Effect of a bicarbonate-buffered peritoneal dialysis solution on clinical and laboratory indices of dialysis
Abstract
Background
Biocompatible fluids for peritoneal dialysis (PD) have been introduced to improve the dialysis adequacyand patient outcomes in end-stage renal disease. However, being buffered with lactate, these fluids may insufficiently correct metabolic acidosis and lead to changes in peritoneal structure. Bicarbonate buffered fluids might mitigatethese complications. The aim of the study was to evaluate the influence of a bicarbonate dialysis fluid on clinical andlaboratory indices of dialysis adequacy.
Methods
Twenty PD patients treated with standard lactate solutions, weredivided into two groups. Patients in the study group started treatment with a 34 mmol/L bicarbonate-buffered solution, whereas those in the control group continued on a lactate-buffered fluid. Assessment of urine output, dialysis ultrafiltration, hydration status as well as metabolic acidosis, dialysis adequacy and potential inflow pain was performed at baseline and at six weeks intervals for 24 weeks.
Results
In the studied group, pH was 7.36 ± 0.03, HCO3 22.1 ± 1.8 mmol/l at baseline and 7.36 ± 0.04, and 21.2 ± 2.3 mmol/l at the end of the study, while in the control group the pH was 7.35 ± 0.12, with HCO3 22.2 ± 1.4 mmol/l, and 7.40 ± 0.03, and 22.3 ± 1.8 mmol/l, respectively. No statistically significant differences were noted. Dialysis effectiveness, measured as urea Kt/V, urine output and dialysis ultrafiltration did not differ between the groups, either at baseline or at the study termination. Only one patient in the studied group reported inflow pain and following conversion to bicarbonate-buffered PD fluid he reported reduction of its intensity.
Conclusion
Bicarbonate-buffered PD solution appears to be similar to standard fluid in terms of the impact on residual renal function and ultrafiltration as well as on acid-base balance and infusion pain. Longitudinal studies are needed to assess the long-term advantages of this biocompatible solution in PD patients.
Citation
Chmielewski M, Bielińska-Ogrodnik D, Jagodziński P, Lichodziejewska-Niemierko M. Effect of a bicarbonate-buffered peritoneal dialysis solution on clinical and laboratory indices of dialysis. Eur J Transl Clin Med. 2020;3(1):11-15Introduction
Peritoneal dialysis (PD) is a method of renal-replacement therapy (RRT) utilized in patients with end-stage renal disease (ESRD). As the name suggests, in PD the peritoneum acts as the dialysis membrane. This mode of treatment is as effective as hemodialysis and can serve as a ‘bridge’ to renal transplantation or a life-long RRT treatment. Peritoneum separates the compartment of dialysis fluid from the blood compartment of peritoneal capillaries. Due to its semi-permeable structure, it allows for removal of uremic toxins as well as for restoring the electrolyte and acid-base balance.
Standard dialysis fluids are sterile solutions containing electrolytes, lactate ions and glucose at various concentrations. Such composition normally allows for sufficient patient dehydration and detoxification in the course of ESRD. However, due to its non-physiologic lactate buffer and the presence of glucose degradation products (GDP) its use is complicated by impairment of immunologic mechanisms of the peritoneum, and progressive fibrosis and thickening of the membrane with a concomitant loss of mesothelial cells [1]. Insufficient correction of metabolic acidosis leads to enhanced muscle catabolism, decreased albumin synthesis, and chronic low-grade inflammation.
New dialysis fluids are buffered with bicarbonates and are thought to mitigate the above complications [2-3]. Moreover, through improvement of the local peritoneal biocompatibility, they might positively affect dialysis adequacy [3]. However, despite being approved for use these new PD fluids have not been utilized in Poland so far. Therefore, the major aim of the current study was to compare novel PD solutions buffered with 34 mmol/l bicarbonates and the standard lactate-buffered fluids, in terms of clinical and biochemical adequacy. Particular aims were to assess the impact of a bicarbonate-buffered solution on the correction of metabolic acidosis and on improving the patient’s hydration status, depending on patient residual renal function, and the presence of co-morbidities, as well as to evaluate the influence of the studied fluids on the sensation of abdominal pain during dialysis.
Materials and Methods
This was a prospective evaluation of 20 patients treated at a single center with continuous ambulatory peritoneal dialysis (CAPD). All patients were initially dialyzed on a standard, neutral pH, 35 mmol/L lactate-buffered fluid (Balance, Fresenius, Germany). The patients who were similar in terms of age, sex, history of diabetes, urine output and dialysis adequacy so far were matched into pairs. Within each pair, the patients were randomly assigned to either start treatment with a 34 mmol/L bicarbonate-buffered solution (BicaVera, Fresenius, Germany) or to continue using the standard PD fluid. This way we obtained 2 equal groups of 10 participants each. All participants provided informed consent to participate in the study, and the study protocol was approved by the local Ethics Committee (NKBBN 212/2019).
Prior to the start of the evaluation (baseline) and at 6-week intervals, the following variables were analyzed: the clinical assessment of hydration status, urine output, dialysis ultrafiltration, acid-base balance (via capillary blood gas tests) as well as routine laboratory parameters. Hydration status was also assessed through bioimpedance spectroscopy (Body Composition Monitor, Fresenius Medical Care, Germany) and was presented as liters of over- or dehydration. Moreover, at 12-week intervals, indices of dialysis adequacy (urea Kt/V, creatinine clearance), protein turnover (normalized protein catabolic ratio, nPCR) and of transmembrane transport status (peritoneal equilibration test) were checked. Inflow pain was assessed using a visual analog scale (VAS). Results were expressed as means with standard deviations or medians with interquartile ranges, as appropriate. The normality of distribution was verified with the Kolmogorov-Smirnov test. A p-value of < 0.05 was considered statistically significant. Comparisons between two groups were assessed with a Student’s unpaired t-test or Mann-Whitney test, as appropriate. The statistical analysis was performed using the Statistica software version 13.3 (StatSoft Inc., United States).
Results
The baseline characteristics of the studied groups are depicted in Table 1. Of the 20 patients in our sample, 11 were female.
Table 1. Baseline clinical characteristics of the studied groups; UF – dialysis ultrafiltration
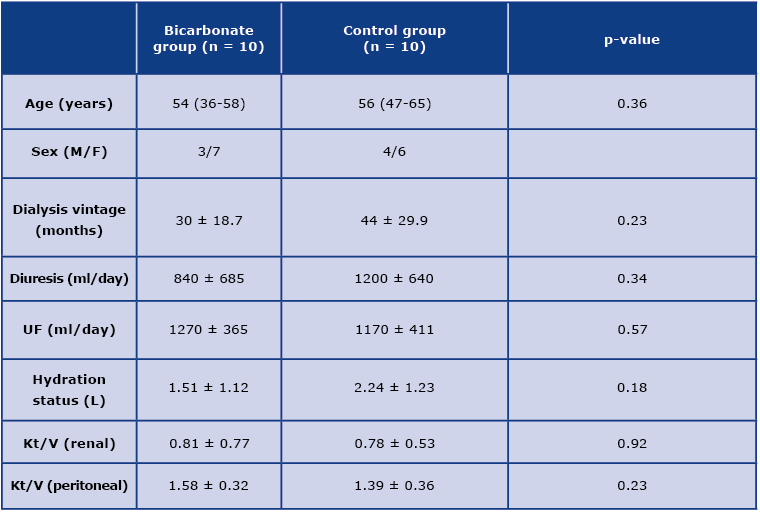
Majority of patients in both groups took calcium carbonate and loop diuretics which affect acid-base balance and diuresis, respectively. However, their doses were not modified during the study period. There was one episode of peritonitis in the studied group, effectively treated with standard therapy, but the patient was excluded from the study. Another patient from the control group underwent kidney transplantation and did not complete the study. Therefore, 18 patients remained for the final evaluation, nine in each group.
Considering capillary blood acid-base balance, in the bicarbonate group, pH equaled 7.36 ± 0.03, while HCO3 was 22.1 ± 1.8 mmol/l, at baseline. At study termination after 24 weeks, pH was 7.36 ± 0.04, and HCO3 21.2 ± 2.3 mmol/l. In the lactate group, pH equaled 7.35 ± 0.12, and HCO3 22.2 ± 1.4 mmol/l at baseline, with the respective values being 7.40 ± 0.03, and 22.3 ± 1.8 mmol/l at the end of the study. There were no statistically significant differences in the presented parameters, within the studied groups or between them.
Urine output has not changed during the study period: after 24 weeks it equaled 910 ml ± 640 ml in the bicarbonate group and 1150 ± 690 ml in the lactate group. Similarly, dialysis ultrafiltration remained stable, as it was 1200 ± 353 ml in the bicarbonate group, and 1220 ± 449 ml in the lactate group, at the end of the study. There were no statistically significant changes in dialysis adequacy, as measured with peritoneal Kt/V, between and within the studied groups. At study termination, peritoneal Kt/V equaled 1.69 ± 0.39 in the bicarbonate group, and 1.49 ± 0.22 in the lactate group. Similarly, renal Kt/V remained stable during the observation period. None of the patients reported inflow abdominal pain in the group with lactate-buffered solution. Whereas only patient from the bicarbonate group experienced inflow pain and reported that it diminished from 2 to 0.
Discussion
In this study we have demonstrated that bicarbonate-buffered peritoneal dialysis solution appears to be similar to standard fluid in terms of the impact on residual renal function and ultrafiltration, as well as on the acid-base balance and the PD fluid inflow pain. Biocompatibility of fluids used for PD has been a topic of intense studies since the very beginning of PD as a method of RRT [4]. The major factors responsible for the relatively low compatibility of available solutions included: low fluid pH, the use of glucose as an osmotic agent and the addition of lactates as a source of endogenously generated bicarbonates. Low fluid pH was necessary, since at physiologic pH the GDPs are formed due to non-enzymatic glucose disintegration during the process of fluid sterilization. Glucose itself, and in particular GDPs, contribute to protein glycation which leads to formation of advanced glycation end-products (AGE) [1]. These are thought to be responsible for the irreversible damage of the peritoneal membrane that results in ultrafiltration failure and the necessity to treat the patient with hemodialysis. Currently, the issue of low pH was overcome with the use of two-compartmental fluids, in which glucose is stored and sterilized in one compartment at a very low pH (2.8-3.1), while the other compartment contains alkaline lactate solution. The solutions from the two compartments are mixed immediately prior to dialysis exchange to form a ready-to-use neutral fluid. However, the use of a lactate buffer is associated with the risk of insufficient correction of metabolic acidosis, as well as with the risk of provoking abdominal pain during the fluid inflow [5-6]. The effects of increased lactate load are associated with a decrease in cellular redox state, thus impairing numerous vital cellular functions [7].
Bicarbonate-buffered dialysis fluids constitute the next step in the quest to obtain an ‘ideal’ biocompatible solution. Actually, studies on bicarbonates as buffers for dialysis fluids started as early as in the 1960s [8]. However, precipitation of calcium and magnesium carbonate has hindered the use of such solutions. As a result, lactate was utilized as a buffer for many years and was regarded as more stable, with no apparent side-effects. Probably, the first studies with bicarbonate-buffered fluids in two-chamber dialysis sets were performed by Feriani et al. [4]. One chamber contained calcium and magnesium, while the other bicarbonates, to avoid the abovementioned precipitation. The studies that followed, confirmed appropriate correction of metabolic acidosis with bicarbonate-based fluids [5-6]. Additionally, a study by Mactier et al. demonstrated better tolerance of such solutions, as compared to lactate buffered ones, with less abdominal pain during fluid inflow [6].
Better correction of metabolic acidosis was associated with an increase in nPCR, suggestive of improved nutrition [7]. The importance of adequate acidosis correction was highlighted in a Korean study in which decreased serum bicarbonates levels turned out as an independent risk factor for mortality in PD patients [9]. Studies on bicarbonate solutions, performed ex vivo demonstrated improved viability of peritoneal mesothelial cells, and decreased concentrations of factors associated with peritoneal fibrosis and neovascularization (as compared to lactate) [10]. Longitudinal evaluations demonstrated less inflammatory cytokines in dialysis effluents of patients treated with bicarbonate-based solutions, as well as decreased amounts of pro-fibrotic factors and chemokines [11]. Increased concentrations of CA125 in dialysis effluents was also suggestive of high mesothelial mass, in comparison to effluents from lactate-based fluids [12]. These indices of decreased peritoneal injury translated into improved preservation of ultrafiltration capacity in long-term observations [2,13].
Better preservation of peritoneal membrane with bicarbonate-based solutions is also thought to be responsible for decreased incidence of peritoneal infections, reported by some authors [14]. In the present study there were no episodes of dialysis-associated peritonitis in either of the groups. However, the observation was limited to 24 weeks. To evaluate the potential impact of bicarbonate-based fluids on the risk of infections and on the membrane function, longer follow-ups are certainly needed.
Conclusion
International guidelines advocate the use of bicarbonate buffered solutions for peritoneal dialysis. Recommendations for adults (issued by the International Society for Peritoneal Dialysis) and for children (European Pediatric Dialysis Working Group) are especially strong for patients with acute kidney injury [15-16]. However, despite these guidelines and national registration, these fluids have not been used in Poland so far. This study might serve as a contribution to our understanding and experience with bicarbonate buffered solutions in the treatment of PD patients. It confirmed that such fluids are safe, well-tolerated, and do not impair the indices of dialysis adequacy. The study limitations include small sample size and a relatively short observation time of 24 weeks. Given their higher price, long-term studies with large patient groups are needed to further verify the longitudinal advantages of these biocompatible solutions in peritoneal dialysis patients.
Disclosures
In the years 2007-2019 DBO, PJ, MLN were employed by Fresenius NephroCare.
References
| 1. |
Holmes CJ, Faict D. Peritoneal dialysis solution biocompatibility: Definitions and evaluation strategies. Kidney Int [Internet]. 2003 Dec;64:S50–6. Available from: https://doi.org/10.1046/j.1523-1755.2003.08806.x.
|
| 2. |
Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int [Internet]. 2009;29(6):647–55. Available from: http://www.pdiconnect.com/content/29/6/647.short.
|
| 3. |
Feriani M. Twenty Years of Bicarbonate Solutions. In: Peritoneal Dialysis-State-of-the-Art 2012 [Internet]. Karger Publishers; 2012. p. 1–5. Available from: https://doi.org/10.1159/000337789.
|
| 4. |
Feriani M, Biasioli S, Borin D, Brendolan A, Gargantini L, Chiaramonte S, et al. Bicarbonate solutions for peritoneal dialysis: a reality. Int J Artif Organs [Internet]. 1985 Jan;8(1):57–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2982743.
|
| 5. |
Coles GA, Gokal R, Ogg C, Jani F, O’donoghue DT, Cancarini GC, et al. A randomized controlled trial of a bicarbonate-and a bicarbonate/lactate-containing dialysis solution in CAPD. Perit Dial Int [Internet]. 1997;17(1):48–51. Available from: http://www.pdiconnect.com/content/17/1/48.short.
|
| 6. |
Mactier RA, Sprosen TS, Gokal R, Williams PF, Lindbergh M, Naik RB, et al. Bicarbonate and bicarbonate/lactate peritoneal dialysis solutions for the treatment of infusion pain. Kidney Int [Internet]. 1998 Apr;53(4):1061–7. Available from: https://doi.org/10.1111/j.1523-1755.1998.00849.x.
|
| 7. |
Feriani M, Kirchgessner J, La Greca G, Passlick-Deetjen J, Bicarbonate CAPD Cooperative Group. Randomized long-term evaluation of bicarbonate-buffered CAPD solution. Kidney Int [Internet]. 1998 Nov;54(5):1731–8. Available from: https://doi.org/10.1046/j.1523-1755.1998.00167.x.
|
| 8. |
Boen ST. Kinetics of peritoneal dialysis: a comparison with the artificial kidney. Medicine (Baltimore) [Internet]. 1961;40(3):243–88. Available from: https://journals.lww.com/md-journal/Citation/1961/09000/KINETICS_OF_PERITONEAL_DIALYSIS__A_comparison_with.1.aspx.
|
| 9. |
Chang TI, Oh HJ, Kang EW, Yoo T-H, Shin SK, Kang S-W, et al. A Low Serum Bicarbonate Concentration as a Risk Factor for Mortality in Peritoneal Dialysis Patients. James LR, editor. PLoS One [Internet]. 2013 Dec 12;8(12):e82912. Available from: https://dx.plos.org/10.1371/journal.pone.0082912.
|
| 10. |
Ogata S, Mori M, Tatsukawa Y, Kiribayashi K, Yorioka N. Expression of vascular endothelial growth factor, fibroblast growth factor, and lactate dehydrogenase by human peritoneal mesothelial cells in solutions with lactate or bicarbonate or both. Adv Perit Dial [Internet]. 2006;22:37–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16983936.
|
| 11. |
Fernández–Perpén A, Pérez–Lozano ML, Bajo M, Albar–Vizcaino P, Correa PS, Del Peso G, et al. Influence of bicarbonate/low-GDP peritoneal dialysis fluid (BicaVera) on in vitro and ex vivo epithelial-to-mesenchymal transition of mesothelial cells. Perit Dial Int [Internet]. 2012;32(3):292–304. Available from: http://www.pdiconnect.com/content/32/3/292.short.
|
| 12. |
Theodoridis M, Thodis E, Tsigalou C, Pappi R, Roumeliotis A, Georgoulidou A, et al. Alterations of dialysate markers in chronic peritoneal dialysis patients treated with the new less bioincompatible bicarbonate solutions. Perit Dial Int [Internet]. 2011;31(2):196–9. Available from: http://www.pdiconnect.com/content/31/2/196.extract.
|
| 13. |
Schmitt CP, Nau B, Gemulla G, Bonzel KE, Hölttä T, Testa S, et al. Effect of the Dialysis Fluid Buffer on Peritoneal Membrane Function in Children. Clin J Am Soc Nephrol [Internet]. 2013 Jan;8(1):108–15. Available from: http://cjasn.asnjournals.org/lookup/doi/10.2215/CJN.00690112.
|
| 14. |
Montenegro J, Saracho R, Gallardo I, Martinez I, Munoz R, Quintanilla N. Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant [Internet]. 2007 Mar 19;22(6):1703–8. Available from: https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfl848.
|
| 15. |
Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int [Internet]. 2014;34(5):494–517. Available from: http://www.pdiconnect.com/content/34/5/494.short.
|
| 16. |
Schmitt CP, Bakkaloglu SA, Klaus G, Schröder C, Fischbach M. Solutions for peritoneal dialysis in children: recommendations by the European Pediatric Dialysis Working Group. Pediatr Nephrol [Internet]. 2011 Jul 1;26(7):1137–47. Available from: https://doi.org/10.1007/s00467-011-1863-4.
|







