Application of X-ray photoelectron spectroscopy (XPS) and a functional near infrared spectroscopy (fNIRS) in medicine
Abstract
The development of modern clinical medicine and the truthful medical diagnosis require hard physicochemical data. Furthermore, the social demands for more accurate and collected data, which refer to human health in real conditions and in real time, force the quest for techniques which would be possible to be applied in a broadly understood clinical field. In this article two techniques are described. A high vacuum technique which is an X-ray photoelectron spectroscopy (XPS), and a functional near infrared spectroscopy (fNIRS), which might be applied in vivo condition. For the both techniques, the results pointing their applicability in the clinical and modern biochemical research are described.
Citation
Szklarczyk M. Application of X-ray photoelectron spectroscopy (XPS) and a functional near infrared spectroscopy (fNIRS) in medicine. Eur J Transl Clin Med. 2021;4(2):86-93Introduction
With the development in science, technique and technology, and above all people’s anticipation and hopes for information about the quality of available products and information about the surrounding environment, as well as the knowledge of our own health condition, a strong demand has arisen for chemical imaging techniques at the end of the 20th century. Imaging techniques permit not only excellent structural research, qualitative and quantitative markings, but they also allow for determination of spatial placement of different substances in the tested samples. Furthermore, due to the eldering of the population and the increasing intensity of brain diseases, there is a call for techniques that allow real-time brain testing, in vivo.
At the turn of the nineteenth and twentieth centuries there was a period of a stormy development in physics and chemistry. At the time many theories were formulated, which have been the basis of modern physicochemical analytical methods. Thanks to the progress in science in the first half of the 20th century, it became possible to design many new solutions in the field of spectroscopy, chromatography and microscopy. The second half of the 20th century appeared as an avalanche development in the production of analytical apparatus enabling the analysis of a chemical composition at an unprecedented level of concentrations even to attmo mol (10-18 – MALDI-TOF spectroscopy). During this period the introduction of the photoelectron spectroscopy method (XPS – X-ray Photoelectron Spectroscopy was a milestone. XPS is a vacuum technique (10-13 Bar), which enables the simultaneous determination of both the qualitative and quantitative elemental chemical composition and the type of bonds between the elements in the samples; both on their surface and in their bulk without having to dissolve them [1]. Another significant achievement at the end of the 20th century was to prove that the non-invasive technique of near-infrared spectroscopy (NIRS –Near InfraRed Spectroscopy) might be used to study the brain activity. It can be applied to study depression, dementia and Alzheimer's disease (atherosclerotic dementia). This technique used in real conditions is called functional technique, i.e. fNIRS [2-3]. The development of medicine, science, technology and environmental protection possibilities in the 21st century depends on the availability of physicochemical spatial information.
The development of modern medicine is increasingly dependent on the availability of information concerning the site of changes and the site of accumulation of a given metabolite in the body, the type of organ and the place where some chemicals accumulate. The knowledge of change of chemical identification and a spatial location of proteins in the human body may enable the development of a method of spatially targeted therapy as well as the preparation for appropriate drugs.
In this article two methods are presented, XPS and fNIRS which for several years become increasingly popular in a widely understood medical field. The first one is a classic in vitro vacuum technique, while the second one is an in vivo technique. These both techniques are discussed in this paper to point out that a similar physical phenomenon might be used in different areas and in different way. This phenomenon is an interaction of incoming radiation with matter. In the case of XPS technique it is X-ray radiation, while in the case of fNIRS technique it is near IR radiation. In both cases, as the result of these interactions, the radiation from the studied sample is outgoing either corpuscular one (XPS) or wave one (fNIRS). The common denominator for both techniques is therefore the applicability of the universal Lambert-Beer equation [4-7] given in the general form as
I/I0 = exp(-axl)
where I is intensity of the radiation coming out from the sample, I0 is intensity of incident radiation, a is so called linear (napierian) absorption coefficient of the radiation and l is the way in which radiation interacts with matter of the sample. The proper description of a coefficient is the most important and difficult in both techniques.
X-ray photoelectron spectroscopy (XPS)
The XPS method uses the effect of electrons ejected from the material during exposure to X-ray radiation. The X-ray radiation transmits its energy to the electrons in the sample during the interaction with the sample. As a result of this process, the electron is kicked out of the sample. The electron obtained in this way is called a photoelectron. Irradiation of the material by X-rays might result in ejected photoelectrons from both; high-energy internal electronic levels as well as outer low-energy valence levels [8-9] (Fig. 1A).
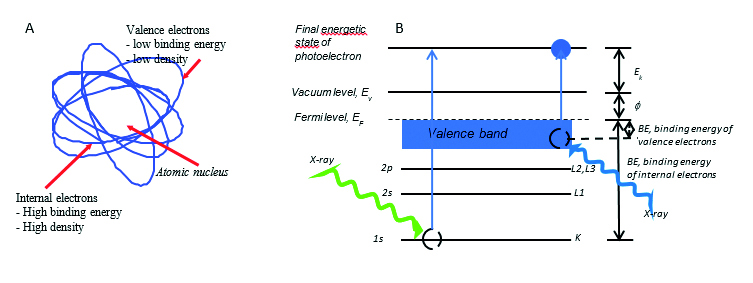
Fig. 1A-B. A. Schematic representation of the structure of the atom. B. Energy scheme of the process of striking the photoelectron from the inner shell (left side of the drawing) and valence band (right side of the drawing). Description: Ev –vacuum level, EF – Fermi level, Ek – electron kinetic energy, ɸ – electronic work function, BE – electronic binding energy, 1s / K, 2s / L1, 2p / L2, L3 – electron shell description
Because the number of internal (core) electrons is usually much higher than valence electrons, the XPS technique of internal electrons is characterized by much a greater sensitivity than the XPS technique of valence electrons. The sensitivity that can be achieved is even tenths of an atomic percentage. On the other hand, valence electrons have more specific energy, i.e. they carry more information about the sample material so that the XPS valence electron technique can be used even to distinguish isomers. The energy of the recorded radiation of photoelectrons enables the chemical identification of atoms and bonds, while its intensity allows the determination of a chemical concentration.
When considering the applicability of the XPS method, it should be taken into account that the phenomenon of ejecting a photoelectron is a one-stage phenomenon (Fig. 1B). The equation of the energy balance of the process of striking of photoelectron by X-ray radiation of hv energy, for example from the 1s shell (K shell) can be represented as follows:
Ek = hv - (EB,K + ɸ)
The EB,K symbol means the electron binding energy at the 1s shell. The experimentally measured kinetic energy of the electron, Ek, is therefore determined by the energy of one electron level, which allows both; an accurate experimental measurement and a theoretical calculation.
The XPS technique is extremely attractive for a chemical analysis and an imaging of sample surfaces. Although the X-ray ionizing sample penetrates the tested material relatively deeply, but the measured objects are photoelectrons, and their so-called free exit way, often called the escape way, due to their much bigger absorbance factor comparing to X-ay radiation penetrates much narrowed layer of studied material. Most often it is below 2 nm, thanks to which the chemical state of even mono layers can be tested with a proper measurement design.
The energy of ejected photoelectrons depends on such physicochemical factors as the absorption coefficient of X radiation and photoelectron, the interaction of photoelectron with other electrons and atoms, the entropy of elemental composition, or the surface roughness. As a result of these dependencies, the energy of the tested photoelectrons coming out of the real sample differs from the energy of those coming out of the pure elemental or calculated sample. These energy differences are called chemical shifts and allow to obtain some information about chemical bonds, concentration and composition dependence on distance from the surface.
The importance of an accurate determination of the chemical energy shift increases with the decreasing of a chemical composition and a spatial distribution diversity variation of individual components in the studied sample. For inorganic samples of large atomic diversity, so also large differences in energy of ejected photoelectrons, it is less important than for organic samples (medicine, biology, biochemistry, biophysics) which mainly consists of carbon and hydrogen, and low atomic concentrations of such elements like oxygen, nitrogen, sulfur and sometimes a limited type of metal. In this case, the ability to determine the energy shifts of the photoelectron accurately is crucial to determine the composition of the sample.
In Fig. 2 the chemical formula and the PET polymer sample spectra is presented [9]. The PET molecule consists of three types of carbon atoms and two types of oxygen atoms, which are perfectly distinguished on the attached spectra due to the possibility of using a high-class XPS spectrometer. The energy differences between the individual peaks are in the order of 1 eV, i.e. 1.602 × 10-19 J, so these are extremely small differences, but thanks to the use of a suitable detection system in this case they are clear.
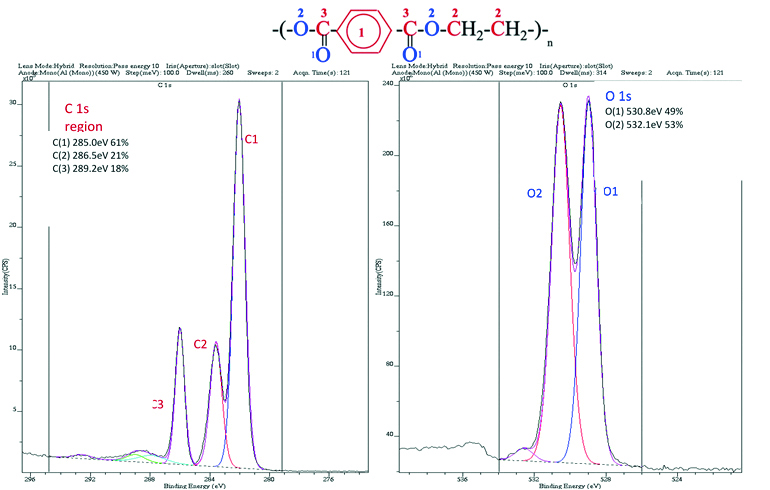
Fig. 2. Chemical formula and PET polymer sample spectra. Numeric energy values for individual types of labeled elements as well as their atomic concentrations are given
The high accuracy of determining the energy of currently produced XPS spectrometers allows, with the help of an appropriate software, to determine even the position of atoms in the Z direction (vertically to the surface of the sample) [10]. In Fig 3. the spectrum in the Z axis direction is shown for the mono layer of the self-organizing film (SAM) containing iron and nitrogen. These results show that the spatial accuracy in the Z axis can be of the order of parts of the nanometer.

Fig. 3. Example of determining the position of atoms in the Z direction for molecule shown at left side of the figure
The great possibilities of the XPS method technique have enabled its application to one of the most difficult samples, i.e. the characteristics of medical samples.
XPS analysis of drug-coated surgical stents
One of the biochemical problems in the use of tubes/stents in cardiac surgery is the pathological response of the human immunological system, which results in the occurrence of thrombosis and hypertrophy, especially in the case of steel stents. Currently, polymer stents made of lactic acid polymer (polylactic acid [PLA]) strengthened with C51HxNO13 compound, are increasingly used and protected by applying special layers about 1.8 µm thick on drugs surface. The key to determining the state of stents is determining the C: O: N ratio indicating their durability or biochemical resistance. In Fig. 4 the results of such tests carried out by means the XPS technique are shown.

Fig. 4A-C. A. Schematic appearance of the B stent. XPS spectrum of the stent sample. C. Dependence of the concentration of elements C, O and N from the distance (C –blue, O –black and N – red). With permission of Kratos Analytical, Manchester, England
XPS analysis of polymer surgical meshes
Woven fabrics of polymer meshes, made of polypropylene (PP) and polyester (PE), are used in a hernia surgery as well as in a surgery for other soft tissues to secure their positioning. The problem which often is faced up is infections and inflammation. The use of plasma functionalization of the applied fabrics as well as the deposition of various adsorbates on the surface of polymer fabrics are tested and used due to the possibility of improving patient’s safety. This type of the procedure results in the preparation of the surface that reduces the adhesion of bacterial biofilms. One possible coating is a polyethylene glycol (PEG) film.
In Fig. 5 results of XPS analysis of a medical fabric covered with PEG are presented [11].
The chemical map shown in Fig. 5 was constructed on the basis of testing C-O bonds concentrations, binding in PEG and C-C bonds, binding in PP. A patient’s safety is likely to increase with PEG concentration, i.e. such tests may be extremely useful for manufacturers of medical fabrics as control tests and in research and the development of the field.
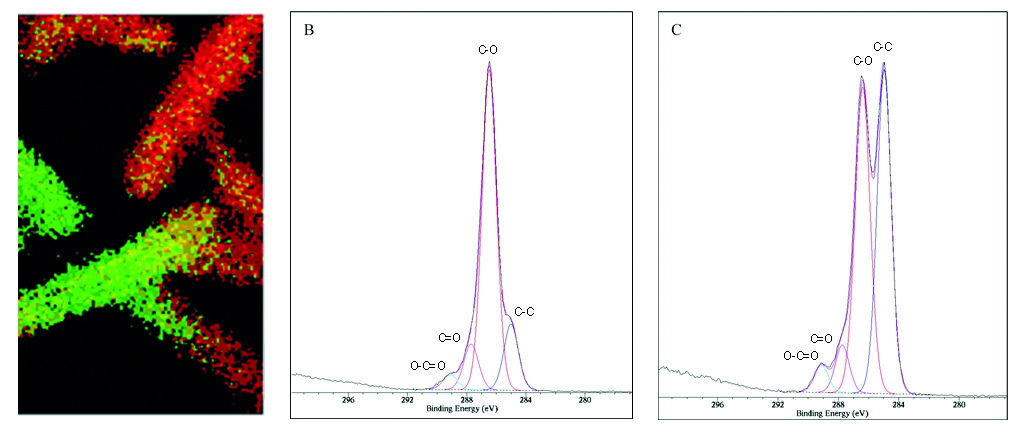
Fig. 5A-C. XPS map of C 1s signals of C-C and C-O bonds for PEG polymer embedded on PP polymer for surgeon mesh. Green regions: good coverage by PEG (high C-O concentration) showed in spectrum B, red regions: poor coverage by PEG (high C-C concentration) showed in spectrum C, respectively. Image 0.8 x 0.8 mm
XPS analysis of caffeine-containing tablets
The XPS technique can also be widely used in pharmacy and the control of manufactured drugs. Fig. 6 shows the three-dimensional distribution of substances which contained in the caffeine tablet.

Fig. 6. Three-dimensional distribution of elements in a tablet containing caffeine. Red, blue and yellow indicate the presence of carbon, oxygen and nitrogen, respectively. Nitrogen is recognized as an indicator of caffeine. With permission of Kratos Analytical, Manchester, England
Functional near infrared spectroscopy method, fNIRS
The 21st century is said to be the age of the brain research. Experimental neurological tests and advanced analytical interdisciplinary techniques will allow enormous progress in knowledge of the mechanism of the brain action under both; static and dynamic conditions. It is anticipated that this progress will influence both; basic and clinical knowledge. This progress may push ahead the preparation of therapy for brain’s and thus nervous system diseases treatment, which will result in psychological, psychiatric, neurological and rehabilitation assistance for hundreds of millions of people. It will be possible to study the processes of thinking, remembering, recognizing, learning, feeling emotions, fatigue or making decisions.
Methods traditionally used to study brain activity processes are electroencephalography (EEG), functional magnetic resonance imaging (fMRI), positron tomography (PET), magneto encephalography (MEG), computed tomography (CAT), by cranial magnetic stimulation (TMS). The functional near infrared (fNIRS) method is new and rapidly gains popularity.
The human body consisting of both soft and hard tissues, is avery good absorber of electromagnetic radiation so it does not pass radiation. However, it turns out that there is a so-called optical window in the range of about 650-950 nm, thanks to which it is possible to examine the light absorption by compounds in the depth of the body when electron transitions are in this energy range. This phenomenon was observed in 1977 by Franz Jobsis during tissue tests [2]. The optical window is formed by radiation absorption on the short wavelength side by oxygenated hemoglobin (deoxy-Hb), and by water (H2O) on the long wavelength side, Fig. 7.
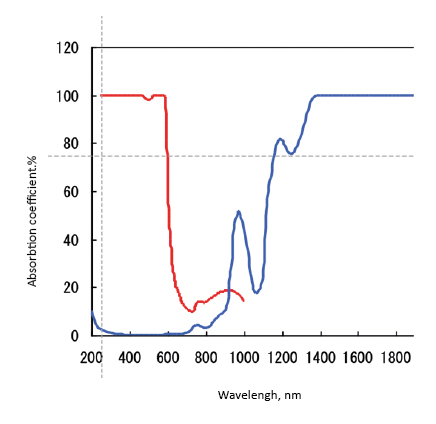
Fig. 7. Spectrophotometric window permeability for infrared radiation of human skull
Measurements using the fNIRS technique involve monitoring the amount of absorbance which results from deoxy-Hb concentration, at the wavelength of about 780 nm, oxy-Hb, at the wavelength of about 830 nm, and for so-called. isobestic point, at 805 nm. At this wavelength, deoxy-Hb and oxy-Hb absorbance should be equal and this point is the reference point (Fig. 7). The knowledge of oxy-Hb and deoxy-Hb absorption coefficients makes it possible to calculate their concentrations and their changes, depending on the object activity. Studying changes in concentration over time allows to determine the effect of mental or physical activity on processes taking place in the brain [12].
The fNIRS method is a non-invasive method, which uses harmless infrared radiation and has significantly less restrictions than other brain imaging methods. This method can be used for both; stationary and mobile objects, for example for children playing, people undergoing rehabilitation, drivers, pilots or athletes. Among brain imaging methods, it is also a cheap method that is easy to be used simultaneously with other methods, such as EEG, so that the two types of tests can be done at the same time. The results obtained using the fNIRS method could also be easily correlated with the results of other methods such as fMRI or PET.
Fig. 8 schematically presents the principle of fNIRS measurement. With the help of optical fibers, light with adequate energy is brought to the head and discharged after interaction with brain tissue.
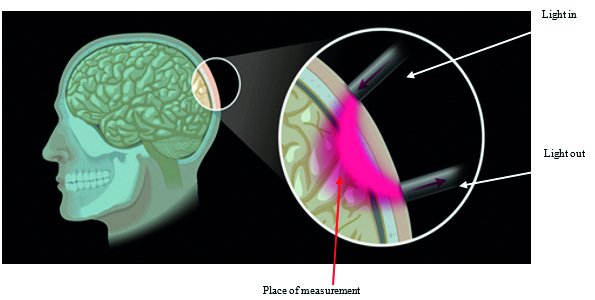
Fig. 8. Graphic representation of the principle of fNIRS measurement of brain activity. With permission of Shimadzu Europe, Duisburg, Germany
Physical activity is one of the methods of monitoring objects after a stroke. In Fig. 9 an example of changes in the brain activity which depends on the time of rehabilitation involving a walk on a treadmill is given [13]. The increasing the area of the activity dependent on exercise time is clearly visible.
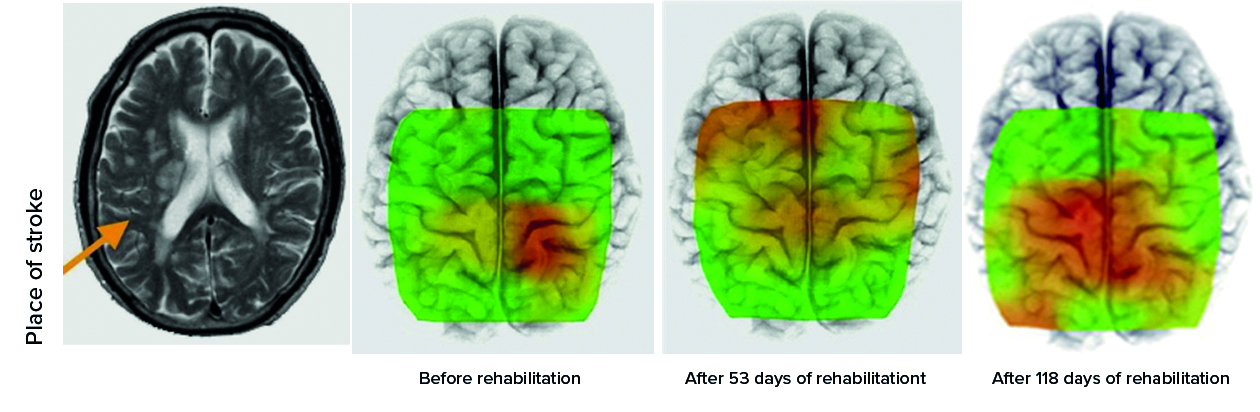
Fig. 9. Studies of influence of time of physical activity on activity of brain
The fNIRS technique can be used practically in all areas, when the brain activity changes, sensitivity to smells, noise, fatigue, impact of security systems on the condition of drivers, or even in studies of differences between diseases such as depression, dementia, or atherosclerotic dementia. Therefore, the technique will definitely become an important tool in psychology and psychiatry.
In Fig. 10 there is given an example presenting the results of the fNIRS studies on distinguishing between objects with depression (D) and Alzheimer’s disease (AD). The VFT (verbal fluency task) test shows significant differences between the healthy controls (HC) and patients with depression and Alzheimer’s disease particularly for frontal cortex studies [14]. Cortical activation for the D and AD group is decreasing in comparison with healthy controls (Fig. 10A, upper row). In the case of parietal cortex, the differences are much smaller (Fig. 10A, lower row). The results of VT (visuospatial task) test shows that the differences in brain activity between healthy and depressed objects are rather small, while between these two groups and AD objects, they are much more significant (Fig. 10B). Summarizing these results, it is clear that fNIRS technique is capable of distinguishing differences in brain activation between healthy, depressed and Alzheimer’s objects.

Fig. 10A-B. Topography of cortical activation in the three tested participant groups; HC – healthy controls; D – depression; AD – Alzheimer’s disease. Superimposed fNIRS images on 3-D MRI represent cortical activation in the verbal fluency task (A) and visuospatial task (B). The upper row shows an activation of the frontal cortex, and the lower row shows the activation of the parietal cortex. The color bar indicates concentration of [oxy-Hb] (mM·cm). The scale differs between figures (A) and (B)
Summary
In the presented material in this article the possibility of application of two extremely different methods are discussed, namely photoelectron spectroscopy, a high vacuum technique, XPS, and technique for brain activity determination under in vivo conditions, i.e. functional near infrared spectroscopy, fNIRS. It has been shown by reporting chosen data that both techniques are suitable for the application to the testing clinical samples and objects and that the results of their application might help solve important problems in the clinical medicine as well as in the fundamental medical research.
XPS technique can be used to determine the chemical consistence from the simple substance to the studies of the phenomenon of a bacteria influence of artificial objects placed in our body; like implants and stents and degradation of bioactive molecules and antibacterial coatings. fNIRS may be used to study the processes taking place in the real time and in the real objects. It is used to monitor an influence on our brain activity of such factors like smell or noise up to such diseases like autism, depression and dementia, or atherosclerotic Alzheimer’s disease.
References
| 1. |
Barr TL. Modern ESCA. The principles and practice of X-ray photoelectron spectroscopy. CRC Press; 1994.
|
| 2. |
Jobsis F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science (80- ) [Internet]. 1977 Dec 23;198(4323):1264–7. Available from: https://www.sciencemag.org/lookup/doi/10.1126/science.929199.
|
| 3. |
Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage [Internet]. 2012 Nov;63(2):921–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1053811912003308.
|
| 4. |
Roberts AJ, Macak K, Takahashi K. Test of the Consistency of Angle Resolved XPS Data for Depth Profile Reconstruction using the Maximum Entropy Method. J Surf Anal [Internet]. 2009;15(3):291–4. Available from: https://www.jstage.jst.go.jp/article/jsa/15/3/15_291/_article.
|
| 5. |
Hollas JM. Modern spectroscopy. John Wiley & Sons; 2004.
|
| 6. |
Vickerman JC, Gilmore IS. Surface analysis: the principal techniques. John Wiley & Sons; 2009.
|
| 7. |
Alford TL, Feldman LC, Mayer JW. Fundamentals of Nanoscale Film Analysis [Internet]. Springer US; 2007. (Springer ebook collection / Chemistry and Materials Science 2005-2008). Available from: https://books.google.pl/books?id=zuOxls8yeNQC.
|
| 8. |
Szklarczyk M. Chemical analysis in the 21st century: Chemical microscopy and analytical multidimensional and conjugated techniques. Warsaw: PWN; 2019.
|
| 9. |
Szklarczyk M. From vacuum analysis to in vivo brain testing [the lecture given on 16th Dec 2020]. Medical University of Gdańsk; 2020.
|
| 10. |
Szklarczyk M, Macak K, Roberts AJ, Takahashi K, Hutton S, Głaszczka R, et al. Sub-nanometer resolution XPS depth profiling: Sensing of atoms. Appl Surf Sci [Internet]. 2017 Jul;411:386–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169433217305950.
|
| 11. |
Roberts AJ, Moffitt CE. Trends in XPS instrumentation for industrial surface analysis and materials characterisation. J Electron Spectros Relat Phenomena [Internet]. 2019 Feb;231:68–74. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0368204817301883.
|
| 12. |
Tsunashima H, Yanagisawa K, Inwadate. Measurement of Brain Function Using Near-Infrared Spectroscopy (NIRS). In: P. Bright, editor. Neuroimaging: Methods [Internet]. IntechOpen; 2012. p. 75. Available from: https://books.google.pl/books?id=iXWfDwAAQBAJ.
|
| 13. |
Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal Optical Imaging Study for Locomotor Recovery After Stroke. Stroke [Internet]. 2003 Dec;34(12):2866–70. Available from: https://www.ahajournals.org/doi/10.1161/01.STR.0000100166.81077.8A.
|
| 14. |
Kito H, Ryokawa A, Kinoshita Y, Sasayama D, Sugiyama N, Ogihara T, et al. Comparison of alterations in cerebral hemoglobin oxygenation in late life depression and Alzheimer’s disease as assessed by near-infrared spectroscopy. Behav Brain Funct [Internet]. 2014;10(1):8. Available from: http://behavioralandbrainfunctions.biomedcentral.com/articles/10.1186/1744-9081-10-8.
|
















