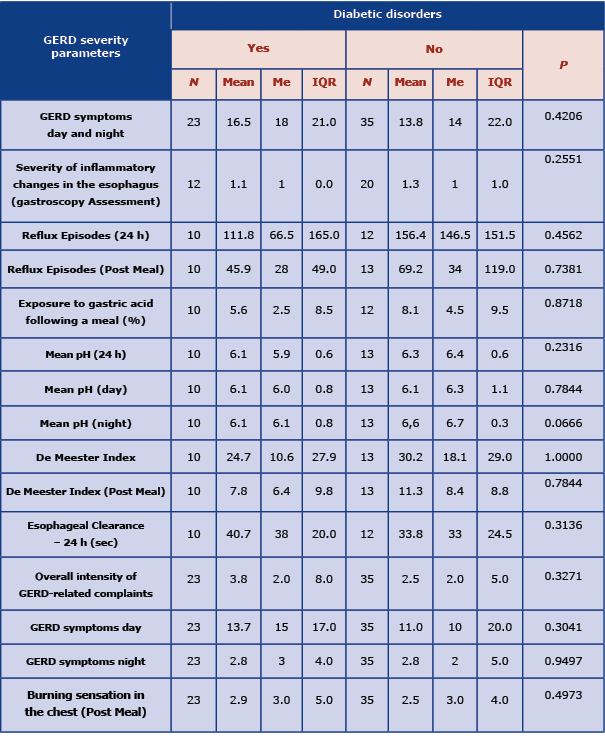
Next, we analyzed the differences between the parameters of GERD severity depending on the diagnosis of carbohydrate disorders. Among the participants with carbohydrate disorders, GERD was diagnosed in 18 patients (78%), of which 12 (86%) had T2DM, and 6 (67%) had pre-diabetes. Whereas in the group without carbohydrate disorders (n=35) GERD was diagnosed in 22 participants (63%). No differences in the severity of GERD were observed between the groups with and without carbohydrate disorders . In our study, the GERD parameters in both groups were very similar (Table 4).
Table 4. Comparison of GERD severity parameters depending on the presence of diabetic disorders (p – test probability values were calculated using the Mann-Whitney test)
The analysis of the probability of GERD depending on the selected variables (BMI, waist circumference, diagnosis of carbohydrate disorders and diagnosis of diabetes) was performed using the logistic regression model. Based on the analyzes performed, no statistical evidence was found that any of the proposed factors had a significant influence on the diagnosis of GERD (Table 5). Moreover, an attempt was made to search for a model containing statistically significant variables. The best model that included only the diagnosis of diabetes was still not statistically significant: OR (95% CI) = 3,429; p = 0.14.
Table 5. Logistic regression model of the probability of GERD diagnosis depending on selected variables
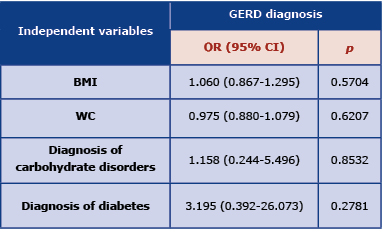
OR – odds ratio; CI – confidence interval; GERD – gastroesophageal reflux disease; BMI – body mass index (kg/m2); waist circumference (cm)
Discussion
All participants in our study were overweight and in this group the occurrence of GERD was much more frequent than in the general population. Obesity is an important risk factor for GERD and numerous studies demonstrated its more frequent occurrence in people with excess weight [11- 17]. In our study, no statistically significant correlation was found between individual obesity measures and GERD parameters. However, there was a trend towards greater severity of postprandial GERD clinical symptoms and worsening of postprandial GERD pH parameters with increasing BMI. Most of the published data demonstrate a more severe course of GERD in people with excess weight.
Obesity, especially the visceral type, causes GERD due to changes in the anatomy and physiology of the gastroesophageal junction (GEJ) [11, 18]. Additionally, it is believed that the pro-inflammatory effects of cytokines synthesized in visceral adipose tissue plays an important role [12, 26]. Akyuz et al. showed a significant correlation between BMI and the severity of GERD in pH measurements. They also found that the severity of esophageal inflammatory changes seen in gastroscopy did not differ significantly in the obese group, however the authors did not assess the severity of GERD in the context of its association with abdominal obesity [11]. In our study, we also did not observe any relationship between obesity and the severity of esophageal inflammatory changes in gastroscopy. The correlation between BMI and GERD severity in pH measurements was also present, but did not reach the level of statistical significance.
We did not observe a correlation between abdominal circumference and the severity of GERD. However, most studies indicate that it does have greater a role than BMI in terms of GERD severity. A study by Wu et al. investigated the correlations of obesity parameters with GERD symptoms and esophageal inflammatory activity via the measurement of glucose metabolism in 18F-Fluorodeoxyglucose positron emission tomography (PET-CT). There was a significant correlation between GERD symptoms and esophageal inflammatory activity in PET-CT with all obesity parameters (BMI, abdominal circumference, and the amount of subcutaneous and visceral adipose tissue) [13]. Nam et al. described the correlations between the amount of visceral fat, concentrations of inflammatory cytokines synthesized within it, and the intensity of esophageal inflammatory changes [12]. Similar results were obtained in large-scale studies in Japan and South Korea. An increased incidence of reflux esophagitis has been observed in obesity and in the metabolic syndrome. Hyperglycemia, high BMI, and in particular, greater abdominal circumference and increased visceral fat, correlated with an increased risk for GERD [14-15].
Gastroesophageal reflux disease is linked to a higher risk of BE [4, 20]. Population-based studies have indicated that the risk of BE and EAC is also significantly increased in obesity, especially in the abdominal type. This risk is increased regardless of the presence of GERD symptoms [21, 23-25]. In a study by Nelsen et al., the risk of developing BE and dysplasia correlated with the amount of visceral adipose tissue and adipose tissue in the GEJ fat area; however, it was independent of the BMI value and the presence of GERD symptoms [24]. Similar conclusions were obtained by El-Seraq et al. [19]. Moreover, in an investigation by Corley et al., abdominal circumference and abdominal obesity (but not BMI) correlated with a greater risk for BE [25].
Gastroesophageal reflux disease is common in T2DM and is more likely to be atypical or present with mild symptoms [29]. In addition, these patients are at an increased risk for developing metaplasia [22]. In our study, no differences in the severity of GERD were found between subjects with T2DM or pre-diabetes and the group without these disorders. Notably, the two groups were very similar in terms of GERD characteristics. Lorentzen et al. compared the features of GERD in patients with a high degree of obesity, depending on the diagnosis of T2DM. As in our study, GERD was more common in the obese group than in the general population. However, a large proportion of the respondents had asymptomatic GERD, regardless of whether or not they suffered from T2DM. In this study, clinical symptoms were reported by approximately 29% of the respondents, but esophagitis in gastroscopy was seen in 58% of patients in the T2DM group and in 47% of patients in the non-T2DM group. Among subjects with inflammatory changes in the esophagus, 68-80% did not report symptoms of GERD. In the T2DM group (only T2DM patients underwent pH-metry), 55% of subjects had pathologic acid reflux, whereas 67% of subjects were asymptomatic [16]. In our study, the severity of GERD clinical symptoms was similar in both groups, which may be due to the lower number of severely obese patients when compared to the cited study.
As reported by Ortiz et al., the asymptomatic course of GERD in obese patients may be related to the decreased esophageal sensitivity to acid content observed in this group. The authors indicated that the absence of typical GERD symptoms in these patients may delay the diagnosis of GERD complications, especially BE [32]. Promberger et al. also observed the frequent occurrence of atypical GERD symptoms in T2DM [33]. Furthermore, Lluch et al. found that GERD was common in diabetic patients, but it was more often asymptomatic [34]. The above conclusions are of clinical significance in the context of a report by Leggett et al, which found an increased risk of BE in patients with metabolic syndrome, regardless of GERD symptoms [23]. The role of carbohydrate disorders in the pathogenesis of GERD is unclear. Gokturk et al. observed a more severe course of GERD in subjects with T2DM, but the presence of reflux episodes was associated with obesity rather than hyperglycemia [17]. In the study by Wang et al., the occurrence of GERD symptoms in diabetes was observed more frequently, and their severity clearly increased in the group of patients with diabetic neuropathy [35].
In our study group, we did not observe an atypical course of GERD in T2DM, but in all cases, these were patients withpre-diabetes or well-controlled diabetes without complications. Although the patients with carbohydrate disorders more often suffer from GERD and its complications, the clinical course does not correlate with the risk of complications. Because of this, they may benefit from early gastroscopic evaluation.
The limitations of our study include the small number of participants and the lack of assessment of other obesity parameters, such as the waist-hip ratio. Moreover, not all patients underwent endoscopic examinations or pH-metry. Another limitation was the inclusion of only hospitalized patients with suspected sleep apnea who were mostly obese, which makes it difficult to transfer the obtained conclusions to the general population. Despite these limitations, the collected results allowed us to demonstrate a greater incidence of GERD in obesity and to show that the presence of carbohydrate disorders was not associated with a more severe clinical course of GERD (in the context of clinical symptoms, changes in gastroscopy, and pH measurement).
Conclusions
In our study group, we observed that GERD is more common in obesity and in T2DM; however, the diagnosis of diabetic disorders was not associated with more severe GERD. Our results and a review of the current literature indicate that due to a mild or atypical course, GERD may be underdiagnosed in the group of severely obese and T2DM patients. Finally, although patients with carbohydrate disorders more often suffer from GERD and its complications, the clinical course does not correlate with the risk of complications, and because of this, these patients may benefit from early gastroscopic evaluation.
Acknowledgments
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the bioethical committee at the Institute of Rural Health (Decision No. 6/2014). Informed consent was obtained from all subjects involved in the study.
Financial Disclosure
None.
Conflicts of interest
There are no conflicts of interest to report for any of the authors.
References
| 1. |
Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. Longo DL, editor. N Engl J Med [Internet]. 2017 Jan 19;376(3):254–66. Available from: http://www.nejm.org/doi/10.1056/NEJMra1514009.
|
| 2. |
El–Serag HB. Time Trends of Gastroesophageal Reflux Disease: A Systematic Review. Clin Gastroenterol Hepatol [Internet]. 2007 Jan;5(1):17–26. Available from: https://linkinghub.elsevier.com/retrieve/pii/S154235650600944X.
|
| 3. |
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract [Internet]. 2010 Jan;87(1):4–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016882270900432X.
|
| 4. |
Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut [Internet]. 2018 Jul;67(7):1351–62. Available from: https://gut.bmj.com/lookup/doi/10.1136/gutjnl-2017-314722.
|
| 5. |
El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut [Internet]. 2014 Jun;63(6):871–80. Available from: https://gut.bmj.com/lookup/doi/10.1136/gutjnl-2012-304269.
|
| 6. |
Promberger R, Spitzer A, Ott J, Lenglinger J, Eilenberg W, Gadenstätter M, et al. Quality of life in type 2 diabetics with gastroesophageal reflux disease: a case control study. Eur Surg [Internet]. 2013 Aug 15;45(4):194–9. Available from: http://link.springer.com/10.1007/s10353-013-0219-7.
|
| 7. |
Hampel H, Abraham NS, El-Serag HB. Meta-Analysis: Obesity and the Risk for Gastroesophageal Reflux Disease and Its Complications. Ann Intern Med [Internet]. 2005 Aug 2;143(3):199–211. Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-143-3-200508020-00006.
|
| 8. |
Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults -The Evidence Report. National Institutes of Health. Obes Res [Internet]. 1998 Sep;6 Suppl 2:51S-209S. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9813653.
|
| 9. |
Savarino E, Bredenoord AJ, Fox M, Pandolfino JE, Roman S, Gyawali CP. Advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol [Internet]. 2017 Nov 27;14(11):665–76. Available from: http://www.nature.com/articles/nrgastro.2017.130.
|
| 10. |
Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol [Internet]. 2022 Jan;117(1):27–56. Available from: https://journals.lww.com/10.14309/ajg.0000000000001538.
|
| 11. |
Akyüz F. Gastroesophageal reflux in asymptomatic obese subjects: An esophageal impedance-pH study. World J Gastroenterol [Internet]. 2015;21(10):3030. Available from: http://www.wjgnet.com/1007-9327/full/v21/i10/3030.htm.
|
| 12. |
Nam SY, Choi IJ, Ryu KH, Park BJ, Kim Y-W, Kim HB, et al. The Effect of Abdominal Visceral Fat, Circulating Inflammatory Cytokines, and Leptin Levels on Reflux Esophagitis. J Neurogastroenterol Motil [Internet]. 2015 Apr 3;21(2):247–54. Available from: http://www.jnmjournal.org/journal/view.html?doi=10.5056/jnm14114.
|
| 13. |
Wu Y-W, Tseng P-H, Lee Y-C, Wang S-Y, Chiu H-M, Tu C-H, et al. Association of Esophageal Inflammation, Obesity and Gastroesophageal Reflux Disease: From FDG PET/CT Perspective. Green J, editor. PLoS One [Internet]. 2014 Mar 18;9(3):e92001. Available from: https://dx.plos.org/10.1371/journal.pone.0092001.
|
| 14. |
Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut [Internet]. 2008 Apr 29;57(10):1360–5. Available from: https://gut.bmj.com/lookup/doi/10.1136/gut.2007.147090.
|
| 15. |
Moki F, Kusano M, Mizuide M, Shimoyama Y, Kawamura O, Takagi H, et al. Association between reflux oesophagitis and features of the metabolic syndrome in Japan. Aliment Pharmacol Ther [Internet]. 2007 Jul 26;26(7):1069–75. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2007.03454.x.
|
| 16. |
Lorentzen J, Medhus AW, Hertel JK, Borgeraas H, Karlsen T-I, Kolotkin RL, et al. Erosive Esophagitis and Symptoms of Gastroesophageal Reflux Disease in Patients with Morbid Obesity with and without Type 2 Diabetes: a Cross-sectional Study. Obes Surg [Internet]. 2020 Jul 19;30(7):2667–75. Available from: http://link.springer.com/10.1007/s11695-020-04545-w.
|
| 17. |
Gokturk S, Akyuz F, Arici S, Alpaslan B, Ormeci A, Soyer OM, et al. Gastroesophageal Reflux in Asymptomatic Patients with Diabetes: An Impedance Study Diabetes, Obesity and Gastroesophageal Reflux. Exp Clin Endocrinol Diabetes [Internet]. 2020 Jan 20;128(01):52–8. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/a-0783-2327.
|
| 18. |
Pandolfino JE, El–Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: A Challenge to Esophagogastric Junction Integrity. Gastroenterology [Internet]. 2006 Mar;130(3):639–49. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508505025266.
|
| 19. |
El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal Obesity and the Risk of Barrett’s Esophagus. Am J Gastroenterol [Internet]. 2005 Oct;100(10):2151–6. Available from: https://journals.lww.com/00000434-200510000-00004.
|
| 20. |
Eusebi LH, Cirota GG, Zagari RM, Ford AC. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut [Internet]. 2021 Mar;70(3):456–63. Available from: https://gut.bmj.com/lookup/doi/10.1136/gutjnl-2020-321365.
|
| 21. |
Singh S, Sharma AN, Murad MH, Buttar NS, El–Serag HB, Katzka DA, et al. Central Adiposity Is Associated With Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol [Internet]. 2013 Nov;11(11):1399-1412.e7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1542356513006885.
|
| 22. |
Iyer PG, Borah BJ, Heien HC, Das A, Cooper GS, Chak A. Association of Barrett’s Esophagus With Type II Diabetes Mellitus: Results From a Large Population-based Case-Control Study. Clin Gastroenterol Hepatol [Internet]. 2013 Sep;11(9):1108-1114.e5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1542356513004643.
|
| 23. |
Leggett CL, Nelsen EM, Tian J, Schleck CB, Zinsmeister AR, Dunagan KT, et al. Metabolic Syndrome as a Risk Factor for Barrett Esophagus: A Population-Based Case-Control Study. Mayo Clin Proc [Internet]. 2013 Feb;88(2):157–65. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025619612010968.
|
| 24. |
Nelsen EM, Kirihara Y, Takahashi N, Shi Q, Lewis JT, Namasivayam V, et al. Distribution of Body Fat and Its Influence on Esophageal Inflammation and Dysplasia in Patients With Barrett’s Esophagus. Clin Gastroenterol Hepatol [Internet]. 2012 Jul;10(7):728–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1542356512003151.
|
| 25. |
Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, et al. Abdominal Obesity and Body Mass Index as Risk Factors for Barrett’s Esophagus. Gastroenterology [Internet]. 2007 Jul;133(1):34–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508507008323.
|
| 26. |
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Metab [Internet]. 2001 May 1;280(5):E745–51. Available from: https://www.physiology.org/doi/10.1152/ajpendo.2001.280.5.E745.
|
| 27. |
Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr [Internet]. 2007 Sep 1;86(3):556–65. Available from: https://academic.oup.com/ajcn/article/86/3/556/4649411.
|
| 28. |
Aune D, Greenwood DC, Chan DSM, Vieira R, Vieira AR, Navarro Rosenblatt DA, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose–response meta-analysis of prospective studies. Ann Oncol [Internet]. 2012 Apr;23(4):843–52. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0923753419346599.
|
| 29. |
Punjabi P, Hira A, Prasad S, Wang X, Chokhavatia S. Review of gastroesophageal reflux disease (GERD) in the diabetic patient. J Diabetes [Internet]. 2015 Sep;7(5):599–609. Available from: https://onlinelibrary.wiley.com/doi/10.1111/1753-0407.12279.
|
| 30. |
Pardak P, Filip R, Woliński J, Krzaczek M. Associations of Obstructive Sleep Apnea, Obestatin, Leptin, and Ghrelin with Gastroesophageal Reflux. J Clin Med [Internet]. 2021 Nov 7;10(21):5195. Available from: https://www.mdpi.com/2077-0383/10/21/5195.
|
| 31. |
Czupryniak L. Zalecenia Polskiego Towarzystwa Diabetologicznego w 2021 roku – by cukrzycę lepiej rozpoznawać, skuteczniej leczyć i zapobiegać jej przewlekłym powikłaniom. Med Prakt Diabetol [Internet]. 2021;(3):44–52. Available from: https://diabetologia.mp.pl/wytyczne/260808,zalecenia-polskiego-towarzystwa-diabetologicznego-2021.
|
| 32. |
Ortiz V, Alvarez-Sotomayor D, Sáez-González E, Díaz-Jaime FC, Iborra M, Ponce J, et al. Decreased Esophageal Sensitivity to Acid in Morbidly Obese Patients: A Cause for Concern? Gut Liver [Internet]. 2017 May 15;11(3):358–62. Available from: http://www.gutnliver.org/journal/view.html?doi=10.5009/gnl16081.
|
| 33. |
Promberger R, Lenglinger J, Riedl O, Seebacher G, Eilenberg WH, Ott J, et al. Gastro-oesophageal reflux disease in type 2 diabetics: symptom load and pathophysiologic aspects - a retro-pro study. BMC Gastroenterol [Internet]. 2013 Dec 23;13(1):132. Available from: https://bmcgastroenterol.biomedcentral.com/articles/10.1186/1471-230X-13-132.
|
| 34. |
Lluch I, Ascaso JF, Mora F, Minguez M, Peña A, Hernandez A, et al. Gastroesophageal Reflux in Diabetes Mellitus. Am J Gastroenterol [Internet]. 1999 Apr;94(4):919–24. Available from: https://journals.lww.com/00000434-199904000-00019.
|
| 35. |
Wang X. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol [Internet]. 2008;14(5):709. Available from: http://www.wjgnet.com/1007-9327/14/709.asp.
|











