Abstract
Biobank Laboratory of the University of Łódź is a unit in the organizational structure of the De- partment of Molecular Biophysics at the Faculty of Biology and Environmental Protection. It was established in 2014 as one of the results of the TESTOPLEK project. One of the main goals of the unit is to collect and share biological material of human origin and related clinical and survey data. Moreover, Biobank Laboratory conducts work in the field of genetics and molecular biology on human biological material. Biobank Laboratory gathers over 40.000 samples such as DNA, FFPE, saliva, together with their data. Data about its material is available for researchers in directories e.g. BBMRI-ERIC Directory 4.0. Since 2014, the unit belongs to the national Consortium BBMRI.pl, and since 2017 it executes a project entitled Research Infrastructure for Biobanks and Biomolecular Resources BBMRI-ERIC, co-creating the Polish Network of Biobanks. Biobank Laboratory is focused on coopera- tion with domestic and foreign scientific institutions and medical units, as well as entities from the local, business and public sector.
Citation
Dobrowolska S, Michalska-Madej J, Słomka M, Sobalska-Kwapis M, Strapagiel D. Biobank Łódź® – population based biobank at the University of Łódź, Poland. Eur J Transl Clin Med. 2019;2(1):85-95Introduction
Biobank name
Biobank Laboratory, Department of Molecular Biophysics, Faculty of Biology and Environmental Protection, University of Łódź [1].
Biobank ID
Biobanking directory: BBMRI-ERIC Directory 4.0 [2].
ID: PL_BLUL
Biobank contact
- Address: 14/16 Pilarskiego Street, 90-231 Łódź, Poland
- URL: www.biobank.uni.lodz.pl
- e-mail: biobank@uni.lodz.pl
- Phone number: +48 42 665 57 02
- Corresponding person: Dr. Dominik Strapagiel, dominik.strapagiel@biol.uni.lodz.pl
Biobank history
Biobank Laboratory of the University of Łódź is a unit in the organizational structure of the Department of Molecular Biophysics at the Faculty of Biology and Environmental Protection, established by the Resolution of the Dean of the Faculty of Biology and Environmental Protection of 25/03/2014.
Biobank Laboratory was created as one of the results of the TESTOPLEK project – full name “Role of multidrug resistance proteins in pharmacokinetics and toxicology – in vitro tests in pharmaceutical and clinical practice” (2008-2014) [3-5].
Over 10.000 individuals nationwide were canvassed to create a retrospective POPULOUS collection (POPUlation - LOdz UniverSity Biobank). The next step was the acquisition of collections of clinical samples, including breast cancer patients (BREAST), patients with pancreatic cancer (PANC), as well as a representative collection of samples from the school-age population from the city of Łódź (PUPILS).
Since 2014, the unit belongs to the national Consortium BBMRI.pl, and since 2017 it executes a project entitled Research Infrastructure for Biobanks and Biomolecular Resources BBMRI-ERIC (Biobanking and BioMolecular Resource Research Infrastructure - European Research Infrastructure Consortium), co-creating the Polish Network of Biobanks [1, 6-7].
Biobank legal status
Public; a part of Department of Molecular Biophysics, Faculty of Biology and Environmental Protection, University of Łódź [8-9].
Current biobanking activity
Longitudinal study; collecting; processing; storage.
Main biobank aim
One of the main goals of the unit is to collect and share biological material of human origin and related clinical and survey data. Moreover, Biobank Laboratory conducts work in the field of genetics and molecular biology on human biological material.
Biobank membership
- BBMRI.pl (Biobanking and BioMolecular resources Research Infrastructure - Consortium) [6],
- BCNet (Biobank and Cohort Building Network) [10],
- ESBB (European and Middle Eastern Society for Biopreservation and Biobanking) [11],
- co-creating the Polish Network of Biobanks [6].
Certification
Biobank Laboratory has European certificates from IBBL (Integrated Biobank of Luxemburg) [12] in the fields of:
- DNA Quantification and Purity,
- RNA Quantification and Purity,
- DNA Extraction from Whole Blood,
- RNA Extraction from Whole Blood,
- Microbial DNA Extraction from Saliva,
- CSF Aliquoting [13].
Some employees of the unit have certification as lead auditors in the field of information security and quality management granted by leading international and national centres [14].
International system standards
In the near future Biobank Laboratory plans to implement international standards related to management systems, such as PN-EN ISO 9001:2015 Quality management systems – Requirements and PN-EN ISO/IEC 27001:2017 Information security management system – Requirements.
Scientific and research projects
TESTOPLEK: “Role of multidrug resistance proteins in pharmacokinetics and toxicology – in vitro tests in pharmaceutical and clinical practice” - POIG grant 01.01.02-10-005/08 TESTOPLEK from the European Regional Development Fund (2008-2014).
BBMRI: "Research Infrastructure for Biobanks and Biomolecular Resources BBMRI-ERIC” – Polish Ministry of Science and Higher Education no. DIR/WK/2017/01 (2017-2021).
POPC: “Digital sharing of biomolecular and descriptive resources of Biobank and Department of Anthropology, University of Łódź – characteristics of populations living in present-day Poland through the ages. Information platform e-Czlowiek.pl” - Polish Ministry of Science and Higher Education no. DIR/WK/2017/01 (Operational Programme Digital Poland for 2014-2020).
Let`s BioIT: "Let's Bio-IT development of professional, bioinformatic, language and entrepreneurial skills of students of the Faculty of Biology and Environmental Protection, University of Łódź" – Project no. POWR.03.01.00-00-K410 / 16-00 (2017-2019).
POLPHARMA: "The study of the importance of epigenetic processes in the pathogenesis of mastocytosis to find new therapeutic options" founded by Polpharma Scientific Foundation (2018-2021).
Organization structure of biobank
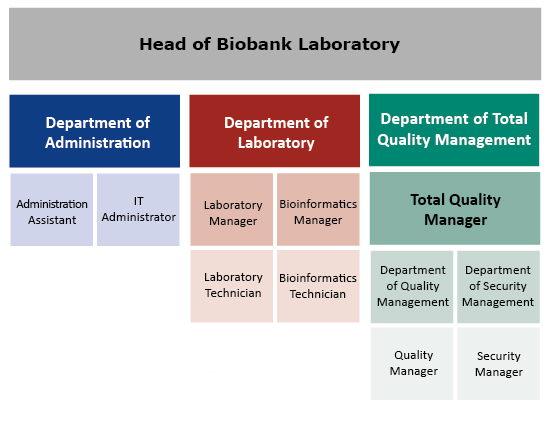
Figure 1. Organization Structure of the Biobank Laboratory
Collection and sample
Biobank category
Population based
Material type
Saliva; DNA; FFPE
Percentage share of the type of biobanked material
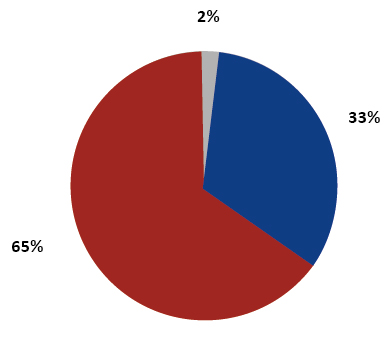
Figure 2. Percentage share of the type of biobanked material
Collections
POPULOUS – saliva; DNA; the collection was created for the project TESTOPLEK; retrospective; anonymized; POPULOUS_BLUL [3-4, 15-23].
BREAST – FFPE; DNA; the collection was created for the project TESTOPLEK; retrospective; anonymized; BREAST_CANCER_COLLECTION_BLUL.
PANC – DNA; saliva; the collection was created for the project TESTOPLEK; retrospective; anonymized; PANCREATIC_CANCER_COLLECTION_BLUL.
PUPILS – saliva; the collection was created within a grant from the Mayor of the City of Łódź (559/VI/11) and project TESTOPLEK; prospective; pseudoanonymized; ANTHROPOLOGICAL_STUDY_PUPILS_BLUL [5, 24-28].
BREAST IBUL – DNA; retrospective; anonymized [29-31].
Geographical origin of samples from the collection
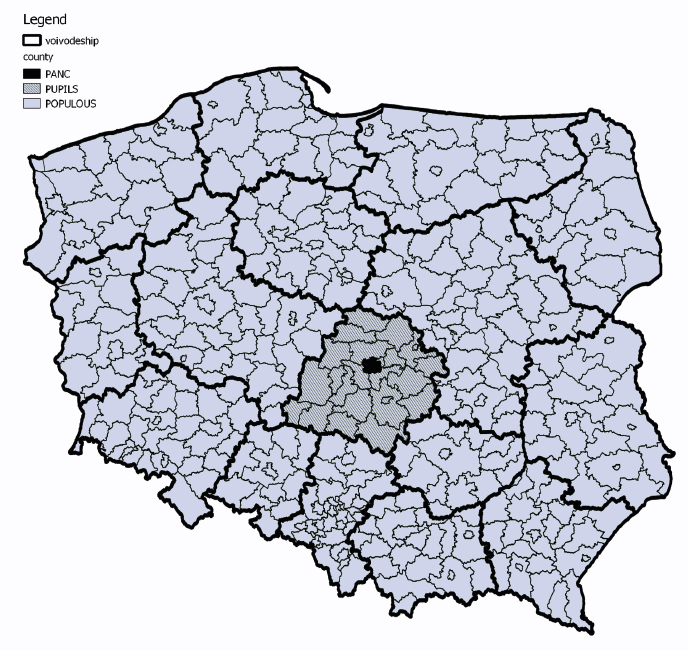
Figure 3. Geographical origin of samples from the collection
BREAST and BREAST IBUL collection have no specific information about geographical origin of donors [32-33].
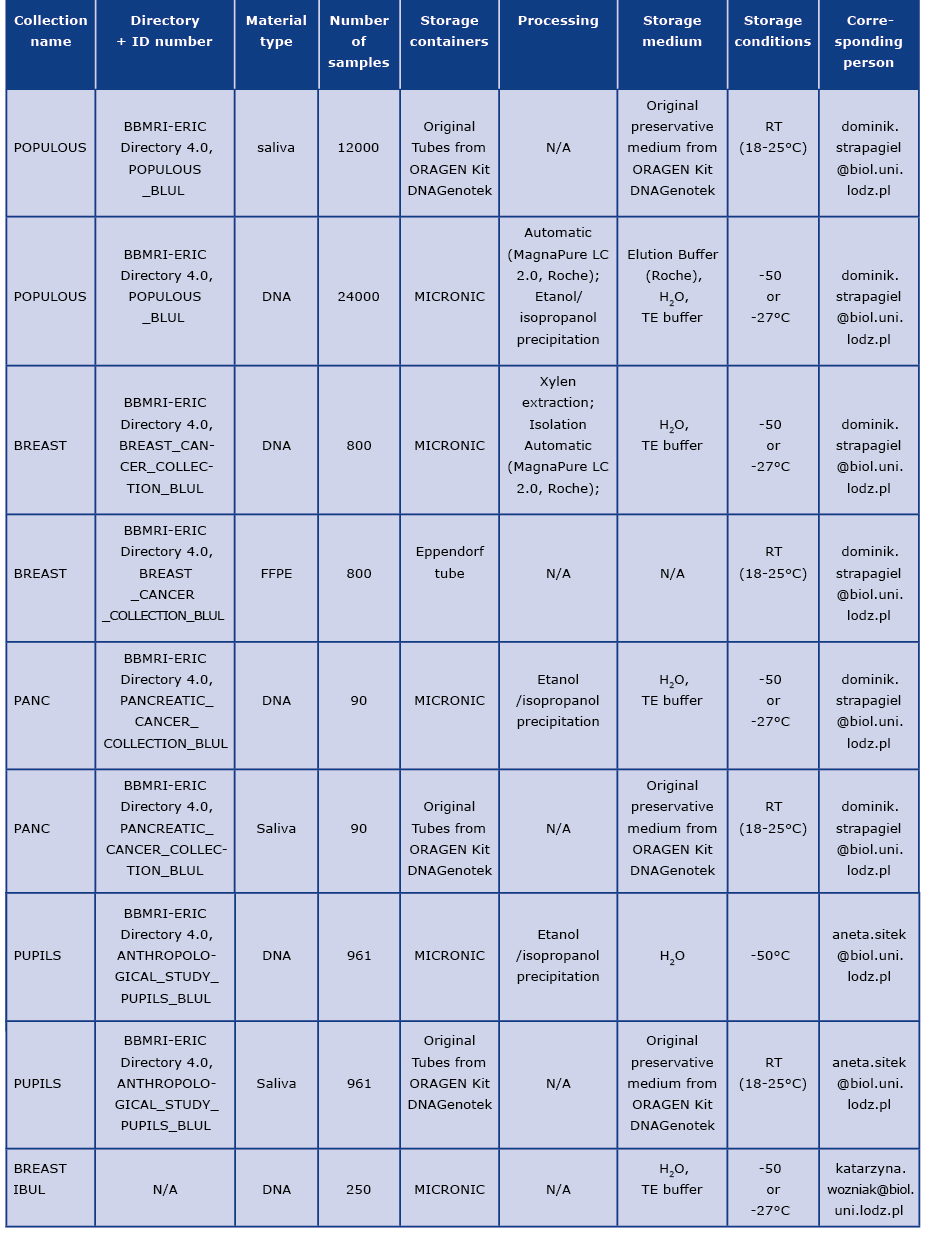
Characteristics of samples in collection
Description of material collected in collections, according to Table 1.
Storage conditions
-50oC; -27oC; room temperature (18-25oC)
Biobank Equipment
- NextSeq500 (Illumina Inc.),
- MinION (Oxford NanoPore Technologies),
- Liquid Handling Robot Janus (Perkin Elmer),
- Liquid Handling Robot MagNa Pure LC 2.0 (Roche),
- Automatic arm for transferring microplates (LabMind),
- TECAN Workstation (Illumina Inc.),
- Scanner iScan (Illumina Inc.),
- ALPS 30 manual heat sealer (Thermo Scientific),
- Low-temperature freezers (-45°C) (Liebherr Medline),
- Low-temperature freezers (-86°C) (New Brunswick Scientific),
- UV3 HEPA PCR Workstations (UVP),
- Laminar Workstations Maxi Safe 2020 (Thermo Scientific),
- Chamber for long storage of DNA in room temperature (BioMatrica),
- ThermalCycler C1000 (BioRad),
- CFX Real-Time PCR Thermal Cycler C1001 (BioRad),
- Automatic liquid handling robot (Perkin Elmer),
- Automatic sample sorter XL20 (BioMicroLab),
- LightScanner (IDAHO Technology)
- Milli-Q (Millipore),
- DNA microplates decapper (Polgen),
- Single TEC control (INHECO),
- Hybex microsample incubators (SciGene),
- Hybridization ovens (Illumina Inc.),
- Qubit 2.0 Fluorometer 2.0 (Invitrogen),
- FastGene Mini centrifuge (NipponGenetics Europe),
- Centrifuge MPW-352R (MPW),
- Pippin Prep (Sage Science),
- DNA Long-Term Stores (BioMatrica).
Donors
POPULOUS – no information about diagnosis, available donor declarations about their diseases (Tumors of soft tissues; Symptomatic atherosclerosis of coronary vessels not treated with stents; Diabetes; Hypothyroidism; Psoriasis; Lung cancer; LDL-C hypercholesterolemia above 130 mg / l; Multiple sclerosis; Symptomatic atherosclerosis of coronary vessels treated with stents; Hyperplasia of the prostate gland; Malignant Hematoma and Non-Hodgkin's Lymphoma; Head and neck region cancer; Breast cancer; Allergy; Myasthenia gravis; Male genital cancers; Cancer of the urinary tract; Osteoporosis; Gastrointestinal neoplasms; Tumors of female genital organs; Leukemia; Thyroid cancer; Tuberculosis, including multi-drug resistant tuberculosis and other mycobacteriosis; Condition after myocardial infarction; Rheumatic diseases; Epilepsy; Parkinson’s disease and syndrome; Alzheimer's disease; Asthma, Chronic obstructive pulmonary disease, eosinophilic bronchitis; Ulcerative colitis and Crohn's disease; Atherosclerosis; Condition after transplantation of a vascularized organ or bone marrow; Peptic ulcer disease – Helicobacter pylori infection detected and treated; Melanomas and skin cancers; Glaucoma); male and female; 18-90 ages.
BREAST – diagnosis: breast cancer / Malignant neoplasm of breast (C50-C50); female; available medical records, information about diagnosis and treatment.
PANC – diagnosis: pancreatic cancer / Malignant neoplasm of pancreas (C25); available medical records, information about diagnosis.
PUPILS – lack of information about diagnosis, available donor declarations about their diseases; male and female; ages 6-16.
BREAST IBUL - diagnosis: breast cancer / Malignant neoplasm of breast (C50-C50); female; available medical records, information about diagnosis.
Directories
- BBMRI-ERIC Directory 4.0 – information about collection [2],
- BioFace - information about biobank’s samples,
- e-Człowiek platform - information about anthropological collection,
- metaBiobank - information about collection [34].
Communication protocol
- BioScoop 1.0 – communication protocol used for BioFace and e-Człowiek platform [35],
- Miabis 2.0 - communication protocol used for BBMRI-ERIC Directory 4.0 [36].
Principles of sharing samples and materials
In order to gain access to biobanked samples, please contact the Biobank Laboratory Manager and present the context of the use of samples in research. The request is subsequently evaluated in terms of formal, legal and ethical compliance with the rules of Biobank Laboratory and Polish law. Following a positive decision, the material is anonymized and transmitted to researcher. Each transfer of samples is contingent upon signing an MTA (Material Transfer Agreement) in which the nature of the access is determined. The researcher may use the samples only for the purposes specified in the MTA.
Samples management system
For the needs of the TESTOPLEK project, Biobank Laboratory created its own system of sample management (SMS). At present, Biobank Laboratory as a member of Polish Biobank Network uses BBMS (BioBank Management System) to manage samples. The SMS system is still used as a form of verifying the correctness of data migration to BBMS [37].
Quality control of samples
Quality control of biobanking samples is based on internal quality procedures (SOP – Standard Operational Procedure). At every step, samples are evaluated by quality tests. Each internal method is verified and validated by qualified Biobank staff. Quality procedures are based on generally accepted standards and the experience and knowledge of employees.
In addition, selected internal biobank procedures are evaluated by external quality tests - Proficiency Testing Program performed by IBBL (Integrated BioBank of Luxembourg) to confirm their quality. The pro-quality testing program is endorsed by ISBER (International Society for Biological and Environmental Repositories) [12, 38].
Collections and samples databases
Format of shared data
Format of genetics/raw sequencing shared data:
- .fastq
- .ped
- .map
- .bam
Format of medical shared data:
- .csv
Format of image shared data:
- .jpg
- .tiff
- .S3Dm
External repository of data
- e-Człowiek.pl platform,
- EGA - European Genome-phenome Archive [39],
- SRA - Sequence Read Archive (available on NCBI) [40],
- WGS – Whole genome sequencing (available on NCBI) [40],
- BioProject (available on NCBI) [40],
- BioSample (available on NCBI) [40].
Principles of sharing data
All data stored by the Biobank Laboratories are subject to strict access control. Access to data can be obtained on the basis of individually negotiated licensing agreements - DTA (Data Transfer Agreement). The DTA determines the detailed scope of data access and processing capabilities. Depending on the scope of the DTA, additional contracts may be required. Shared data should be kept confidential and should only be used for tasks related to the implementation of the agreement. In order to obtain access to data, please contact the Manager of the Biobank Laboratory specifying the use of the data.
Ways of sharing data
Encoded external disc, FTP servers, commercial data sharing services.
Data management system
BBMS – BioBank Management System [37].
Ethics
Institutional Review Board
Any activity that uses biological material collected by the Biobank Laboratory is controlled by the Institutional Review Board of the University of Łódź (IRB). The Head of Biobank Lab is an active member and participates in the legislative work of the IRB.
In order to use the material each researcher should submit an appropriate application to the IRB. IRB gives its opinion on the proposal in terms of its scientific validity and feasibility, and respecting the rights of participants in the research experiment.
After receiving a positive opinion from the IRB, the researcher may start recruiting donors to create a new collection or start research on the already created collection.
Regulations
Every activity of the Biobank Laboratory in the area of biobanking conforms to the following regulations:
- Declaration of Helsinki: ethical principles for medical research involving human subjects [41],
- Data Protection Code, Polish Data Protection Authority [42],
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) [43],
- Resolution of Institutional Review Board of the University of Łódź.
Other information
Form of contact for those interested in cooperation with biobank
Information about collections, samples and data can be found in the catalogues described above. In order to get direct access to resources, please use the data below.
- e-mail: biobank@uni.lodz.pl,
- Phone number: +48 42 665 57 02,
- Corresponding person: Dr. Dominik Strapagiel, dominik.strapagiel@biol.uni.lodz.pl
External Biobank activity
Biobank Laboratory has a modern and very well-equipped laboratory, adapted to perform research in the field of genetics and molecular biology.
The unit cooperates with the public and private sector, offering services such as:
- DNA, RNA tests (DNA and RNA isolation from various biological materials),
- study of genetic variation using classical methods of molecular biology such as: PCR, real-time PCR, Sanger sequencing [18], high-resolution melting of PCR products (HRM) [3-4, 17, 21, 26, 44-45], Taq-Man probes,
- measurements of selected parameters of the tested material (fluorometric testing of the DNA concentration),
- separation of nucleic acids using horizontal, vertical, capillary electrophoresis,
- genomic DNA testing using microarrays (e.g. GWAS) [15-16, 20, 22-23],
- next generation sequencing (Illumina Platform, Nanopore Sequencing),
- gene expression testing (RNA-seq, microarray and qPCR) [46-51],
- epigenetic analysis (Chip-Seq, DNA methylation analysis, methylation RNA),
- microorganism genome analysis (de novo sequencing, variant calling) [44, 52-57],
- eukaryotes whole genome and targeting sequencing,
- aDNA analysis [58-59],
- human genome analysis (variant calling, NGS Target Enrichment, whole exome sequencing),
- amplicon sequencing,
- metagenomics and metabarcoding,
- bioinformatics analysis.
Biobank Laboratory applies an open data policy that allows broad access to data for interested researchers.
Biobank Laboratory conducts teaching and educational activities, engaging in numerous social projects for University of Łódź students and local community in Łódź.
Patent
- Method for identification of pathogenic fungi species contained in a sample taken from a patient – number P.408 734 [60],
- Method for determination of gender – number P. 406 569 [61]
- Method for determination of gender – number P. 423 420 [62],
- Method and a set for the detection of genetic predisposition to having a certain hair colour – number P. 403 360 [63].
Vision and plans for future
In the near future, Biobank Laboratory plans to expand its headquarters and acquire more highest class infrastructure for biobanking. Moreover, the unit plans to implement international standards related to management systems such as PN-EN ISO 9001:2015 Quality management systems – Requirements and PN-EN ISO/IEC 27001:2017 Information security management system – Requirements.
Major barrier
The main challenge for our unit is to recruit donors of biological material. Biobank Laboratory as a university unit is not a medical facility, creating difficulties in contact with potential donors. In addition, considering the population-based character of the biobank, Polish legislation lacks regulations regulating this area of biobanking.
Acknowledgments
The authors would like to thank Łukasz Pułaski for language editing, Jakub Lach and Justyna Jarczak for methodical supporting which improved the man- uscript. This work was supported by Polish Ministry of Science and Higher Education - Project no. DIR/ WK/2017/01
Conflict of interests
The authors declare no conflict of interest.
Table 1. Basic information about material stored in biobank
References
| 1. |
Biobank Łódź. [cited 2019 Feb 15]; Available from: http://www.biobank.uni.lodz.pl/index.php.
|
| 2. |
BBMRI- ERIC Directory 4.0. [cited 2019 Feb 15]; Available from: https://directory.bbmri-eric.eu/menu/main/dataexplorer?entity=eu_bbmri_eric_collections&mod=data&attrs%5B%5D=country&attrs%5B%5D=biobank&attrs%5B%5D=collection&hideselect=true&query%5Bq%5D%5B0%5D%5Boperator%5D=SEARCH&query%5Bq%5D%5B0%5D%5Bvalue%5D=biobank%20lodz.
|
| 3. |
Koszarska M, Kucsma N, Kiss K, Varady G, Gera M, Antalffy G, et al. Screening the expression of ABCB6 in erythrocytes reveals an unexpectedly high frequency of Lan mutations in healthy individuals. PLoS One. 2014;9(10):e111590; Available from: http://doi.org/10.1371/journal.pone.0111590.
|
| 4. |
Słomka M, Sobalska-Kwapis M, Korycka-Machała M, Bartosz G, Dziadek J, Strapagiel D. Genetic variation of the ABC transporter gene ABCC1 (Multidrug resistance protein 1-MRP1) in the Polish population. BMC Genet. 2015;16:114; Available from: http://doi.org/10.1186/s12863-015-0271-3.
|
| 5. |
Sitek A, Rosset I, Strapagiel D, Majewska M, Ostrowska-Nawarycz L, Żądzińska E. Association of FTO gene with obesity in Polish schoolchildren. 2014;77(1):33; Available from: https://doi.org/10.2478/anre-2014-0003.
|
| 6. |
BBMRI.pl. [cited 2019 Feb 15]; Available from: http://www.bbmri.pl/pl/.
|
| 7. |
Witoń M, Strapagiel D, Gleńska-Olender J, Chróścicka A, Ferdyn K, Skokowski J, et al. Organization of BBMRI.pl: The Polish Biobanking Network. Biopreservation and Biobanking. [Review]. 2017;15(3):264-9; Available from: http://doi.org/10.1089/bio.2016.0091.
|
| 8. |
Faculty of Biology and Environmental Protection, University of Łódź. [cited 2019 Feb 15]; Available from: http://www.biol.uni.lodz.pl/pl.
|
| 9. |
University of Łódź. [cited 2019 Feb 15]; Available from: https://www.uni.lodz.pl/.
|
| 10. |
Biobank and Cohort Building Network. [cited 2019 Feb 15]; Available from: http://bcnet.iarc.fr/.
|
| 11. |
European and Middle Eastern Society for Biopreservation and Biobanking. [cited 2019 Feb 15]; Available from: https://esbb.org/.
|
| 12. |
Integrated Biobank of Luxembourg - Biospecimen Proficiency Testing. [cited 2019 Feb 15]; Available from: https://www.ibbl.lu/ibbl-bioservices/biospecimen-proficiency-testing/.
|
| 13. |
Lewczuk P, Gaignaux A, Kofanova O, Ermann N, Betsou F, Brandner S, et al. Interlaboratory proficiency processing scheme in CSF aliquoting: implementation and assessment based on biomarkers of Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):87, Available from: https://doi.org/10.1186/s13195-018-0418-3.
|
| 14. |
CQI-IRCA. [cited 2019 Feb 15]; Available from: https://www.quality.org/.
|
| 15. |
Sobalska-Kwapis M, Smolarz B, Słomka M, Szaflik T, Kępka E, Kulig B, et al. New variants near RHOJ and C2, HLA-DRA region and susceptibility to endometriosis in the Polish population-The genome-wide association study. Eur J Obstet Gynecol Reprod Biol. 2017;217:106-12; Available from: http://doi.org/10.1016/j.ejogrb.2017.08.037.
|
| 16. |
Sobalska-Kwapis M, Suchanecka A, Słomka M, Siewierska-Górska A, Kępka E, Strapagiel D. Genetic association of FTO/IRX region with obesity and overweight in the Polish population. PLoS One. 2017;12(6):e0180295; Available from: http://doi.org/10.1371/journal.pone.0180295.
|
| 17. |
Ratajewski M, Słomka M, Karaś K, Sobalska-Kwapis M, Korycka-Machała M, Sałkowska A, et al. Functional Analysis of the rs774872314, rs116171003, rs200231898 and rs201107751 Polymorphisms in the Human RORgammaT Gene Promoter Region. Genes (Basel). 2017;8(4): 126; Available from: http://doi.org/10.3390/genes8040126.
|
| 18. |
Siewierska-Górska A, Sitek A, Żądzińska E, Bartosz G, Strapagiel D. Association of five SNPs with human hair colour in the Polish population. Homo. 2017;68(2):134-44; Available from: http://doi.org/10.1016/j.jchb.2017.02.002.
|
| 19. |
Rosset I, Żądzińska E, Strapagiel D, Grzelak A, Henneberg M. Association between body height and month of birth among women of European origin in northern and southern hemispheres. Am J Hum Biol. 2017;29(3):e22967; Available from: http://doi.org/10.1002/ajhb.22967.
|
| 20. |
Nedoszytko B, Siemińska A, Strapagiel D, Dabrowski S, Słomka M, Sobalska-Kwapis M, et al. High prevalence of carriers of variant c.1528G>C of HADHA gene causing long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) in the population of adult Kashubians from North Poland. PLoS One. 2017;12(11):e0187365; Available from: http://doi.org/10.1371/journal.pone.0187365.
|
| 21. |
Słomka M, Sobalska-Kwapis M, Wachulec M, Bartosz G, Strapagiel D. High Resolution Melting (HRM) for High-Throughput Genotyping-Limitations and Caveats in Practical Case Studies. Int J Mol Sci. 2017;18(11):2316; Available from: http://doi.org/10.3390/ijms18112316.
|
| 22. |
Romanowicz H, Strapagiel D, Słomka M, Sobalska-Kwapis M, Kępka E, Siewierska-Górska A, et al. New single nucleotide polymorphisms (SNPs) in homologous recombination repair genes detected by microarray analysis in Polish breast cancer patients. Clin Exp Med. 2017;17(4):541-6; Available from: http://doi.org/10.1007/s10238-016-0441-2.
|
| 23. |
Jarczak J, Grochowalski L, Marciniak B, Lach J, Słomka M, Sobalska-Kwapis M, et al. Mitochondrial DNA variability of the Polish population. Eur J Hum Genet. 2019; Available from: http://doi.org/10.1038/s41431-019-0381-x.
|
| 24. |
Sitek A, Rosset I, Żądzińska E, Siewierska-Górska A, Pietrowska E, Strapagiel D. Selected gene polymorphisms effect on skin and hair pigmentation in Polish children at the prepubertal age. Anthropol Anz. 2016;73(4):283-93; Available from: http://doi.org/10.1127/anthranz/2016/0632.
|
| 25. |
Pruszkowska-Przybylska P, Sitek A, Rosset I, Sobalska-Kwapis M, Słomka M, Strapagiel D, et al. Association of the 2D:4D digit ratio with body composition among the Polish children aged 6-13years. Early Hum Dev. 2018;124:26-32; Available from: http://doi.org/10.1016/j.earlhumdev.2018.08.001.
|
| 26. |
Rosset I, Strapagiel D, Sitek A, Majewska M, Ostrowska-Nawarycz L, Żądzińska E. Association of FTO and TMEM18 polymorphisms with overweight and obesity in the population of Polish children. Anthropological review. 2016;79(1):17-33; Available from: http://doi.org/10.1515/anre-2016-0002.
|
| 27. |
Pruszkowska-Przybylska P, Sitek A, Rosset I, Żądzińska E, Sobalska-Kwapis M, Słomka M, et al. The association between socioeconomic status, duration of breastfeeding, parental age and birth parameters with BMI, body fat and muscle mass among prepubertal children in Poland. Anthropol Anz. 2019; Available from: http://doi.org/10.1127/anthranz/2019/0955.
|
| 28. |
Kukla-Bartoszek M, Pośpiech E, Spólnicka M, Karłowska-Pik J, Strapagiel D, Żądzińska E, et al. Investigating the impact of age-depended hair colour darkening during childhood on DNA-based hair colour prediction with the HIrisPlex system. Forensic Sci Int Genet. 2018;36:26-33; Available from: http://doi.org/10.1016/j.fsigen.2018.06.007.
|
| 29. |
Sassi A, Popielarski M, Synowiec E, Morawiec Z, Woźniak K. BLM and RAD51 genes polymorphism and susceptibility to breast cancer. Pathol Oncol Res. 2013;19(3):451-9; Available from: http://doi.org/10.1007/s12253-013-9602-8.
|
| 30. |
Synowiec E, Krupa R, Morawiec Z, Wasylecka M, Dziki L, Morawiec J, et al. Efficacy of DNA double-strand breaks repair in breast cancer is decreased in carriers of the variant allele of the UBC9 gene c.73G>A polymorphism. Mutat Res. 2010;694(1-2):31-8; Available from: http://doi.org/10.1016/j.mrfmmm.2010.09.002.
|
| 31. |
Synowiec E, Stefańska J, Morawiec Z, Błasiak J, Woźniak K. Association between DNA damage, DNA repair genes variability and clinical characteristics in breast cancer patients. Mutat Res. 2008;648(1-2):65-72; Available from: http://doi.org/10.1016/j.mrfmmm.2008.09.014.
|
| 32. |
GIS Support. [cited 2019 Feb 15]; Available from: http://gis-support.com/.
|
| 33. |
QGIS. [cited 2019 Feb 15]; Available from: http://qgis.org.
|
| 34. |
metaBiobank. [cited 2019 Feb 15]; Available from: https://biobank.plgrid.pl/.
|
| 35. |
BioSCOOP. [cited 2019 Feb 15]; Available from: https://github.com/BiobankLab/BioSCOOP.
|
| 36. |
Merino-Martinez R, Norlin L, van Enckevort D, Anton G, Schuffenhauer S, Silander K, et al. Toward Global Biobank Integration by Implementation of the Minimum Information About BIobank Data Sharing (MIABIS 2.0 Core). Biopreserv Biobank. 2016;14(4):298-306; Available from: http://doi.org/10.1089/bio.2015.0070.
|
| 37. |
Michalska-Madej J, Dobrowolska S, Strapagiel D. Application of BBMS in the biobanks storage management. Eur J Trans Clin Med. 2018;1(Suppl.4):72; Available from: https://ejctm.gumed.edu.pl/issues/5.
|
| 38. |
ISBER. 2012 best practices for repositories collection, storage, retrieval, and distribution of biological materials for research international society for biological and environmental repositories. Biopreserv Biobank. 2012;10(2):79-161; Available from: http://doi.org/10.1089/bio.2012.1022.
|
| 39. |
EGA. [cited 2019 Feb 15]; Available from: https://www.ebi.ac.uk/ega/home.
|
| 40. |
National Center for Biotechnology Information. [cited 2019 Feb 15]; Available from: https://www.ncbi.nlm.nih.gov/.
|
| 41. |
World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4; Available from: http://doi.org/10.1001/jama.2013.281053.
|
| 42. |
Polish Data Protection Authority. [cited 2019 Feb 15]; Available from: http://prawo.sejm.gov.pl/isap.nsf/home.xsp.
|
| 43. |
Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) 2016 [cited 2019 Feb 15]; Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32016R0679.
|
| 44. |
Caban M, Strapagiel D, Dziadek J, Korycka-Machała M, Grzelak A. Principles of a New Protocol for Prediction of Azole Resistance in Candida albicans Infections on the Basis of ERG11 Polymorphisms. Curr Microbiol. 2016;73(2):172-82; Available from: http://doi.org/10.1007/s00284-016-1039-3.
|
| 45. |
Sitek A, Rosset I, Strapagiel D, Majewska M, Ostrowska-Nawarycz L, Żądzińska E. Association of FTO gene with obesity in Polish schoolchildren. Anthropological review. 2014;77(1):33-44; Available from: http://doi.org/10.2478/anre-2014-0003.
|
| 46. |
Karaś K, Salkowska A, Sobalska-Kwapis M, Walczak-Drzewiecka A, Strapagiel D, Dastych J, et al. Digoxin, an Overlooked Agonist of RORgamma/RORgammaT. Front Pharmacol. 2018;9:1460; Available from: http://doi.org/10.3389/fphar.2018.01460.
|
| 47. |
Pułaski L, Jatczak-Pawlik I, Sobalska-Kwapis M, Strapagiel D, Bartosz G, Sadowska-Bartosz I. 3-bromopyruvate induces expression of antioxidant genes. Free Radic Res. 2018;53(2):170-178; Available from: http://doi.org/10.1080/10715762.2018.1541176.
|
| 48. |
Antczak M, Płocińska R, Płociński P, Rumijowska-Galewicz A, Żaczek A, Strapagiel D, et al. The NnaR orphan response regulator is essential for the utilization of nitrate and nitrite as sole nitrogen sources in mycobacteria. Sci Rep. 2018;8(1):17552; Available from: http://doi.org/10.1038/s41598-018-35844-z.
|
| 49. |
Reczyńska D, Witek B, Jarczak J, Czopowicz M, Mickiewicz M, Kaba J, et al. The impact of organic vs. inorganic selenium on dairy goat productivity and expression of selected genes in milk somatic cells. J Dairy Res. 2019;86(1):48-54; Available from: http://doi.org/10.1017/S0022029919000037.
|
| 50. |
Karwaciak I, Salkowska A, Karaś K, Sobalska-Kwapis M, Walczak-Drzewiecka A, Pułaski L, et al. SIRT2 Contributes to the Resistance of Melanoma Cells to the Multikinase Inhibitor Dasatinib. Cancers (Basel). 2019;11(5):673; Available from: http://doi.org/10.3390/cancers11050673.
|
| 51. |
Płociński P, Macios M, Houghton J, Niemiec E, Płocińska R, Brzostek A, et al. Proteomic and transcriptomic experiments reveal an essential role of RNA degradosome complexes in shaping the transcriptome of Mycobacterium tuberculosis. Nucleic Acids Res. 2019; Available from: http://doi.org/10.1093/nar/gkz251.
|
| 52. |
Kolsut J, Borowka P, Marciniak B, Wojcik E, Wojtasik A, Strapagiel D, et al. In silico Analysis of Virulence Associated Genes in Genomes of Escherichia Coli Strains Causing Colibacillosis in Poultry. J Vet Res. 2017;61(4):421-6; Available from: http://doi.org/10.1515/jvetres-2017-0051.
|
| 53. |
Bakuła Z, Brzostek A, Borówka P, Żaczek A, Szulc-Kiełbik I, Podpora A, et al. Molecular typing of Mycobacterium kansasii using pulsed-field gel electrophoresis and a newly designed variable-number tandem repeat analysis. Sci Rep. 2018;8(1):4462; Available from: http://doi.org/10.1038/s41598-018-21562-z.
|
| 54. |
Borówka P, Lach J, Bakuła Z, van Ingen J, Safianowska A, Brzostek A, et al. Draft Genome Sequences of Mycobacterium kansasii Clinical Strains. Genome Announc. 2017;5(22):e00406-17; Available from: http://doi.org/10.1128/genomeA.00406-17.
|
| 55. |
Labudda L, Strapagiel D, Karczewska-Golec J, Golec P. Complete Annotated Genome Sequences of Four Klebsiella pneumoniae Phages Isolated from Sewage in Poland. Genome Announc. 2017;5(45):e00919-17; Available from: http://doi.org/10.1128/genomeA.00919-17.
|
| 56. |
Karczewska-Golec J, Strapagiel D, Sadowska M, Szalewska-Pałasz A, Golec P. Draft Genome Sequence of Shewanella baltica M1 Isolated from Brackish Surface Water of the Gulf of Gdansk. Genome Announc. 2016:4(3):e00611-16; Available from: http://doi.org/10.1128/genomeA.00611-16.
|
| 57. |
Strapagiel D, Borówka P, Marciniak B, Bakuła Z, van Ingen J, Safianowska A, et al. Draft Genome Sequences of Mycobacterium kansasii Strains 1010001454, 1010001458, 1010001468, 1010001493, 1010001495, and 1010001469, Isolated from Environmental Sources. Genome Announc. 2016 Jun;4(3):e00456-16; Available from: http://doi.org/10.1128/genomeA.00456-16.
|
| 58. |
Fernandes DM, Strapagiel D, Borówka P, Marciniak B, Żądzińska E, Sirak K, et al. A genomic Neolithic time transect of hunter-farmer admixture in central Poland. Sci Rep. 2018;8(1):14879; Available from: http://doi.org/10.1038/s41598-018-33067-w.
|
| 59. |
Lorkiewicz W, Płoszaj T, Jędrychowska-Dańska K, Żądzińska E, Strapagiel D, Haduch E, et al. Between the Baltic and Danubian worlds: the genetic affinities of a middle neolithic population from central Poland. PLoS One. 2015;10(2):e0118316; Available from: http://doi.org/10.1371/journal.pone.0118316.
|
| 60. |
Caban M, Strapagiel D, Bartosz G, Stączek P, Ciesielska A, Gadzalski M, et al., inventors; Univeristy of Lodz, assignee. The method of identifying pathogenic fungus species contained in a sample taken from a patient. Poland patent P. 408 734. 2018.
|
| 61. |
Strapagiel D, Słomka M, Majewska M, Janik K, Sobalska M, Bartosz G, inventors; University of Łódź, assignee. Method for determination of gender. Poland patent P. 406 569. 2019.
|
| 62. |
Strapagiel D, Słomka M, Majewska M, Janik K, Sobalska M, Bartosz G, inventors; University of Łódź, assignee. Method for determination of gender. Poland patent P. 423 420. 2019.
|
| 63. |
Strapagiel D, Siewierska-Górska A, Bartosz G, Żądzińska E, Sitek A, inventors; University of Łódź, assignee. Method and a set for the detection of genetic predisposition to having a certain hair colour. Poland patent P. 403 360. 2013.
|










