Abstract
We are presenting the ablation of parasympathetic ganglia in the atria as a new method of treatment of vasovagal and other neurocardiogenic syncope. This method, shifting the balance of the autonomic nervous system in the sympathetic direction, is directed to the immediate cause of syncope which is excessive activation of the vagus nerve. Its effectiveness in the annual observation is within 80-100%. This method offers a great chance to improve the quality of life in patients with reflex syncope what have not been prevented by conventional treatment.
Citation
Koźluk E, Piątkowska A, Rodkiewicz D, Opolski G. Cardioneuroablation in neurocardiogenic syncope – hype or hope?. Eur J Transl Clin Med. 2022;5(1):47-52Introduction
Syncope is defined as total loss of consciousness characterized by a rapid onset, short duration, and spontaneous complete recovery [1]. Its direct cause is cerebral hypoperfusion [1-2]. There are many reasons of syncope and they are listed in the current ESC guidelines [1]. The most common, however with the lower risk for the patient is the neurocardiogenic syncope. Although they do not significantly increase the risk of sudden death, episodes of syncope can significantly reduce the quality of life and in some patients may be difficult to treat [3-5]. Neurocardiogenic syncope (also called reflex syncope), is caused by a pathological cardiovascular autoregulation. There are two types of syncope: peripheral and central. In the peripheral type, syncope is caused by prolonged standing. In the central type, the cause of syncope is emotional stress [6].
Pathophysiology of the reflex syncope
The most typical reflex syncope is vasovagal syncope [1] caused by orthostatic stress associated with decreased venous return, resulting in insufficient filling of the ventricles and reflex increase in their contractility. This causes activation of the left ventricular mechanoreceptors that send an impulse via the vagus nerve to the vasomotor center in the medulla oblongata. The efferent pathways consist of the vagus nerve (increased function) to the heart and sympathetic fibers (decrease function) to the heart and blood vessels. The result of this is a sudden and sharp drop in blood pressure and/or heart rate (including asystole), which causes a reduction in cerebral blood flow, leading to the total loss of consciousness [7]. In the central type, the stimulation of centers in the hypothalamus and the cerebral cortex is caused by an emotional stress factor and leads to a vasovagal reaction. [6-8]. Reflex syncope also includes carotid sinus hypersensitivity, excessive orthostatic reaction, and situational fainting, usually occurring in such situations as: coughing, sneezing, stimulation of the back of the throat, micturition, defecation, visceral pain, playing brass instruments, loss of weight or after a heavy meal [1, 4, 8]. Abnormal reactivity of the autonomic nervous system may also predispose to functional disturbances of automatism (some patients with sinus node disease) or atrioventricular conduction [9].
Current treatment methods
Lifestyle modification and non-pharmacological treatment in the prevention of neurocardiogenic syncope are effective in about 80% of patients. The next step is pharmacological treatment, yet there is no clear evidence for its effectiveness [1]. It should also be noted that when choosing a drug, contraindications should be considered, particularly in female patients of childbearing age [10]. Invasive treatment may be considered in patients with refractory syncope or who do not tolerate pharmacological methods. According to the guidelines, in people over 40 years of age with cardiodepressive syncope resistant to the above-mentioned treatment, implantation of a dual-chamber cardiac pacing system may be considered [1].
Genesis of cardioneuroablation
Recently, a new treatment option was introduced: ablation of the atrial parasympathetic ganglia (ganglion plexi – GP) [11-14]. Its genesis was an observation made during ablation of atrial fibrillation [15-16], specifically isolation of the pulmonary veins (PV), that damage of the parasympathetic ganglia (manifested during ablation with bradycardia/sinus asystole or in the course of second- or third-degree atrioventricular block or decrease in arterial pressure; after ablation with an acceleration of sinus rhythm) correlates with higher ablation effectiveness [15]. Hence, the next step was to use targeted ablation of the parasympathetic ganglia to treat reflex syncope [11-14].
Anatomical and histological background
The intrinsic cardiac autonomic nervous system is comprised of an extensive epicardial neural network of nerve axons, interconnecting neurons and clusters of autonomic ganglia, known as GP [17] (Fig. 1). Most of them are located within epicardial fat pads. Ganglion plexi vary in size, contain from just a few neurons to over several hundred neurons [18-19]. The highest density of autonomic innervation is found at the posterior wall of the left atrium, particularly at the pulmonary vein–atrial junction [18]. The most important anterior right GP is located immediately anterior to the right superior PV and often extends inferiorly, to the region anterior to the right inferior PV. Other important are the superior left GP located at the roof of the left atrium, medial to the left superior PV, the right and left inferior GP located at the inferior aspect of the posterior wall of the left atrium, below the right and left PVs [20].
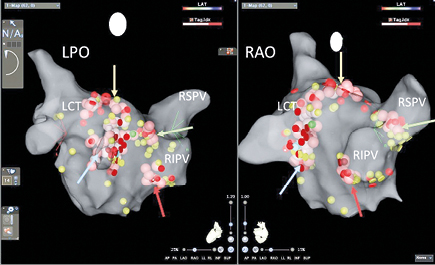
Fig.1. Anatomical CARTO map of the left atrium and pulmonary vein ostia in patient during GP ablation. This patient has anatomical variant with left common trunk. LAO – left posteriori oblique view, LCT – left common trunk, RAO – right anterior oblique view, RIPV – right inferior pulmonary vein, RSPV – right superior pulmonary vein; yellow dots – pacing points without neurocardiogenic reaction, red dots – pacing points with sinus bradycardia induction, green dots – pacing points with vasodepressive reaction, blue dots – pacing points with mixt-type reaction, brown/pink/white – dots – ablation point; red arrow – right inferior GP, green arrow – right superior GP, blue arrow – left inferior GP, yellow arrow – left superior GP
Cardioneuroablation – challenges for clinicians and electrophysiologists
Cardioneuroablation is a relatively new technique for managing patients with vasovagal syndrome, hence there are no clear indications and contraindications for this procedure [1]. There are many different criteria to qualify patients and many techniques of the procedure, and we need some time to determine in which clinical situations what type of the procedure is extremely effective, beneficial or not at all. So far, several studies were carried out to prove that cardioneuroablation is an effective and safe method of treating vasovagal syndrome [11-14]. The first clinical results are very promising. However, because of the small size of the treatment groups, short observation period and the lack of control groups, the available data are insufficient to confirm the efficacy and safety of ablation of the atrial ganglion plexi [1]. The longest follow-up was published by Sun W. et al. [21] with total cohort of 57 patients over the course of 12-102 months. Syncope and syncope prodromes recurred in 5 and 16 participants, respectively [21]. Because of small study group there were no statistically significant differences between group with ablation based on high-frequency stimulation and based on anatomy. However, all 5 recurrences of syncope were in anatomical group [21]. The time to first recurrent syncopal episode after ablation ranged from 2 to 17days [21] and such early recurrence suggests that anatomical approach ablation was not enough in some patients. Further studies, particularly randomized multicenter trials, should be performed to assess the long-term effects of the GP ablation strategy in neurocardiogenic syncope [1]. Similar results with 34.1 ± 6.1 months of follow-up were published by Calo L. et al. [22] according to ablation of the right atrial GP only. During this time only 3 of 18 patients (16.6%) experienced syncopal episodes and 5 patients (27.7%) only prodromal episodes (including during the head-up tilt test).
Contrary to pharmacotherapy or pacemaker implantation, ablation aims to get to the source of the problem: disturbances in the intrinsic cardiac autonomic nervous system [17, 23]. The main goal of cardioneuroablation is parasympathetic denervation of the heart (more precisely, shift of the autonomic balance into the sympathetic direction) to treat reflex (neurocardiogenic syncope) or functional (sinus node dysfunction and functional atrioventricular block) bradycardia/asystole [11]. In contrast to the permanent cardiac pacing, the effectiveness of this method is not limited only to cardiodepressive syncope [11, 14, 24], but is also effective in mixed-type of syncope [13, 21, 25]. There are no data on the efficacy of cardioneuroablation in vasovagal syndrome of the vasodepressive type. Theoretically, the chances for success are much lower here, as the effector of this form of syncope is mainly in the vessels. However, it has been shown that the same patient can have various forms of vasovagal syncope (e.g. some episodes vasodepressive, while others cardiodepressive or mixed) [3, 26]. Also, during the isolating pulmonary veins we sometimes observe a blood pressure reaction only, so the pathophysiology of cardioneuroablation in this group is ambiguous.
In most centers, the atropine test is performed prior to cardioneuroablation and only patients with a correct response to the drug are qualified for the procedure [11, 14]. The theoretical basis for this way of proceeding seems to be very strong. However, the question about the dose of atropine remains open (an insufficient dose may lead to a negative test result). Besides, cardiodepressive syncope has an effector in the heart, so in theory it should be sensitive to ablation in all patients. At present it is difficult to predict which treatment approach is appropriate.
Cardioneuroablation – methodology
In order to find GP ganglia and identify the targets of ablation, electroanatomical maps of the right atrium (RA) and left atrium (LA) are created. High-frequency stimulation or anatomical site selection or characteristics of the atrial electrograms can be used to select the site for GP ablation. Various combinations of these methods can be used [14, 21-25]. In our center, we prefer mapping with pacing (100ms cycle length) (Fig. 2A, 2B). If there is no response to stimulation (we observe this in about half of the patients), we perform ablation using the anatomical method observing the heart rate and pressure response to the applications. If during applications we observe the reaction from GP, we prolong it to obtain a permanent effect [24-25].
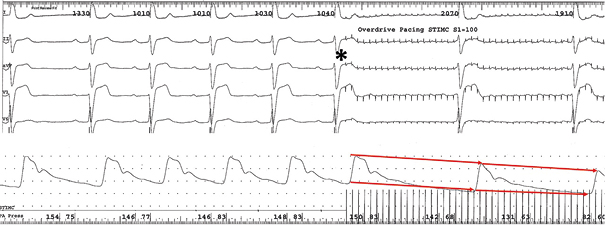
Fig.2A. High frequency pacing (CL 100ms) of GP with mixt type naurocardiogenic reaction. Beginning of pacing marked with the star. After that there is a significant decreasing of sinus rhythm frequency. Blood pressure line present decreasing of the systolic and diastolic arteriar pressure (red arrow)
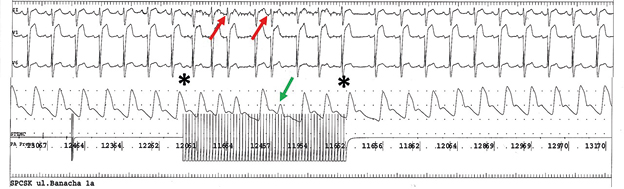
Ryc.2B. High frequency pacing (CL 100ms) of GP region after ablation without important decreasing of sinus rhythm frequency and blood pressure. Stars indicate beginning and termination of pacing. There are two supraventricular extrasystolies induced by pacing (red arrows). After the second one we can observed pulse wave deficyt (green arrow).
Ganglion plexi ablation shifts the balance of the autonomic system towards the sympathetic side. This is related to the improvement in the parameters of automatism and conduction, also observed in our patients [11-14, 24]. In some cases, excessive sinus tachycardia is observed, requiring temporary treatment with a beta-blocker, occasionally in combination with ivabradine (our experience) [24]. Usually, this drug therapy is temporary (few months) because the heart rhythm slows down during follow-up, although it is not as strong as it was before the procedure (probably partial reinnervation) [12, 21, 24]. In study by Sun W. et al [21] the heart rate variability and heart rate demonstrated significant changes at 3 months that persisted at 12 months after the procedure. Compared with the baseline measurement, the time‐ and frequency‐domain heart rate variability was significantly lower (except at low frequency), whereas the minimum, mean, and maximum heart rates were significantly higher (P < 0.01). After an average period of 28.7 ± 9.8 months after ablation, only the minimum heart rate remained higher than before the ablation (p = 0.022). This also confirms partial reinnervation hypothesis [12].
Cardioneuroablation – outcomes
At present, the effectiveness of GP ablation in preventing recurrence of vasovagal syncope is within the range of 80-100% [11-14, 21, 23]. In order to assess the direct effectiveness of the procedure, it is worth considering several endpoints, defined as an increase the frequency of the sinus rhythm or a decrease in the degree of atrioventricular (AV) block [12, 14] or lack of response to stimulation in places where it induced a neurocardiogenic reaction [12, 21, 24-25]. It may also be useful to record the lower variability of automatism and conduction parameters observed in patients with vasovagal syndrome [27]. We can also receive valuable information from HRV analysis based on 24-hours ECG Holter monitoring [28]. Performing a control head-up tilt tests raises doubts. Due to the fact that this tests provokes reflex reaction in a very aggressive, exceeding the real-life conditions manner, it is not recommended to assess the effectiveness of any form of treatment of vasovagal syncope [1].
In most studies, however, the control head-up tilt test is performed because for research purposes. Changes in test results that were interpreted as a benefit of cardioneuroablation include a change in the detected hemodynamic response to a vasodepressive form or a completely negative test result, as well as an increase in the time of symptoms or syncope onset [17]. The behavior of hemodynamic parameters during upright standing may also be helpful in the assessment [29-23].
Summary
At present, cardioneuroablation is still an experimental method, although it has a strong pathophysiological basis and much indicates its high effectiveness. For this reason, it should be reserved for patients with multiple syncope, especially traumogenic, which cannot be prevented by lifestyle modification. The effectiveness of this method has been documented in patients with cardiodepressive and mixed syncope.
Funding
None.
Conflicts of interest
None.
References
| 1. |
Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, et al. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J [Internet]. 2018 Jun 1;39(21):e43–80. Available from: https://doi.org/10.1093/eurheartj/ehy071.
|
| 2. |
Szufladowicz E, Maniewski R, Nosek A, Bodalski R, Kozluk E, Zbiec A, et al. Changes in cerebral oxygenation during vasovagal syncope inducted by tilt-table test. Folia Cardiol [Internet]. 2005;12(suppl D):184–6. Available from: https://www.researchgate.net/profile/Edward-Kozluk/publication/267843149_CHANGES_IN_CEREBRAL_OXYGENATION_DURING_VASOVAGAL_SYNCOPE_INDUCED_BY_TILT_-_TABLE_TEST/links/548b64760cf2d1800d7db70b/CHANGES-IN-CEREBRAL-OXYGENATION-DURING-VASOVAGAL-SYNCOPE-INDUCED-BY-TILT-TABLE-TEST.pdf.
|
| 3. |
Koźluk E, Zastawna I, Piątkowska A. Omdlenia neurokardiogenne – kiedy stawką jest jakość życia [in Polish]. Kardiol w Prakt. 2004;(1):16–21.
|
| 4. |
Koźluk E, Kozłowski D. Omdlenia i utraty przytomności jako problem interdyscyplinarny [in Polish]. Kardiol w Prakt. 2004;(1):3–6.
|
| 5. |
Rudnicki J, Zyśko D, Kozłowski D, Kuliczkowski W, Koźluk E, Lelonek M, et al. The Choice of Surgical Specialization by Medical Students and Their Syncopal History. Biondi-Zoccai G, editor. PLoS One [Internet]. 2013 Jan 31;8(1):e55236. Available from: https://dx.plos.org/10.1371/journal.pone.0055236.
|
| 6. |
Janiec I, Werner B, Pietrzak R. Omdlenia wazowagalne u dzieci [in Polish]. Nowa Pediatr. 2010;3:84–6.
|
| 7. |
Kozłowski D, Koźluk E, Krupa W. Patomechanizm omdleń wazowagalnych. Folia Cardiol [Internet]. 2000;7(2):83–6. Available from: https://journals.viamedica.pl/folia_cardiologica/article/download/24787/19849.
|
| 8. |
da Silva RMFL. Syncope: epidemiology, etiology, and prognosis. Front Physiol [Internet]. 2014 Dec 8;5. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2014.00471/abstract.
|
| 9. |
Adan V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician [Internet]. 2003;67(8):1725–32. Available from: https://www.aafp.org/afp/2003/0415/p1725.html.
|
| 10. |
Hutt-Centeno E, Mayuga KA. What can I do when first-line measures are not enough for vasovagal syncope? Cleve Clin J Med [Internet]. 2018 Dec;85(12):920–2. Available from: https://www.ccjm.org//lookup/doi/10.3949/ccjm.85a.17112.
|
| 11. |
Pachon M JC, Pachon M EI, Pachon M JC, Lobo TJ, Pachon MZ, Vargas RNA, et al. “Cardioneuroablation” – new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. EP Eur [Internet]. 2005 Jan 1;7(1):1–13. Available from: https://academic.oup.com/europace/article/7/1/1/432294.
|
| 12. |
Pachon M JC, Pachon M EI. Differential effects of ganglionic plexi ablation in a patient with neurally mediated syncope and intermittent atrioventricular block: a commentary. Europace [Internet]. 2016 Jun 14;euw133. Available from: https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/euw133.
|
| 13. |
Yao Y, Shi R, Wong T, Zheng L, Chen W, Yang L, et al. Endocardial Autonomic Denervation of the Left Atrium to Treat Vasovagal Syncope. Circ Arrhythmia Electrophysiol [Internet]. 2012 Apr;5(2):279–86. Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.111.966465.
|
| 14. |
Piotrowski R, Baran J, Kułakowski P. Cardioneuroablation using an anatomical approach: a new and promising method for the treatment of cardioinhibitory neurocardiogenic syncope. Kardiol Pol [Internet]. 2018 Dec 17;76(12):1736–8. Available from: https://journals.viamedica.pl/kardiologia_polska/article/view/83108.
|
| 15. |
Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, et al. Circumferential Pulmonary-Vein Ablation for Chronic Atrial Fibrillation. N Engl J Med [Internet]. 2006 Mar 2;354(9):934–41. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa050955.
|
| 16. |
Lemery R, Birnie D, Tang ASL, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Hear Rhythm [Internet]. 2006 Apr;3(4):387–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1547527106000658.
|
| 17. |
Stavrakis S, Po S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythmia Electrophysiol Rev [Internet]. 2017;6(4):186. Available from: https://www.aerjournal.com/articles/ganglionated-plexi-ablation-physiology-and-clinical-applications.
|
| 18. |
Armour JA, Murphy DA, Yuan B-X, MacDonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec [Internet]. 1997 Feb 1;247(2):289–98. Available from: https://doi.org/10.1002/(SICI)1097-0185(199702)247:2%3C289::AID-AR15%3E3.0.CO.
|
| 19. |
Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec [Internet]. 2000 Aug 1;259(4):353–82. Available from: https://doi.org/10.1002/1097-0185(20000801)259:4%3C353::AID-AR10%3E3.0.CO.
|
| 20. |
Po SS, Nakagawa H, Jackman WM. Localization of Left Atrial Ganglionated Plexi in Patients with Atrial Fibrillation. J Cardiovasc Electrophysiol [Internet]. 2009 Oct;20(10):1186–9. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1540-8167.2009.01515.x.
|
| 21. |
Sun W, Zheng L, Qiao Y, Shi R, Hou B, Wu L, et al. Catheter Ablation as a Treatment for Vasovagal Syncope: Long‐Term Outcome of Endocardial Autonomic Modification of the Left Atrium. J Am Heart Assoc [Internet]. 2016 Jul 6;5(7). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.116.003471.
|
| 22. |
Calo L, Rebecchi M, Sette A, Sciarra L, Borrelli A, Scara A, et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: a long-term follow-up prospective study. J Interv Card Electrophysiol [Internet]. 2021 Sep 6;61(3):499–510. Available from: https://link.springer.com/10.1007/s10840-020-00840-9.
|
| 23. |
Aksu T. Cardioneuroablation in the treatment of neurally mediated reflex syncope: a review of the current literature. Turk Kardiyol Dern Arsivi-Archives Turkish Soc Cardiol [Internet]. 2016; Available from: http://archivestsc.com/jvi.aspx?un=TKDA-55250.
|
| 24. |
Koźluk E, Piątkowska A, Rodkiewicz D, Kowalczuk N, Opolski G. Kardioneuroablacja w omdleniach odruchowych — nowa nadzieja dla trudnych pacjentów [in Polish]. Forum Med Rodz [Internet]. 2019;13(5):223–31. Available from: https://journals.viamedica.pl/forum_medycyny_rodzinnej/article/view/65347.
|
| 25. |
Koźluk E, Piątkowska A, Rodkiewicz D. Omdlenia – jak wejść na właściwą ścieżkę diagnostyczna i leczniczą? [in Polish]. Stany Nagłe po Dyplomie [Internet]. 2021;5(2). Available from: https://podyplomie.pl/snpd/categories/2323.
|
| 26. |
Koźluk E, Kozłowski D, Szufladowicz E. Results of head-up tilt test with nitroglicerin depends on used protocol. In: Thomsen PE, editor. Europace 2001. Bologna: Monduzzi Editore; 2001. p. 143–7.
|
| 27. |
Graff B, Graff G, Koźluk E, Tokarczyk M, Piątkowska A, Budrejko S, et al. Electrophysiological features in patients with sinus node dysfunction and vasovagal syncope. Arch Med Sci [Internet]. 2011;6:963–70. Available from: http://www.termedia.pl/doi/10.5114/aoms.2011.26607.
|
| 28. |
Pachon-M JC, Pachon-M EI, Pachon CTC, Santillana-P TG, Lobo TJ, Pachon-M JC, et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ Arrhythmia Electrophysiol [Internet]. 2020 Dec;13(12). Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.120.008703.
|
| 29. |
Buszko K, Piątkowska A, Koźluk E, Fabiszak T, Opolski G. Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope. Entropy [Internet]. 2018 Dec 16;20(12):976. Available from: http://www.mdpi.com/1099-4300/20/12/976.
|
| 30. |
Koźluk E, Cybulski G, Piątkowska A, Zastawna I, Niewiadomski W, Strasz A, et al. Early hemodynamic response to the tilt test in patients with syncope. Arch Med Sci [Internet]. 2014;6:1078–85. Available from: http://www.termedia.pl/doi/10.5114/aoms.2014.47820.
|
| 31. |
Buszko K, Piatkowska A, Kozluk E. Entropy in description of vasovagal syndrome. In: 2015 Computing in Cardiology Conference (CinC) [Internet]. IEEE; 2015. p. 981–4. Available from: http://ieeexplore.ieee.org/document/7411077/.
|
| 32. |
Zastawna I, Lodziński P, Koźluk E. Parametry hemodynamiczne w 5 minucie testu pionizacyjnego pozwalają przewidzieć wynik testu pionizacyjnego [in Polish]. 63–65. Pol Przegląd Kardiol. 2004;6(suppl.1):63–5.
|









