SARS-CoV-2 infection in vaccinated maintenance hemodialysis patients despite anti-spike seroconversion: a report of 3 breakthrough cases
Abstract
Chronically hemodialyzed (HD) patients are vulnerable population during a SARS-COV-2 pandemic. They are at high risk of developing very severe forms of COVID-19 disease. In this article we describe, for the first time to our knowledge, three HD patients (all males, aged 70, 70 and 74 years) vaccinated intramuscularly with two doses of the mRNA BNT162b2 vaccine (BionTech/Pfizer Comirnaty) in whom subsequent breakthrough SARS-CoV-2 infections developed. All patients achieved post-vaccine seroconversion for anti-spike antibodies with IgG titers of 227 AU/mL (cut-off, 13 UA/ml). SARS-CoV-2 infection was diagnosed 28, 44 and 48 days after the second dose of BNT162b2 and confirmed with the polymerase-chain-reaction (PCR) test. Two patients were asymptomatic of COVID-19 and didn't require hospitalization. The third patient reported only non-significant drop in oxygen saturation and was hospitalized. All patients were characterized by a moderate or even low post-vaccination neutralizing antibody titer but on the contrary a high production of these antibodies after infection. Perhaps this production of antibodies by memory B cells is responsible for the mild course of the disease and the likely reduction of mortality. These breakthrough cases in no way undermine the importance of the vaccinations and on the contrary, argue for their urgency.
Citation
Biedunkiewicz B, Tylicki L, Puchalska-Reglińska E, Dąbrowska M, Ślizień W, Kubanek A, Rąbalski Ł, Kosiński M, Grzybek M, Renke M, Dębska-Ślizień M A. SARS-CoV-2 infection in vaccinated maintenance hemodialysis patients despite anti-spike seroconversion: a report of 3 breakthrough cases. Eur J Transl Clin Med. 2022;5(1):12-16Patients chronically hemodialyzed (HD) in-center are a unique and vulnerable population during a SARS-COV-2 pandemic. Due to the high rate of comorbidity, older age and impaired immunity, they are at high risk of developing very severe forms of COVID-19 disease with fatality rates varying from 16% to 32% [1-2]. Consequently, in most countries HD patients are prioritized to receive vaccines against COVID-19. Vaccinations follow the same schedule as in people without chronic kidney disease. Recently, we showed that the majority of dialyzed patients achieve high seroconversion rates after full vaccination with BNT162b2, with few and mild side effects [3-5]. On the other hand, Grupper et al. demonstrated that such vaccine seroconversion is definitely lower than that observed in the general population [6]. Therefore, it is uncertain whether vaccinating with standard schedules will result in sufficient immune response in this population and, by consequence, protection against infection.
In this report, we describe our experience with 3 breakthrough SARS-COV-2 cases in HD patients. Their characteristics are presented in table 1. They had received a two-dose vaccination with the mRNA BNT162b2 vaccine (BionTech/Pfizer Comirnaty) intramuscularly with a 3-week interval between the first and the second doses. All our patients were routinely monitored for anti-spike and anti-nucleocapsid (N)-specific antibody titer before the vaccination, after the first, and the second, dose of BNT162b2. The patients achieved post-vaccine seroconversion for anti-spike antibodies with IgG titers of 227 AU/ mL (cut-off, 13 UA/mL); 452 AU/mL and 92.5 AU/mL, assessed 14-21 days after the second dose of BNT162b2 using chemiluminescent immunoassay (The LIAISON® SARSCoV-2 Trimetric-S IgG, test Diasorin, Italy). SARS-CoV-2 infection was diagnosed 28, 44 and 48 days after the second dose of BNT162b2 and confirmed with the polymerase-chain-reaction (PCR) test. Viral genome sequencing performed in one patient revealed B.1.1.7 – currently the most common variant in Poland.
Table 1. Demographic, clinical, laboratory and radiological features
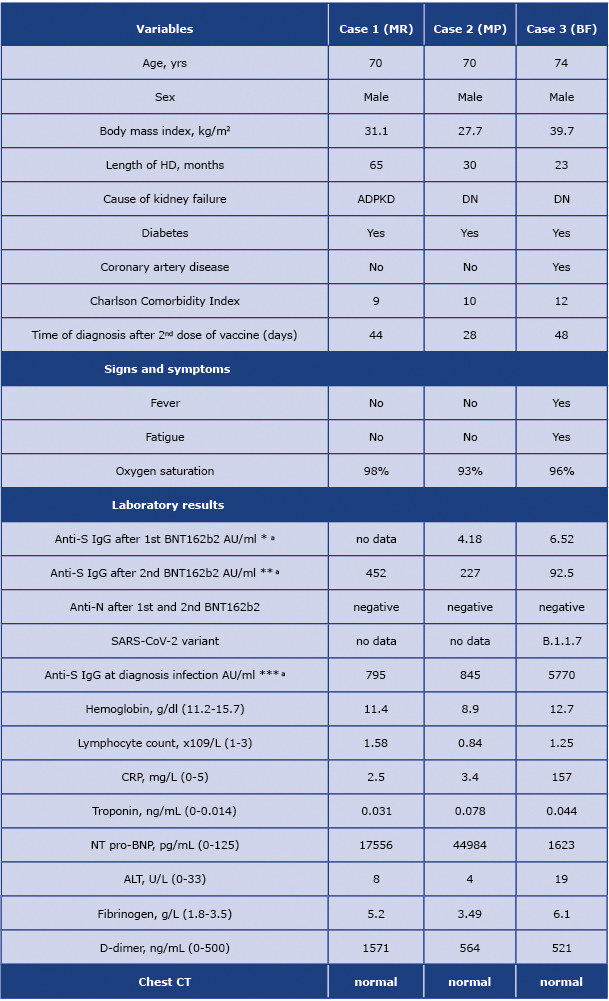
DN, diabetic Nephropathy; ADPKD, autosomal dominant polycystic kidney disease; * 21 days after the first dose of vaccine; ** 14-21 days after the second dose of vaccine; *** in the first week after diagnosis of breakthrough infection; a - to convert AU/ml into BAU/ml multiple by 2.6
All three patients were male and suffered from diabetes, two of them had diabetic nephropathy as the underlying renal disease. The Charlson Comorbidity Index was high in all of them, and ranged from 9 to 12. The patients had negative computed tomography. Two patients did not have any symptoms of SARS-CoV-2 infection and did not require hospitalization. The third patient reported fever, fatigue, and a temporary, non-significant drop in oxygen saturation. He was the only one with an elevated inflammation marker (CRP) and was hospitalized. He received antiviral treatment with remdesivir (total dose 600 mg), dexamethasone (6 mg for 10 days), LMWH (enoxaparin 40 mg) and an antibiotic (ceftriaxone). The patient showed a quick improvement in his general condition and on the twentieth day of his hospitalization, he clinically recovered and was discharged home.
In terms of laboratory findings, all patients showed a high titer of anti-spike IgG antibodies, elevated d-dimer level and significantly increased NT pro-BNP, at the time of diagnosis. Fibrinogen level elevation was detected in two out of three patients and only one patient had lymphopenia (lymphocytes < 1.0 x 109 /L). Oxygen therapy was not required in any of the patients. We observed SARS-CoV-2 clearance (PCR negative) in all patients within 4 weeks of infection. None of the patients had developed any serious complications, and no residual symptoms were observed in any of them.
To our knowledge, this is the first description of three fully vaccinated HD patients in whom subsequent breakthrough SARS-CoV-2 infections developed. It is noteworthy that the infection occurred in patients with confirmed vaccine seroconversion to spike protein. Previous studies demonstrated that sera from individuals vaccinated with BNT162b2, were similarly potent against the B.1.1.7 variant when compared to the reference D614G strain of virus [7]. Although rare, breakthrough infection can occur because vaccines against SARS-CoV-2 do not offer 100% protection according to the pivotal studies. In the recent study the breakthrough infection rate among 2916 fully vaccinated residents of skilled nursing facilities and staff members did not exceed 1%. Like our report, the majority of the cases were asymptomatic, the rest had a mild or moderate course [8]. Interestingly, all our patients were characterized by a moderate or even low post-vaccination neutralizing antibody titer but on the contrary a high production of these antibodies after becoming infected. Perhaps this production of antibodies by memory B cells is responsible for the mild course of the disease and the likely reduction of mortality. That’s why these breakthrough cases in no way undermine the importance of the vaccinations and on the contrary argue for their urgency. However, the described cases in this report raise several new questions: (i) is the breakthrough infection due to the decreased immunity of HD patients or rather immune evasion of new variants, and their ability to create higher viral loads? [9]; what is the optimal vaccination schedule for HD patients? and what is the titer of neutralizing antibodies that protects patients against COVID-19? Clinical studies answering these questions should be a priority.
Disclosures
All authors have no conflicts of interest to disclose.
Funding
None.
Conflicts of interest
None.
References
| 1. |
Francis A, Baigent C, Ikizler TA, Cockwell P, Jha V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: a call to action. Kidney Int [Internet]. 2021 Apr;99(4):791–3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0085253821001770.
|
| 2. |
Puchalska-Reglińska E, Dębska-Ślizień A, Biedunkiewicz B, Tylicki P, Polewska K, Jagodziński P, et al. Extremely high mortality rates among hemodialysis patients with COVID-19 before the era of SARS-CoV-2 vaccination: results from a large database from the North of Poland. Polish Arch Intern Med [Internet]. 2021;131(7–8):643–8. Available from: http://europepmc.org/abstract/MED/34105917.
|
| 3. |
Tylicki L, Biedunkiewicz B, Dąbrowska M, Ślizień W, Tylicki P, Polewska K, et al. Humoral response to SARS-CoV-2 vaccination promises to improve the catastrophic prognosis of hemodialysis patients as a result of COVID-19. The COViNEPH Project. Polish Arch Intern Med [Internet]. 2021 Aug 5; Available from: https://www.mp.pl/paim/issue/article/16069.
|
| 4. |
Tylicki L, Piotrowska M, Biedunkiewicz B, Zieliński M, Dąbrowska M, Tylicki P, et al. Humoral response to COVID-19 vaccination in patients treated with peritoneal dialysis: the COViNEPH Project. Polish Arch Intern Med [Internet]. 2021 Oct 11; Available from: https://www.mp.pl/paim/issue/article/16091.
|
| 5. |
Polewska K, Tylicki P, Biedunkiewicz B, Rucińska A, Szydłowska A, Kubanek A, et al. Safety and Tolerability of the BNT162b2 mRNA COVID-19 Vaccine in Dialyzed Patients. COViNEPH Project. Medicina (B Aires) [Internet]. 2021 Jul 19;57(7):732. Available from: https://www.mdpi.com/1648-9144/57/7/732.
|
| 6. |
Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin J Am Soc Nephrol [Internet]. 2021 Jul 1;16(7):1037 LP – 1042. Available from: http://cjasn.asnjournals.org/content/16/7/1037.abstract.
|
| 7. |
Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med [Internet]. 2021;27(5):917–24. Available from: https://doi.org/10.1038/s41591-021-01318-5.
|
| 8. |
Teran RA, Walblay KA, Shane EL, Xydis S, Gretsch S, Gagner A, et al. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members — Chicago, Illinois, December 2020–March 2021. Am J Transplant [Internet]. 2021 Jun 1;21(6):2290–7. Available from: https://doi.org/10.1111/ajt.16634.
|
| 9. |
G. DN, Sam A, C. BR, I. JC, J. KA, D. MJ, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science (80- ) [Internet]. 2021 Apr 9;372(6538):eabg3055. Available from: https://doi.org/10.1126/science.abg3055.
|







