Difference in demand for analgesic and sedative medication according to the type of catheter ablation for atrial fibrillation
Abstract
Background
Our aim was to determine if there is a difference in demand for analgesic and sedative medication according to the type of catheter ablation for atrial fibrillation (AF).
Material and methods
We collected data from protocols of 1144pts, who underwent ablation of AF. We excluded 275pts, at most due to electrocardioversion during the procedure. We divided them into 4 groups: cryoballoon ablation group (CB, n = 101), single-point radiofrequency ablation group (RFth-, n = 541), single-point radiofrequency ablation group with thermocool catheter (RFth+, n = 156) and Multielectrode Pulmonary Vein Ablation Catheter group (PVAC, n = 71). We used fentanyl and midazolam for pain control. The dose was adjusted by the operator, accord-ing to patients’ request.
Results
The median dose of fentanyl 0.04 mg (0.00-0.08) and midazolam 1.00 mg (0.00-2.00) in CB group was lower than in other groups (p < 0.001). The median dose of fentanyl 0.12 mg (0.08-0.17) was lower in RFth- than in in RFth+ group: 0.15 mg (0.1-0.2) (p < 0.001). The demand for analgesia was higher when PVAC was used, with median dose of fentanyl 0.15 mg (0.1-0.2) (p < 0.0024).
Conclusions
The demand for analgesic/sedative medication was lower among patients who underwent CB. Among those who underwent RF ablation it was higher in groups with thermocool and multielectrode catheters.
Citation
Koźluk E, Rzechorzek W, Piątkowska A, Rodkiewicz D, Opolski G. Difference in demand for analgesic and sedative medication according to the type of catheter ablation for atrial fibrillation. Eur J Transl Clin Med. 2021;4(1):35-42Abbreviations
- AF – atrial fibrillation
- PVAC – Multielectrode Pulmonary
- Vein Ablation Catheter
- RF – radiofrequency
- Th – thermocool
- IQR – interquartile
- SD – standard deviation
Introduction
Atrial fibrillation (AF) is a common health care problem with a prevalence of over 30 million and increasing incidence worldwide [1]. In comparison with pharmacotherapy alone, catheter ablation of AF reduces all-cause mortality, cardiovascular hospitalizations and recurrences of atrial arrhythmia [2]. Recent meta-analysis of 14 randomized clinical trials shows reduced inci-dence of AF recurrence, shorter procedural time, a higher rate of phrenic nerve palsy, and a lower rate of pericardial effusion and cardiac tamponade in cryoballoon ablation (CB) in com-parison with radiofrequency (RF) ablation [3]. The choice of ablation type is often based on the experience of the operator and specific circumstances. The analgesic medication most used dur-ing cardiac procedures is the short-acting fentanyl. In most cases, it is co-administered with mid-azolam to minimize the patient’s anxiety and movements [4]. The aim of our study was to de-termine the difference in demand for analgesic and sedative medication according to the type of catheter ablation, as this could become a factor to consider while choosing the method of AF ablation.
Material and methods
Patients
We collected data from protocols of patients, who underwent catheter ablation of AF. We divid-ed the cohort into four groups depending on the type of catheter ablation including CB, single-point radiofrequency ablation (RFth-), single-point radiofrequency ablation with active cooling of the catheter tip with the Thermocool Irrigated Tip Catheter (RFth+) and the Multielectrode Pulmonary Vein Ablation Catheter (PVAC) which delivers cycled bipolar and unipolar radiofre-quency energy through multiple electrodes.
Anaesthesia
We used midazolam and fentanyl to reduce the patients' anxiety, movements and pain during the procedure. The doses were adjusted by the operator based on patients' request, responsive-ness, and movements as well as objective parameters including heart rate, oxygen saturation and continuous arterial blood pressure.
Ablation Procedure
We performed the first ablation of AF in our center in 2003. To reduce the learning curve bias, we excluded data from procedures performed between 2003 and 2006. We selected the type of ablation strategy based on the available staff, the anatomy of patients’ heart, type of AF and the patients’ co-morbidities. We performed the ablation procedure according to standard protocol, described in previous publications and the latest HRS/EHRA/ECAS recommendations [5-8]. Initially, we performed ablation, using the Lasso catheter and 4 mm tip ablation catheter, method described by Haïssaguerre et al. Subsequently, we introduced the CARTO anatomical isolation with a thermocool catheter [9]. In 2008 we introduced CB ablation and in 2009 PVAC ablation.
In the single tip RF ablation, we placed the circular mapping catheter and ablation catheter with a 4 mm tip (Marinr – Medtronic) in the left atrium after transseptal puncture or via the persistent foramen ovale, if present. We set the temperature and power of the ablation catheter at 50°C and 30 W respectively. In RFth+ group, the ablation catheter was irrigated with a heparinized saline solution using a thermocool technology to lower the electrode and tissue surface tempera-ture and to reduce the possibility of thrombus formation. The procedure was performed with 3D CARTO system (primary system, since 2010 CARTO 3). We set the temperature and power of the catheter at 48°C and 30-35 W (on the posterior wall 25-30 W). The flow rate was 20-30 ml/min for classic thermocool catheters and 8-14 for THERMOCOOL SMARTTOUCH® Sur-roundflow (SF) catheters. We used the 12-Fr sheath (Flex-CATH® Steerable Sheath, Abbott) to introduce the multielectrode catheter. The leading 0.0032-inch wire was positioned in all PVs to stabilize and support the circular, decapolar ablation catheter. The RF energy was delivered in a combination of 1 to 5 bipolar channels. The target temperature and maximum powers were 50-60°C and 8-10 W respectively [6]. We used different combinations of bipolar to unipolar pro-portions depending on the observed effect. We preferred 4:1 proportion on the posterior wall and 2:1 in other regions.
In CB ablation after a single transseptal puncture, we replaced the 8-Fr sheath with a 12-Fr sheath and introduced a 28 mm double-lumen cryoballoon (Arctic Front – Cryocath, Medtron-ic). We used N2O cooling temperature of –35°C to –60°C. During right pulmonary vein isolation the catheter placed in the superior vena cava was used to stimulate the phrenic nerve at a rate of 30/min to prevent phrenic nerve palsy [7].
Statistical Analysis
We presented all the categorical data as percentages. Continuous variables with non-parametric distribution were presented as median and interquartile ranges (IQR) and those with normal dis-tribution as mean value and standard deviation (SD). We used Kruskal-Wallis equality-of-populations rank test to compare multiple non-parametric variables. We performed Wilcoxon Two-Sample Test to detect difference between two non-parametric variables. We used Spearman's Rank-Order Correlation to assess the relationship between nonparametric variables. All statistical tests were 2-tailed, and a p < 0.05 was considered significant. The analysis was performed using the SAS 9.4 software (SAS Institute Inc., Cary, 2013).
Results
The total number of patients included in our database was 1144, from which we excluded 275 patients. 197 patients underwent electrical cardioversion during the procedure, which required general anesthesia with propofol and influenced the doses of fentanyl and midazolam. In 72 cases data were not available. Finally, in 6 cases ablation was discontinued due to tamponade and in one case due to cryoballoon failure (Figure 1).
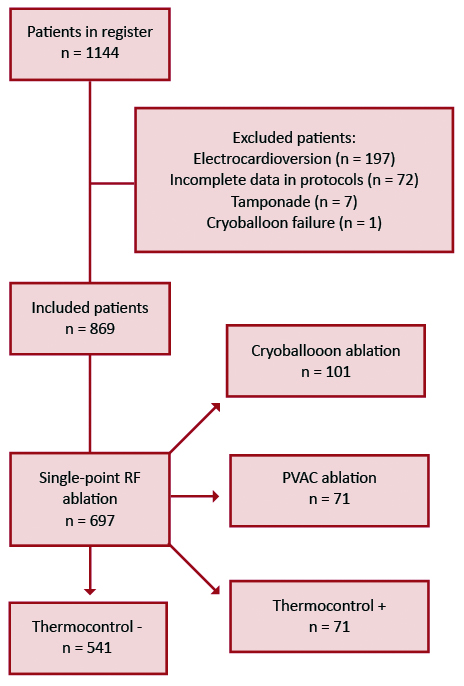
Figure 1. Patient flowchart
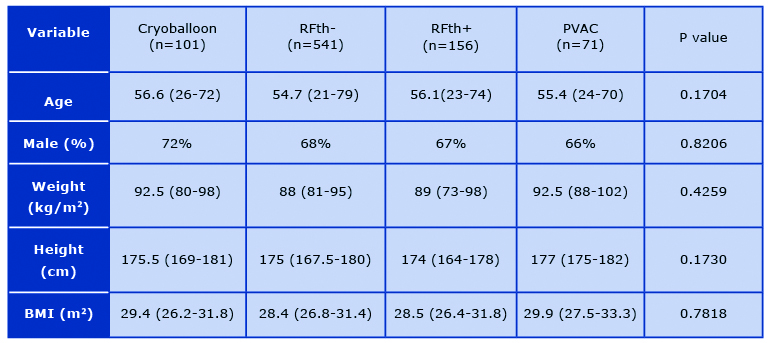
Finally, 869 patients, were included in our study and divided in following groups based on abla-tion technique: CB (n = 101, 11.62%), RFth- (n = 541, 62.26%), RFth+ (n = 156, 17.95%), and PVAC (n = 71, 8.17%). The mean age in our patient population was 55 (21- 79) and 594 (68%) of patients were male. There was no difference in demographic and anthropomorphic parame-ters between the groups (Table 1). 160 patients had persistent atrial fibrillation (18.41%). AF ablation was attempted for the first time in 518 cases.
The median dose of fentanyl 0.04 mg (0.00-0.08 mg) and midazolam 1.00 mg (0.00- 2.00 mg) was significantly lower in CB in comparison with other groups (p < 0.001). Median dose of fen-tanyl and midazolam in PVAC was 0.15 mg (0.1-0.2 mg), and 2.00 mg (1-3 mg), RFth+ 0.15 mg (0.1- 0.2 mg), and 2 mg (1.5-3 mg), RFth0.13 mg (0.08-0.17 mg), and 2.00 mg (1-3 mg) for fentanyl and midazolam respectively (Figures 2 and 3). Midazolam was not required in 37 (37%), fentanyl in 29 (29%), and neither of the drugs in 22 (22%) in group; 5 (7%), 1 (1%), and 1 (1%) in PVAC group; 9 (6%), 2 (1%), and 2 (1%) in RFth+ group, and 54 (10%), 24 (4%), and 22 (4%) in RFth- group respectively.
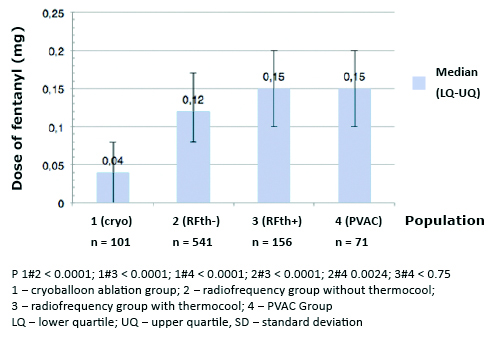
Figure 2. Total doses of fentanyl (mg) used during particular ablation procedures
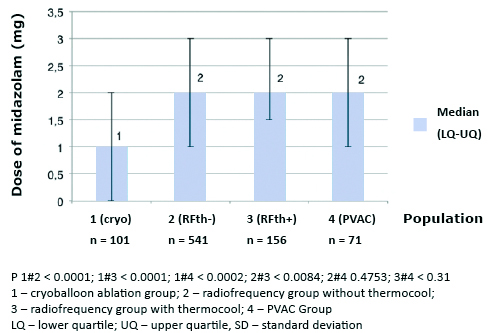
Figure 3. Total doses of midazolam (mg) used during particular ablation procedures
Table 1. Comparison of population basic characteristics
The median dose of fentanyl 0.12 mg (0.08-0.17 mg) was significantly lower in the RFth- in comparison with the RFth+ group 0.15 mg (0.1- 0.2 mg) with p < 0.001. Patients in PVAC group 0.15 mg (0.1-0.2 mg) required more fentanyl than patients in RFth- group 0.12 mg (0.08-0.17 mg) with p < 0.0024.
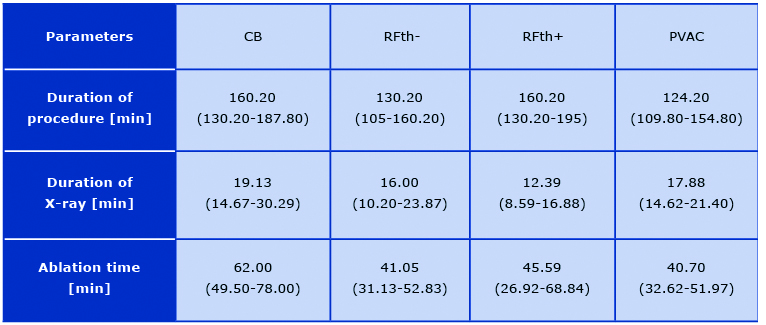
There was no difference in demand for sedation and analgesia in groups with first and those with subsequent ablation procedures with p = 0.37 for both of administered drugs. Time of ap-plication correlated with the dose of fentanyl (r = 0.38, p < 0.0001) and midazolam (r = 0.30, p > 0.0002) in RFth+ group and in RFth- group (r = 0.3, p < 0.0001, r = 0.25, p < 0.0001) (Figure 4). There was a trend in correlation for midazolam (p = 0.08) and fentanyl (p < 0.13) in PVAC group. The dose of fentanyl (p < 0.96) and midazolam (p < 0.68) did not correlate with time of application in CB group. The median total application time was longer in cryoballoon ablation 62.00 (49.50-78.00) in comparison to other groups: PVAC 40.70 (32.62-51.97, p < 0.0001), RFth+ 45.59 (26.92- 68.84, p < 0.0001), RFth- 41.05 (31.13- 52.83, p < 0.0001). There was no statistically significant difference in the application time between RFth+ and RFth- (p < 0.2221), RFth+, and PVAC (p < 0.3655), RFth- and PVAC (p < 0.9823) (Table 2). The median total pro-cedure time was longer in cryoballoon ablation 160.20 min (130.20-187.80) in comparison to PVAC 124.20 min (109.80-154.80) and RFth- 130.20 min (105-160.20) (p < 0.0001) and there was no significant difference in comparison with RFth+ group 160.20 min (130.20-195, p < 0.3941). The procedure was longer in RFth+ group than in PVAC and in RFth- (p < 0.0001). There was no statistically significant difference between RFth- and PVAC (p < 0.7342) (Table 2). The median fluoroscopy time was longer in cryoballoon ablation group 19.13 min (14.67-30.29) than in other groups: RFth- 16.00 min (10.20-23.87), RFth+ 12.39 min (8.59-16.88, p < 0.0001), and close to statistical significance for PVAC group 17.88 (14.62-21.40, p < 0.065) (Table 2).
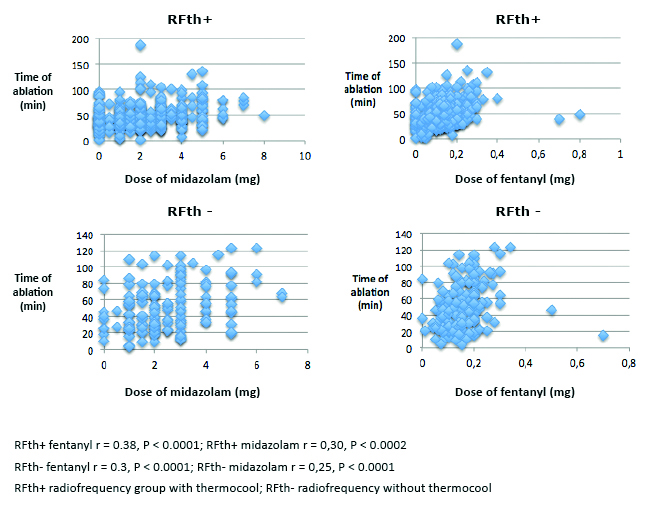
Figure 4. Correlation of ablation time with the doses of midzolam and fentanyl used in particular ablation techniques
Table 2. Procedural data
Discussion
Administration of benzodiazepines and opioids during cardiac electrophysiological procedures is considered safe mostly due to the broad therapeutic range and reversal agents [4]. According to Kezershvili et al. administration of intravenous sedation in 9.558 cardiac procedures, was associated with only 9 complications, with 6 of them related to electrophysiological procedures [10]. We found that patients, who underwent CB ablation, required lower doses of fentanyl and midazolam in comparison with RF ablation. In a similar study, Defaye et al. show a lower dose of morphine administered to patients in CB group (3 ± 1.53 mg/m2 ) in comparison with RF group (2.09 ± 1.02 mg/m2 , p < 0.01) with no difference in the administered dose of midazolam (p < 0.135) [11]. Patients in this study had similar demographic and anthropometric parameters and the lack of significant difference in midazolam dose was likely related to smaller n-size (n = 60). In a small randomized trial including 32 patients, Collins et al. found no difference in the dosing of fentanyl and midazolam. However, based on a numeric scale, he described much lower procedural discomfort among patients, who underwent cryoballoon ablation in comparison to RF ablation [12]. Lowe et al. reported increased patient satisfaction with CB ablation in comparison with RF ablation in patients who underwent ablation for supraventricular arrhythmi-as (1.3 ± 2.2 vs 6.1 ± 3.5, p < 0.01) [13]. In a recent analysis of 71 patients, Miśkowiec D et al. show that CB ablation for AF is safe and efficacious with only one case of transient phrenic palsy, 4.2% of patients developing a hematoma and an acute procedural success rate of 95.8% [14]. Attanasio et al. examined painful ablation sites in patients sedated with midazolam and propofol and found that 92% of patients, who underwent RF ablation had a ≥1 pain reaction in comparison with only 13% in CB group [15]. Furthermore, patients in the RF group had more pain reactions (3.6 ± 4.7) than in CB group (1.3 ± 0.6, p = 0.005) [15].
The reason for the reduced demand for analgesia and sedation in cryoballoon ablations might be due to different character of lesion in this ablation type. Hypothermia generated in the tissue causes a three- -phase response including: freeze/thaw phase, the hemorrhagic-inflammatory phase, and the replacement fibrosis phase [16]. Consequently smaller and partially reversibility lesions caused by the cooling process as well as more stable energy delivery might indicate bet-ter preservation of tissue integrity [13, 16].
Recent studies support the use of propofol, administered by either cardiologist or anesthesiologist for unconscious sedation during ablation of AF. Wutzler et al. reported reduced motion of patients during ablation and no complications related to sedation [17]. In a study of 152 patients, Yamagutchi et al show feasibility of total intravenous anesthesia by a cardiologist with support from an anesthesiologist with no major anesthesia-associated complications, 4% of ablation- -associated complications and a success rate of 85% at 12 months [18]. Some of the theoretical risks of this approach include the use of muscle relaxants during the general anesthesia, which might prevent muscle contraction in response to pacing of the phrenic nerve and increase the rate of phrenic nerve palsy. General anesthesia may also delay the recognition of cerebrovascular events as well as decrease alertness of physicians for patients’ pain. In a study of 120 cases, Tang et al. reported more hypotension and hypoxia (21.7% vs 6.7%) in the propofol group in comparison with the midazolam and fentanyl group [19]. In a randomized trial Di Biase et al. reported a better success rate of AF ablation under general anesthesia with a higher rate of esophageal injuries [20]. The use of propofol for elective electric cardioversion is a standard of care and because it impacts doses of fentanyl and midazolam administered during ablation we have excluded all of the patients who underwent this procedure from our study.
We noticed longer application time and fluoroscopy time in cryoballoon ablation, which is con-sistent with the results of Schmidt et al [21]. We found no difference in procedure time, in con-trast to Ciconte et al., who found shorter procedure time and radiation exposure with second-generation cryoballoon technique in comparison with RF ablation [22]. We found a positive correlation between application time and doses of midazolam and fentanyl in RFth+ and RFth- groups and no correlation in CB group. We did not adjust our analysis for application time since it would only emphasize our results. Increased dose of fentanyl in RFth+ group could be related to deeper lesion formation of irrigated tip ablation catheter in comparison with standard single-point RF catheter due to higher power, which is reached be lowering the temperature [23-24]. The higher demand for analgesia in PVAC ablation group could be related to higher cumulative energy of electrodes.
Limitations
Our study has several limitations. We used a standard protocol for the administration of analge-sic and sedative medications based on the subjective perception of pain and objective data in-cluding patients’ motion and vital signs observed by the operator. Yet, some confounding fac-tors including individual variance in perception of pain and biases of the operator could be con-tributing to the patient’s demand for analgesics. At the time our study, there was no literature suggesting any type of ablation to be more painful, which limits the operator bias. Taking into consideration the complex and subjective character of pain, even a prospective study would face limitations e.g. the standardization bias.
Patients receive sedation during ablation, which limits their ability to manage patient controlled analgesia or respond to a VAS scale questionnaire.
Conclusions
The dose of fentanyl and midazolam was lower among patients who underwent CB in comparison with RF. This suggests that CB is a less painful technique and should be considered in patients at high risk for general anesthesia. Patients in the RFth+ group required higher dose of fentanyl in comparison with RFth-, which likely reflects increased destruction of the tissue during single application.
References
| 1. |
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation [Internet]. 2014 Feb 25;129(8):837–47. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.113.005119.
|
| 2. |
Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2019 Sep;12(9):e007414. Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.119.007414.
|
| 3. |
Fortuni F, Casula M, Sanzo A, Angelini F, Cornara S, Somaschini A, et al. Meta-Analysis Comparing Cryoballoon Versus Radiofrequency as First Ablation Procedure for Atrial Fibrillation. Am J Cardiol [Internet]. 2020 Apr;125(8):1170–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914920300576.
|
| 4. |
Thomas SP, Thakkar J, Kovoor P, Thiagalingam A, Ross DL. Sedation for Electrophysiological Procedures. Pacing Clin Electrophysiol [Internet]. 2014 Jun;37(6):781–90. Available from: http://doi.wiley.com/10.1111/pace.12370.
|
| 5. |
Kozluk E, Gawrysiak M, Lodzinski P, Kiliszek M, Winkler A, Kochanowski J, et al. The LocaLisa system as the key to shortening the procedure duration and fluoroscopy time during ablation of atrial fibrillation. Kardiol Pol [Internet]. 2008;66(6):624. Available from: https://www.mp.pl/kardiologiapolska/en/node/10424/pdf.
|
| 6. |
Koźluk E, Balsam P, Peller M, Kiliszek M, Lodziński P, Piątkowska A, et al. Efficacy of multi-electrode duty-cycled radiofrequency ablation in patients with paroxysmal and persistent atrial fibrillation. Cardiol J [Internet]. 2013 Dec 11;20(6):618–25. Available from: http://czasopisma.viamedica.pl/cj/article/view/36496.
|
| 7. |
Koźluk E, Gaj S, Piątkowska A, Kiliszek M, Lodziński P, Dąbrowski P, et al. Evaluation of safety and the success rate of cryoballoon ablation of the pulmonary vein ostia in patients with atrial fibrillation: A preliminary report. Kardiol Pol [Internet]. 2010;68(2):175–80. Available from: https://www.mp.pl/kardiologiapolska/en/node/9682/pdf.
|
| 8. |
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J [Internet]. 2021 Feb 1;42(5):373–498. Available from: https://academic.oup.com/eurheartj/article/42/5/373/5899003.
|
| 9. |
Haïssaguerre M, Jaïs P, Shah DC, Garrigue S, Takahashi A, Lavergne T, et al. Electrophysiological End Point for Catheter Ablation of Atrial Fibrillation Initiated From Multiple Pulmonary Venous Foci. Circulation [Internet]. 2000 Mar 28;101(12):1409–17. Available from: https://www.ahajournals.org/doi/10.1161/01.CIR.101.12.1409.
|
| 10. |
Kezerashvili A, Fisher JD, DeLaney J, Mushiyev S, Monahan E, Taylor V, et al. Intravenous sedation for cardiac procedures can be administered safely and cost-effectively by non-anesthesia personnel. J Interv Card Electrophysiol [Internet]. 2008 Jan 14;21(1):43–51. Available from: http://link.springer.com/10.1007/s10840-007-9191-0.
|
| 11. |
Defaye P, Kane A, Jacon P, Mondesert B. Cryoballoon for pulmonary vein isolation: Is it better tolerated than radiofrequency? Retrospective study comparing the use of analgesia and sedation in both ablation techniques. Arch Cardiovasc Dis [Internet]. 2010 Jun;103(6–7):388–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1875213610001373.
|
| 12. |
Collins NJ, Barlow M, Varghese P, Leitch J. Cryoablation versus Radiofrequency Ablation in the treatment of Atrial Flutter trial (CRAAFT). J Interv Card Electrophysiol [Internet]. 2006 Nov 10;16(1):1–5. Available from: http://link.springer.com/10.1007/s10840-006-9027-3.
|
| 13. |
Lowe MD, Meara M, Mason J, Grace AA, Murgatroyd FD. Catheter Cryoablation of Supraventricular Arrhythmias:. A Painless Alternative to Radiofrequency Energy. Pacing Clin Electrophysiol [Internet]. 2003 Jan;26(1p2):500–3. Available from: http://doi.wiley.com/10.1046/j.1460-9592.2003.00081.x.
|
| 14. |
Miśkowiec D, Kasprzak JD, Wejner-Mik P, Szymczyk E, Qawoq HD, Życiński P, et al. Conscious sedation during cryoballoon ablation of atrial fibrillation: a feasibility and safety study. Minerva Cardioangiol. 2018;66(2):143–51.
|
| 15. |
Attanasio P, Huemer M, Shokor, PARWANI A, Boldt L-H, Mugge A, Haverkamp W, et al. Pain Reactions during Pulmonary Vein Isolation under Deep Sedation: Cryothermal versus Radiofrequency Ablation. Pacing Clin Electrophysiol [Internet]. 2016 May;39(5):452–7. Available from: http://doi.wiley.com/10.1111/pace.12840.
|
| 16. |
Andrade JG, Dubuc M, Guerra PG, Macle L, Mondedert B, RivardI L, et al. The Biophysics and Biomechanics of Cryoballoon Ablation. Pacing Clin Electrophysiol [Internet]. 2012 Sep;35(9):1162–8. Available from: http://doi.wiley.com/10.1111/j.1540-8159.2012.03436.x.
|
| 17. |
Wutzler A, Rolf S, Huemer M, Parwani AS, Boldt L-H, Herberger E, et al. Safety Aspects of Deep Sedation during Catheter Ablation of Atrial Fibrillation. Pacing Clin Electrophysiol [Internet]. 2012 Jan;35(1):38–43. Available from: http://doi.wiley.com/10.1111/j.1540-8159.2011.03260.x.
|
| 18. |
Yamaguchi T, Shimakawa Y, Mitsumizo S, Fukui A, Kawano Y, Otsubo T, et al. Feasibility of total intravenous anesthesia by cardiologists with the support of anesthesiologists during catheter ablation of atrial fibrillation. J Cardiol [Internet]. 2018 Jul;72(1):19–25. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0914508718300017.
|
| 19. |
Tang R-B, Dong J-Z, Zhao W-D, Liu X-P, Kang J-P, Long D-Y, et al. Unconscious sedation/analgesia with propofol versus conscious sedation with fentanyl/midazolam for catheter ablation of atrial fibrillation: a prospective, randomized study. Chin Med J (Engl) [Internet]. 2007;120(22). Available from: https://journals.lww.com/cmj/Fulltext/2007/11020/Unconscious_sedation_analgesia_with_propofol.18.aspx.
|
| 20. |
Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CD, et al. Esophageal Capsule Endoscopy After Radiofrequency Catheter Ablation for Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2009 Apr;2(2):108–12. Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.108.815266.
|
| 21. |
Schmidt M, Dorwarth U, Andresen D, Brachmann J, Kuck K-H, Kuniss M, et al. Cryoballoon versus RF Ablation in Paroxysmal Atrial Fibrillation: Results from the German Ablation Registry. J Cardiovasc Electrophysiol [Internet]. 2014 Jan;25(1):1–7. Available from: http://doi.wiley.com/10.1111/jce.12267.
|
| 22. |
Ciconte G, Baltogiannis G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace [Internet]. 2015 Apr 1;17(4):559–65. Available from: https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/euu350.
|
| 23. |
Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy. J Am Coll Cardiol [Internet]. 2000 Jun;35(7):1905–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S073510970000615X.
|
| 24. |
Skrumeda LL, Mehra R. Comparison of Standard and Irrigated Radiofrequency Ablation in the Canine Ventricle. J Cardiovasc Electrophysiol [Internet]. 1998 Nov;9(11):1196–205. Available from: http://doi.wiley.com/10.1111/j.1540-8167.1998.tb00092.x.
|












