Abstract
Background
Adrenal tumors are common neoplasms and majority of them are small, benign, hormonally inactiveadrenocortical adenomas. Whereas adrenal cancer is a rarely occurring (5% of adrenal tumors) but highly aggressive neoplasm. The early diagnosis and complete surgical resection is the only effective treatment option. Laparoscopic adrenalectomy is the gold standard for small and medium tumors. Whereas for large tumors classic adrenalectomy is considered a procedure of choice with a proven better oncological outcome.
Material and methodsThere were 245 patients with adrenal tumors operated in the Department of General, Endocrine and Transplant Surgery, Medical University of Gdańsk, Poland between 2014and 2018. Results: Out of entire series, there was one case of a 57-year-old female diagnosed with a large, advanced left adrenal tumor with invasion of vena cava. It was diagnosed in computer tomography and proven in core biopsy. Open adrenalectomy with thoracotomy was conducted to completely resect the tumor by the interdisciplinary team.
Conclusions
In case of large adrenal tumour with vessel infiltration, a successful R0 resection can be performed by multi-specialist approach. For adrenal cancer early diagnosis based on the clinical, biochemical and imaging features and successful surgical treatment is crucial in significant prolongation of patient survival.
Citation
Dobrzycka M, Spychalski P, Rusek U, Brzeziński M, Kobiela J, Kłosowski P, Berendt-Obołończyk M, Łachiński A, Sworczak K, Śledziński Z. Interdisciplinary treatment of large adrenocortical carcinoma infiltrating inferior vena cava. Eur J Transl Clin Med. 2019;2(2):38-42Introduction
Adrenocortical cancer (ACC) is a rare malignancy. The incidence is estimated to be 1 to 2 per million per year [1]. There is a predilection for the female sex with 2.5-fold higher prevalence [1]. Despite its rare occurrence, According is especially noteworthy due to its aggressive nature. The prognosis of ACC is poor with a 5-year overall survival rates below 30% in most series [2]. In addition, lymph node and/or distant organ metastases are already present in about 60% of patients at the time of diagnosis [3]. Early diagnosis of hormone-secreting adrenocortical tumor depends on the occurrence of clinical symptoms of excess of adrenocortical hormones. Whereas the diagnosis of non-secreting tumors is usually accidental in patients due to non-specific abdominal pain, nausea, vomiting and feeling of abdominal fullness [1-4].
The primary treatment of ACC is surgical excision [5]. Furthermore, chemotherapy with steroidogenesis inhibitors (especially mitotane) is crucial. The application of mitotane can be considered in adjuvant treatment and is the basis for the management of patients with diffused ACC. In the treatment of advanced disease, multi-drug chemotherapy is also used alone or in combination with mitotane. Currently, the basic treatment regimen is the combination of etoposide, doxorubicin and cisplatin (EDP) [1].
Series analysis
There were 245 patients with adrenal tumors operated in the Department of General, Endocrine and Transplant Surgery, Medical University of Gdańsk, Poland between 2014 and 2018. In 211 (86%) of them laparoscopic adrenalectomy was performed, whereas in 34 patients with large tumors classic adrenalectomy was performed. In histopathological examination there were 5 cases of ACC. In the presented case report, we describe the clinical picture of a patient with highly advanced, metastatic ACC. Furthermore, we present a highly specialized interdisciplinary approach towards patient care in case of a rare cancer.
Case report
A 57 year old woman (0.4%) was admitted to the Department of General, Endocrine and Transplant Surgery at the Medical University of Gdańsk (Poland) due to presence of a left adrenal tumor. The patient’s history included autoimmune polyendocrine syndrome type 2 (APS2) diagnosed 10 years prior. Since then she received hormonal substitution for Hashimoto's disease and adrenal insufficiency (thyroxine, prednisone and fludrocortisone). Furthermore, she was diagnosed with hypertension one month before the admission to hospital. The patient's family history included her mother's death from adrenal cancer at the age of 69.
An outpatient abdominal ultrasound incidentally discovered a 97x75x94 mm tumor located in the left adrenal gland (near the superior pole of the left kidney). It had mixed echogenicity, peripheral and central flows, fluid spaces and fine calcifications. The diagnosis was confirmed in abdominal CT examination (Fig. 1). The lesion was 143x85mm in transverse dimension and 98 mm (cephalocaudal dimension). In addition, the CT images revealed infiltration and thrombosis of the left renal vein, ovarian vein and the inferior vena cava from the left renal vein inflow to the diaphragm level. Furthermore, a slight 5 mm hypodense focal lesion was identified in the liver segment IVa and a 4 mm nodule was revealed in the right lung segment 8 near the fissure. Endocrine diagnostics showed increased androgen concentration without any clinical symptoms of hyperandrogenism. The androstendione level was 29.7 ng/ml, (normal range 0.3-3.5 ng/ml), the dehydroepiandrosterone sulphate (DHEA-S) was also markedly elevated to 960 ug/dl, (normal range 26-200 ug/dl), the testosterone was elevated to 6.6 nmol/l (normal range 0.43-1.24 nmol/l) and the 17(OH)-progesterone was 7.7 ng/ml. The excretion of meta- and normetanephrine in a 24-hour collection of urine was normal. Loss of the circadian rhythm of cortisol secretion (morning cortisol level was 16 ug/dl and nighttime was 17 μg/dl) in this case was not very diagnostic because of adrenal insufficiency and hormonal substitution.
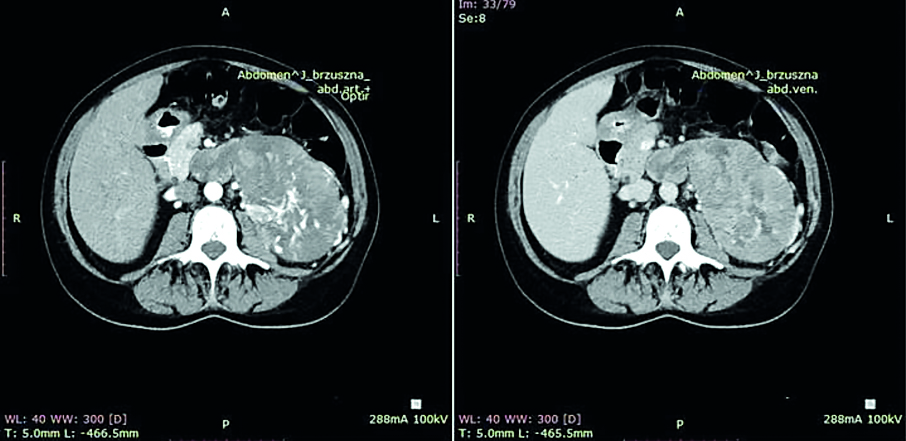
Figure 1. Abdomen CT. Left adrenal gland tumor. Infiltration of left renal vein, vena cava inferior and left kidney
A core biopsy revealed the formation of a tumor originating from the adrenal cortex, with the following pathological characteristics: IHC:inhibin +/-, MelanA +, Ki67 7%, no signs of increased atypia, atypical divisions or necrosis. Together these features result in Weiss score >3, indicating the malignant nature of the lesion. The patient was qualified for curative surgical treatment planned by an interdisciplinary team consisting of general and cardiac surgeons.
After the laparotomy, a 20cm tumor displacing the kidney downwards was found in the left adrenal field. It was growing into the inferior vena cava through the left renal vein. Moreover, a solid 8mm lesion in the left lobe of the liver was exposed. No further signs of tumor spread were revealed. Then a right-sided thoracotomy was performed by a cardiac surgeon to obtain access to the inferior vena cava through the apex of the right atrium. Following this, IVC below the renal veins and the right renal vein were clamped. The tumor was extracted by incising the left renal vein at the entrance to the inferior vena cava. Later, the adrenal gland with the tumor, the left kidney, the retroperitoneal tissues and the spleen were resected en bloc (Fig 2). Next, a non-anatomical tumor resection of the left lobe of the liver with margins was performed. The surgery lasted 284 minutes. The estimated intraoperative blood loss was 1700 ml. Histopathological examination of the extracted tissue revealed ACC cells with atypical mitosis, hemorrhage and extensive necrosis (Fig 3). There was no evidence of invasion of the organ capsule. Cancer was resected within the limits of the organ pouch. In addition, the tumor expanded into the renal vein. Metastasis was found in the removed liver tumor. The postoperative period was complicated by hematoma in right pleural cavity resulting from bleeding from the branch of right internal mammary artery and from the splenic artery stump (successfully treated with transarterial embolization). Hydrocortisone replacement therapy was conducted perioperatively. On postoperative day 15 the patient was discharged home. In laboratory follow-up 1 month after the surgery, the total testosterone and DHEA-S levels returned to normal (0.8 ng/ml and 15 ug/dl respectively). Later the patient was treated for metastasis found in CT with chemotherapy (mitotane, etoposide and cisplatin) and transarterial chemoembolization of the liver metastasis with lipiodol and doxorubicin.
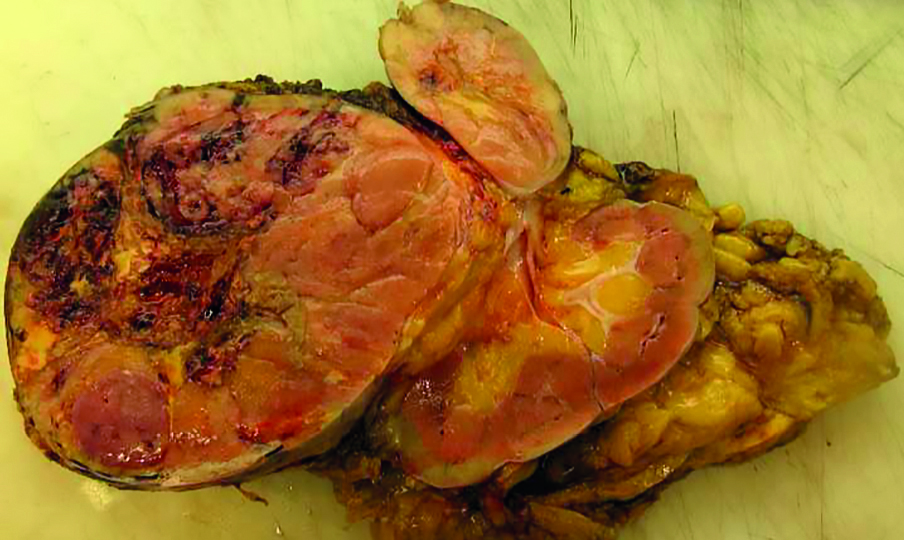
Figure 2. Adrenal cancer resected en bloc with left kidney and vascular invasion to inferior vena cava
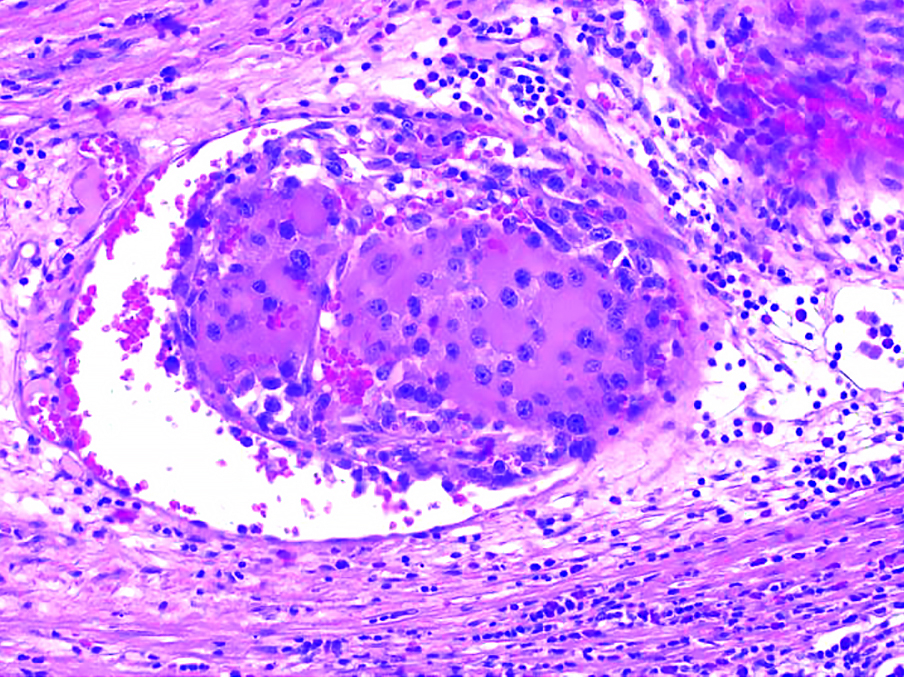
Figure 3. Microscopic intravascular invasion of adrenal cancer
Discussion
The diagnosis of adrenal mass should focus on distinguishing between benign and non-secreting masses from malignant or hormonally active lesions that require further treatment. It consists of a complete physical examination, biochemical evaluation of hormone secretion, and careful examination of radiological imaging tests. Adrenal tumor biopsy is contraindicated in the case of suspected ACC (due to the risk of local spread after damage to the tumor capsule) and pheochromocytoma (danger of hypertensive crisis). Its implementation is justified in the case of suspicion of metastases to the adrenal glands if imaging studies do not state clearly whether the lesion is benign or malignant and the unequivocal determination of the etiology of focal lesions will affect further therapeutic treatment. About 80% of the tumors are benign adenomas or adrenal tuberous hyperplasia. According to The European Network for the Study of Adrenal Tumors (ENSAT) classification, the patient described above was diagnosed with stage IV tumor with high grade malignant features in histological Weiss criteria [6-7].
The basic screening tests for hypercortisolism include test of inhibition of cortisol secretion after oral administration of 1 mg dexamethasone, daily excretion of free cortisol in the urine and a midnight salivary cortisol level. In women with adrenal tumors and with hyperandrogenisation syndrome, it is recommended to measure total testosterone, DHEA-S and 17(OH)-progesterone. High levels of testosterone (>200 ng/dl), DHEA-S (>800 μg/dl) and 17(OH)-progesterone are more often associated with ACC [8]. Our patient had an increased androgen concentration (total testosterone, 17(OH)-progesterone, androstendione and DHEA-S) and no circadian rhythm of cortisol secretion.
The coexistence of ACC with the history of APS2 might change the standard diagnostic process. In such cases, non-circadian secretion of cortisol should be considered because it is already disturbed as a result of APS2. Also, our patient's normal level of aldosterone seems intriguing. Before the tumor developed, the patient might have had reduced aldosterone levels due to the adrenal insufficiency, whereas secretion by the ACC brought this hormone to its normal level.
For localized ACC the only curative method is a complete resection (R0) of the tumor and it is considered as the treatment of choice. Superiority of open vs. laparoscopic adrenalectomy remains controversial. Laparoscopic adrenalectomy is the gold standard in adrenal tumors surgery but recent research has shown that open adrenalectomy is superior to laparoscopic approach in terms of disease-free survival and rate of 2-year disease-free survival, in spite of the larger maximum diameter of tumors and lesser benefit during the perioperative period [9- 10]. Laparoscopic surgery is still not recommended in case of large tumor (>6cm) and if there is a suspicion of local invasion or the presence of metastases in regional lymph nodes. About 30-40% of patients are in the IV clinical stage at the time of diagnosis. The prognosis in these patients is significantly worse than in stage I and II, but with the predicted resectability R0 of the primary site and the resection of lung and liver metastases, they are still eligible for surgery. Due to the relatively frequent recurrences after treatment (range 19% - 30% depending on the tumor stage), adjuvant therapy should be administered after the surgery [3]. It includes using mitotane and tumoral bed irradiation. In cases of oligometastatic disease, >80% tumor mass resection and slow tumor progression, radiotherapy, radiofrequency ablation or transarterial chemoembolization with mitotane can be conducted [1].
Conclusion
In conclusion, the presented case has shown that in case of large, advanced ACC with vessel infiltration a successful R0 resection can be performed by multidisciplinary surgical team. For adrenal cancer early diagnosis based on the clinical, biochemical and imaging features and successful surgical treatment is crucial in order to achieve significant prolongation of patient survival.
References
| 1. |
Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab [Internet]. 2009;23(2):273–89. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1521690X08001413.
|
| 2. |
Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet]. 2012;23(suppl 7):vii131–8. Available from: https://academic.oup.com/annonc/article-lookup/doi/10.1093/annonc/mds231.
|
| 3. |
Fay AP, Elfiky A, Teló GH, McKay RR, Kaymakcalan M, Nguyen PL, et al. Adrenocortical carcinoma: The management of metastatic disease. Crit Rev Oncol Hematol [Internet]. 2014;92(2):123–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1040842814000882.
|
| 4. |
Dworakowska D, Drabarek A, Wenzel I, Babińska A, Świątkowska-Stodulska R, Sworczak K. Rak kory nadnercza (ACC) — przegląd piśmiennictwa i doświadczenia własne. Endokrynol Pol [Internet]. 2015;65(6):492–512. Available from: http://czasopisma.viamedica.pl/ep/article/view/38591.
|
| 5. |
Gaujoux S, Mihai R. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg [Internet]. 2017;104(4):358–76. Available from: http://doi.wiley.com/10.1002/bjs.10414.
|
| 6. |
Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, Isbarn H, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: A North American validation. Eur J Cancer [Internet]. 2010;46(4):713–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0959804909009198.
|
| 7. |
Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A. Weiss criteria in large adrenocortical tumors: A validation study. Indian J Pathol Microbiol [Internet]. 2010;53(2):222. Available from: http://www.ijpmonline.org/text.asp?2010/53/2/222/64325.
|
| 8. |
McDuffie LA, Aufforth RD. Adrenocortical carcinoma: modern management and evolving treatment strategies. Int J Endocr Oncol [Internet]. 2016;3(2):161–74. Available from: https://www.futuremedicine.com/doi/10.2217/ije-2015-0003.
|
| 9. |
Zheng G-Y, Li H-Z, Deng J-H, Zhang X-B, Wu X-C. Open adrenalectomy versus laparoscopic adrenalectomy for adrenocortical carcinoma: a retrospective comparative study on short-term oncologic prognosis. Onco Targets Ther [Internet]. 2018;11:1625–32. Available from: https://www.dovepress.com/open-adrenalectomy-versus-laparoscopic-adrenalectomy-for-adrenocortica-peer-reviewed-article-OTT.
|
| 10. |
Autorino R, Bove P, De Sio M, Miano R, Micali S, Cindolo L, et al. Open Versus Laparoscopic Adrenalectomy for Adrenocortical Carcinoma: A Meta-analysis of Surgical and Oncological Outcomes. Ann Surg Oncol [Internet]. 2016;23(4):1195–202. Available from: http://link.springer.com/10.1245/s10434-015-4900-x.
|









